Vanadium Pentoxide Nanobelt-Reduced Graphene Oxide Nanosheet Composites as High-Performance Pseudocapacitive Electrodes: ac Impedance Spectroscopy Data Modeling and Theoretical Calculations
Abstract
:1. Introduction
2. Methodology, Materials, Methods and Characterization
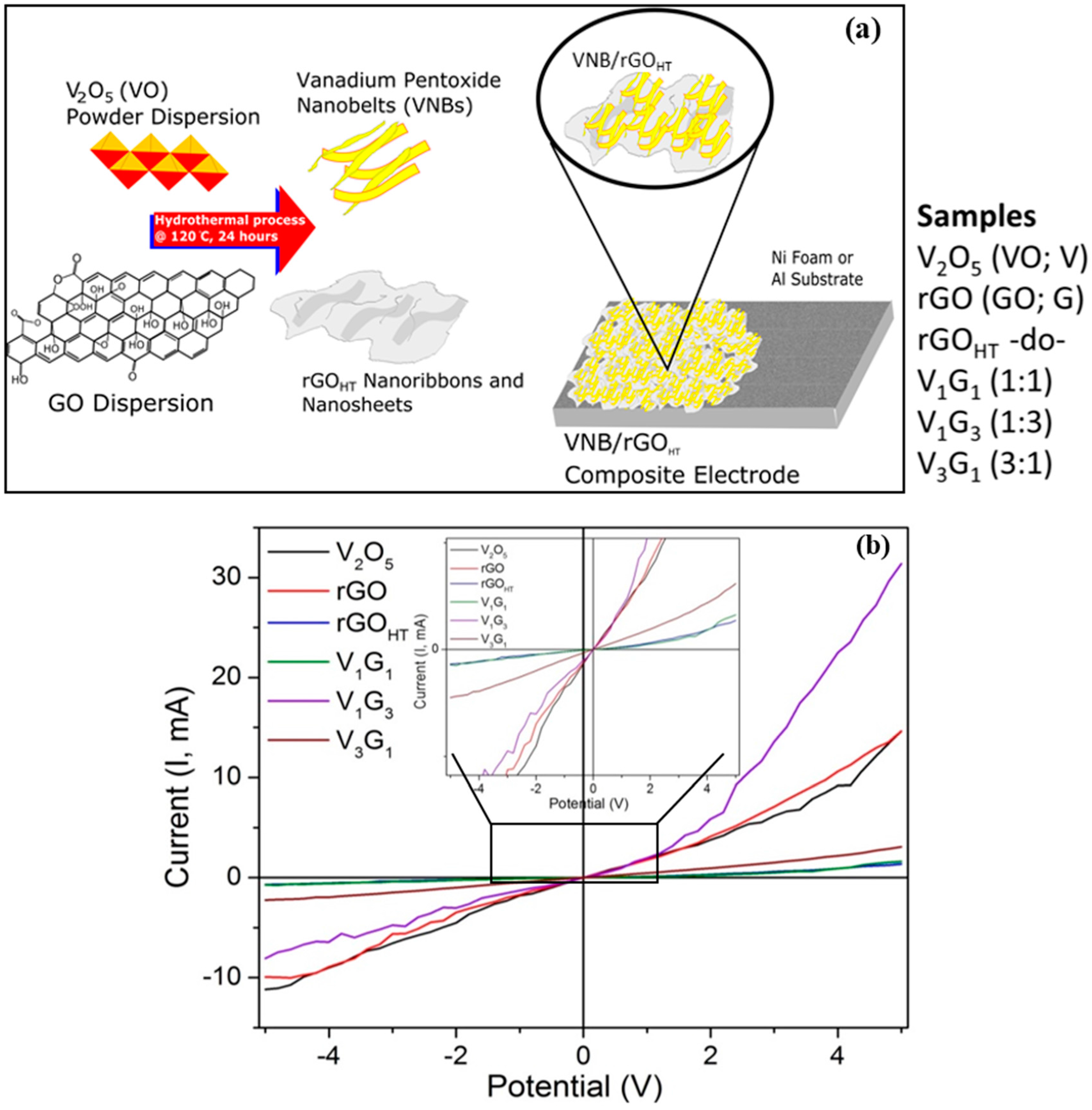
2.1. Hydrothermal Synthesis and Electrode Preparation
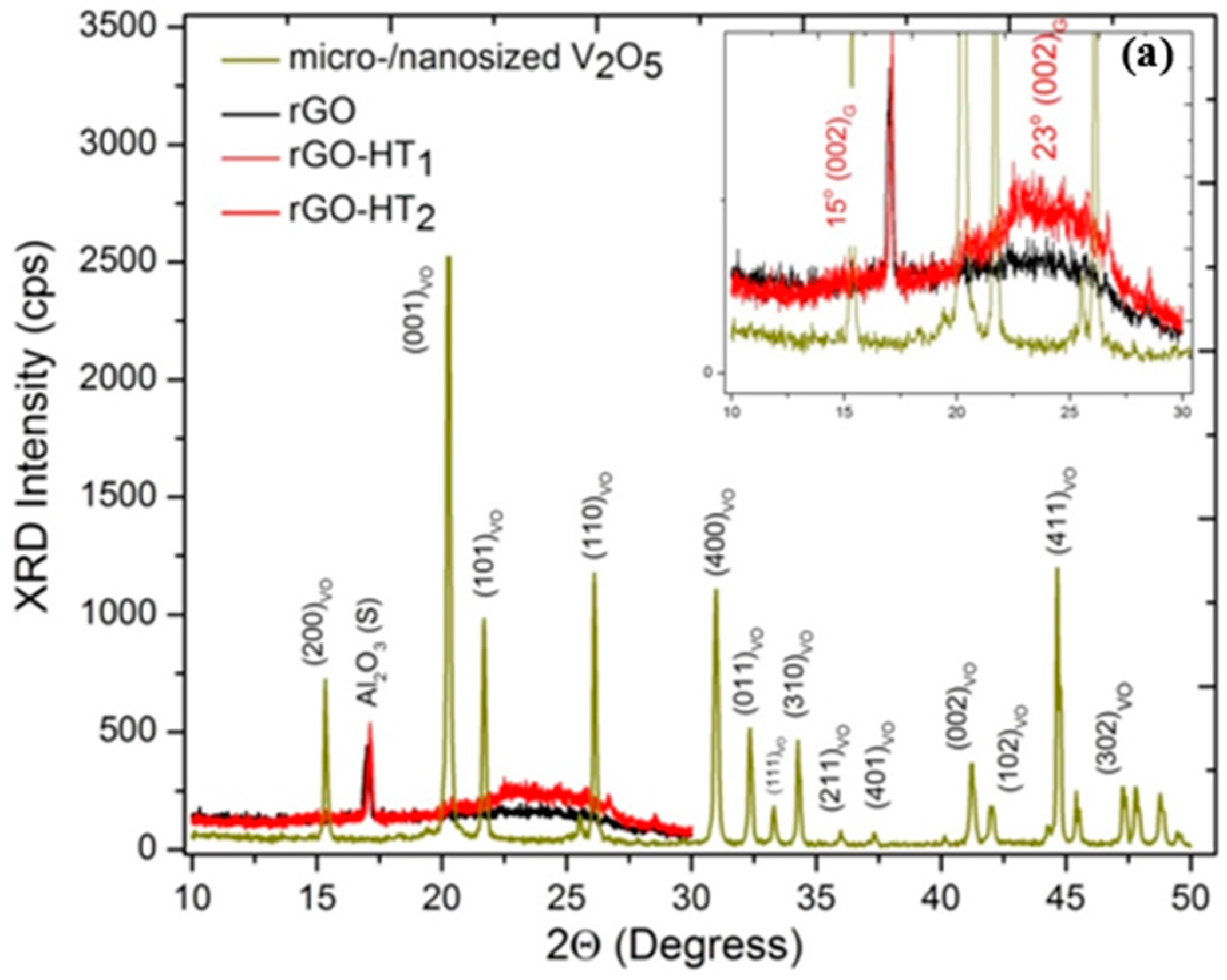
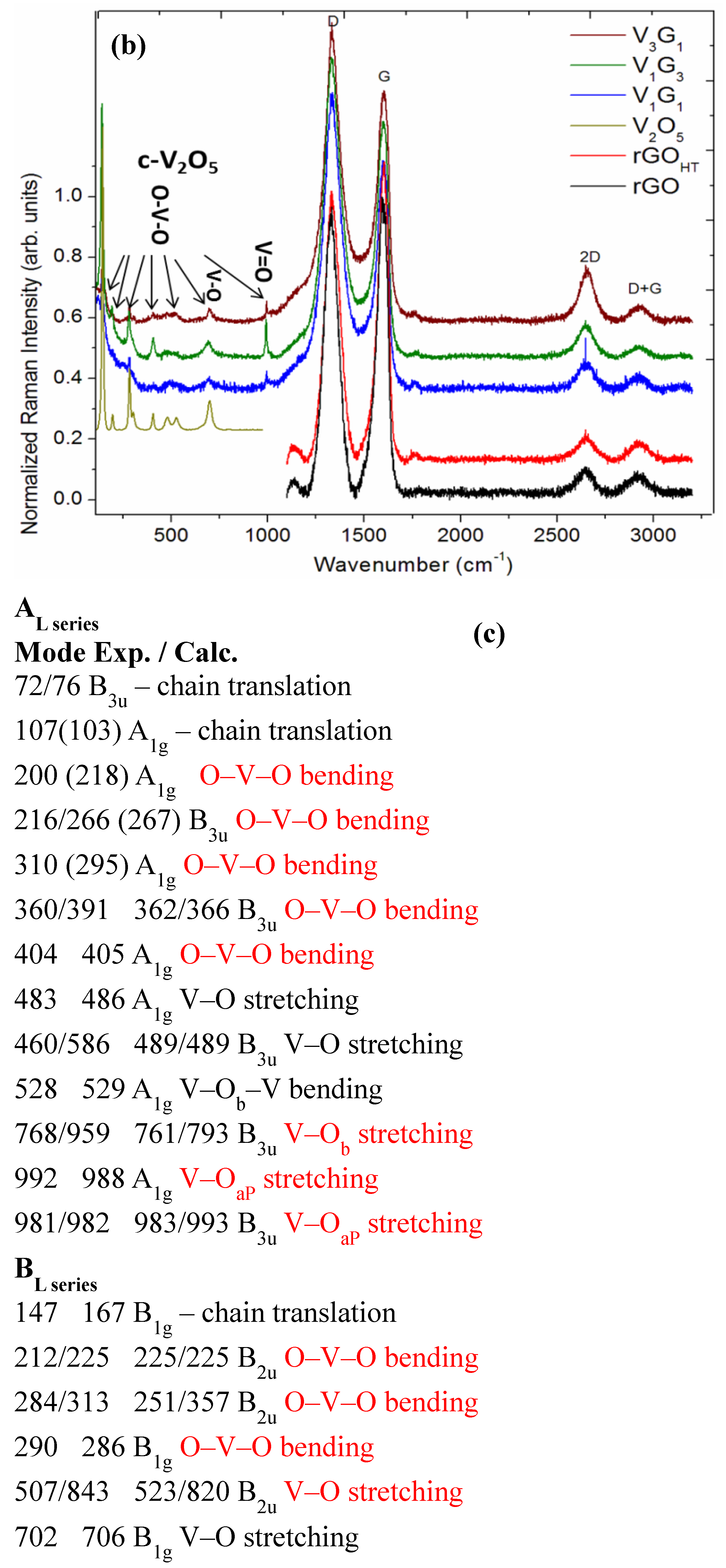
2.2. Sample Characterization
3. Results and Discussion
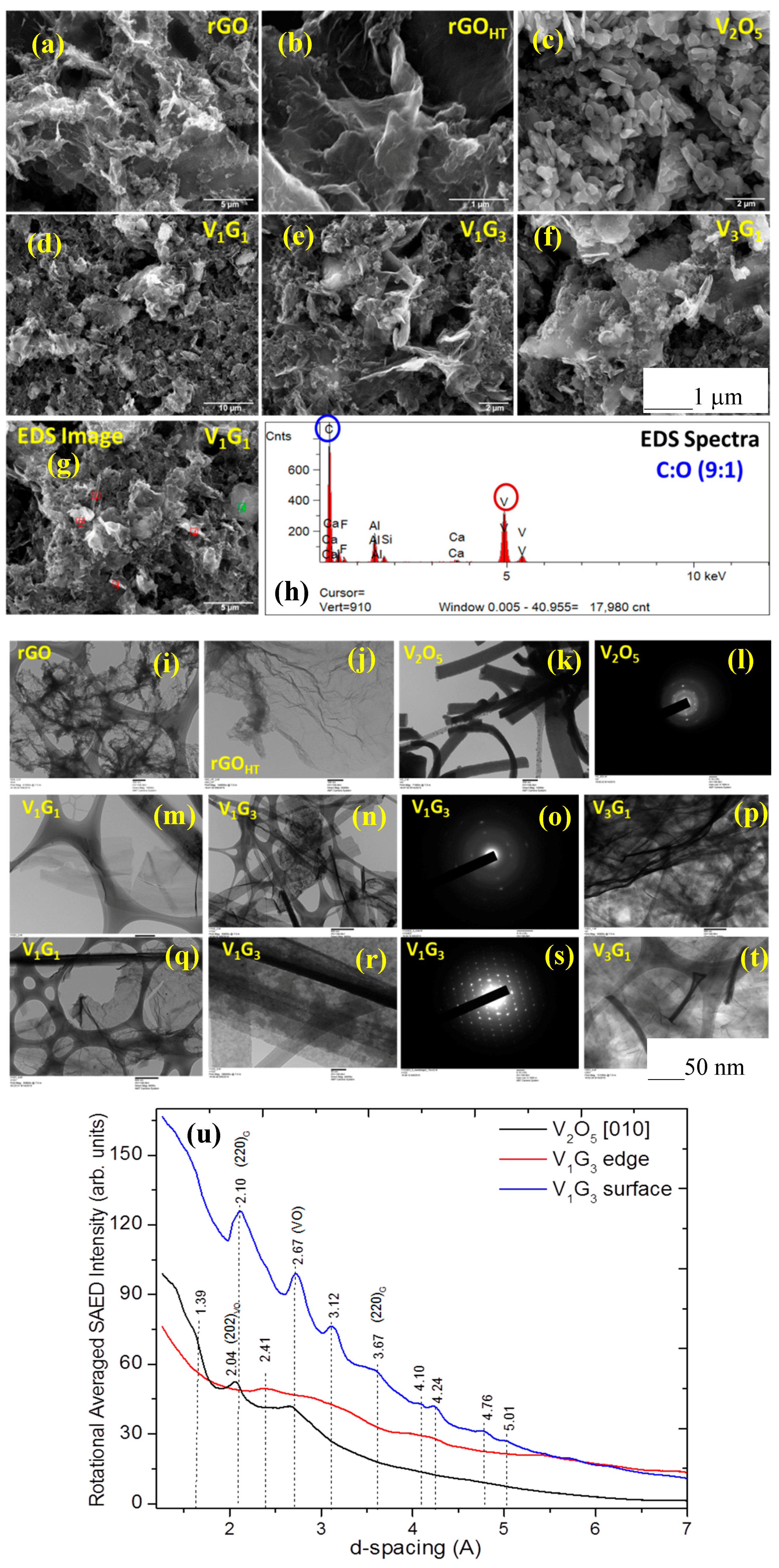
3.1. Microscopic Structural Characterization
3.2. Electrochemical Properties and Impedance Data Simulation
3.3. Density Functional Theory Simulations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and thermal reduction of graphene oxide: Reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Makino, S.; Yamauchi, Y.; Sugimoto, W. Synthesis of electro-deposited ordered mesoporous RuOx using lyotropic liquid crystal and application toward micro-supercapacitors. J. Power Sources 2013, 227, 153–158. [Google Scholar] [CrossRef]
- Ren, Z.; Li, J.; Ren, Y.; Wang, S.; Liu, Y.; Yu, J. Large-scale synthesis of hybrid metal oxides through metal redox mechanism for high-performance pseudocapacitors. Sci. Rep. 2016, 6, 20021:1–20021:10. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; van Meveren, M.; Jasinski, J. Graphene-based hybrids with manganese oxide polymorphs as tailored interfaces for electrochemical energy storage: Synthesis, processing, and properties. J. Electron. Mater. 2015, 44, 62–78. [Google Scholar] [CrossRef]
- Conway, B.E.; Bliss, V.; Wojtowicz, J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Sources 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Kluwer Academic Plenum: New York, NY, USA, 1999. [Google Scholar]
- Lee, T.; Yun, T.; Park, B.; Sharma, B.; Song, H.-K.; Kim, B.-S. Hybrid multilayer thin film supercapacitor of graphene nanosheets with polyaniline: Importance of establishing intimate electronic contact through nanoscale blending. J. Mater. Chem. 2012, 22, 21092–21099. [Google Scholar] [CrossRef] [Green Version]
- Ellis, B.L.; Knauth, P.; Djenizian, T. Three-dimensional self-supported metal oxides for advanced energy storage. Adv. Mater. 2014, 26, 3368–3397. [Google Scholar] [CrossRef] [PubMed]
- Dai, L. Functionalization of graphene for efficient energy conversion and storage. Acc. Chem. Res. 2012, 46, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-C.; Xu, H.; Wang, X.-W.; Huang, Y.-X.; Park, M.B.C.; Zhang, H.; Wang, L.-H.; Huang, W.; Chen, P. 3D Graphene–cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qi, X.Y.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Zhu, H.; Vajtai, R.; Ajayan, M.P.; Wei, B. Hydrothermal synthesis and pseudocapacitance properties of MnO2 nanostructures. J. Phys. Chem. B 2005, 109, 20207–20214. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Carrizosa, S.B. Graphene–inorganic hybrids with cobalt oxide polymorphs for electrochemical energy systems and electrocatalysis: Synthesis, processing and properties. J. Electron. Mater. 2015, 44, 4492–4509. [Google Scholar] [CrossRef]
- Gupta, S.; van Meveren, M.; Jasinski, J. Investigating electrochemical properties and interfacial processes of manganese oxides/graphene hybrids as high-performance supercapacitor electrodes. Int. J. Electrochem. Sci. 2015, 10, 10272–10291. [Google Scholar]
- Bai, S.; Zhang, K.; Wang, L.; Sun, J.; Luo, R.; Li, D.; Chen, A. Synthesis mechanism and gas-sensing application of nanosheet-assembled tungsten oxide microspheres. J. Mater. Chem. A 2014, 2, 7927–7933. [Google Scholar] [CrossRef]
- Lee, M.; Balasingham, S.K.; Jeong, H.Y.; Hong, W.G.; Lee, H.-B.-R.; Kim, B.H.; Jun, Y. One-step hydrothermal synthesis of graphene decorated V2O5 nanobelts for enhanced electrochemical energy storage. Sci. Rep. 2015, 5, 8151–8176. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Vu, A.; Smyrl, W.; Stein, A. Facile preparation and electrochemical properties of V2O5-graphene composite films as free-standing cathodes for rechargeable lithium batteries. J. Electrochem. Soc. 2012, 159, A1135–A1145. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Z.-F.; Liu, Y.; Zhang, H.; Ren, Y.; Sun, C.-J.; Lu, W.; Zhou, Y.; Stanciu, L.; Stach, E.A.; et al. Graphene-modified nanostructured vanadium pentoxide hybrids with extraordinary electrochemical performance for Li-ion batteries. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-F.; Zhang, H.; Liu, Q.; Liu, Y.; Stanciu, L.; Xie, J. Novel Pyrolyzed polyaniline-grafted silicon nanoparticles encapsulated in graphene sheets as li-ion battery anodes. ACS Appl. Mater. Interfaces 2014, 6, 18894–18900. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A. Transition Metal Oxides: An Introduction to Their Electronic Structure and Properties; Clarendon Press: Oxford, UK, 1992. [Google Scholar]
- Tang, Y.; Rui, X.; Zhang, Y.; Lim, T.M.; Dong, Z.; Hng, H.H.; Chen, X.; Yan, Q.; Chen, Z. Vanadium pentoxide cathode materials for high-performance lithium-ion batteries enabled by a hierarchical nanoflower structure via an electrochemical process. J. Mater. Chem. A 2013, 1, 82–86. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Sathiya, M.; Prakash, A.S.; Ramesha, K.; Tarascon, J.M.; Shukla, A.K. V2O5-anchored carbon nanotubes for enhanced electrochemical energy storage. J. Am. Chem. Soc. 2011, 133, 16291–16299. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dai, H. Strongly coupled inorganic–nano-carbon hybrid materials for energy storage. Chem. Soc. Rev. 2013, 42, 3088–3113. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, X.; Luo, W.; Huang, Y.J. Ultrathin CoO/Graphene hybrid nanosheets: A highly stable anode material for Lithium-ion batteries. Phys. Chem. C 2012, 116, 20794–20799. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Wang, H.; Tu, F.; Fang, D.; Li, Y. Facile and green synthesis of Co3O4 nanoplates/graphene nanosheets composite for supercapacitor. J. Sol. Stat. Electrochem. 2012, 16, 3593–3602. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, S.; Wu, L.; Sun, S. Co/CoO Nanoparticles assembled on graphene for electrochemical reduction of oxygen. Angew. Chem. Int. Ed. 2012, 51, 11770–11773. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Wang, H.; Dai, H. Strongly coupled inorganic/nanocarbon hybrid materials for advanced electrocatalysis. J. Am. Chem. Soc. 2013, 135, 2013–2036. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; An, J.; Potts, R.J.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite- and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Byrappa, K.; Yoshimura, M. Handbook of Hydrothermal Technology; Noyes Publications: Park Ridge, NJ, USA, 2001. [Google Scholar]
- Roy, R. Acceleration the kinetics of low-temperature inorganic syntheses. J. Sol. Stat. Chem. 1994, 111, s11. [Google Scholar] [CrossRef]
- Wesselowski, W.S.; Wassiliew, K.W. Zwillingsbildungen Bei Graphitkristallen, Zeitschrift für Kristallographie; DE GRUYTER: Münster, Germany, 1934; Volume 89, pp. 494–496. [Google Scholar]
- Dovesi, R.; Saunders, V.R.; Roetti, C.; Orlando, R.; Zicovich-Wilson, C.M.; Pascale, F.; Civalleri, B.; Doll, K.; Harrison, N.M.; Bush, I.J.; et al. CRYSTAL09 User’s Manual (University of Torino, Torino, 2009). Available online: http://www.crystal.unito.it (accessed on 19 July 2016).
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Peintinger, M.F.; Oliveira, D.V.; Bredow, T. Consistent Gaussian basis sets of triple-zeta valence with polarization quality for solid-state calculations. J. Comput. Chem. 2013, 34, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Häser, M.; Patzelt, H.; Ahlrichs, R. RI-MP2: Optimized auxiliary basis sets and demonstration of efficiency. Chem. Phys. Lett. 1998, 294, 143–152. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Gilat, G.; Raubenheimer, L.J. Accurate Numerical method for calculating frequency-distribution functions in solids. Phys. Rev. 1966, 144, 390–395. [Google Scholar] [CrossRef]
- Gilat, G. Analysis of methods for calculating spectral properties in solids. J. Comput. Phys. 1972, 10, 432–465. [Google Scholar] [CrossRef]
- Anderson, D.G. Iterative procedures for nonlinear integral equations. J. Assoc. Comput. Mach. 1965, 12, 547–560. [Google Scholar] [CrossRef]
- He, T.; Chen, D.R.; Jiao, X.L.; Wang, Y.L. Co3O4 Nanoboxes: Surfactant-templated fabrication and microstructure characterization. Adv. Mater. 2006, 18, 1078–1082. [Google Scholar] [CrossRef]
- Xia, X.H.; Tu, J.P.; Zhang, Y.Q.; Mai, Y.J.; Wang, X.L.; Gu, C.D.; Zhao, X.B. Freestanding Co3O4 nanowire array for high performance supercapacitors. RSC Adv. 2012, 2, 1835–1841. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Pearson: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Avansi, W., Jr.; Ribeiro, C.; Leite, E.R.; Mastelaro, V.R. Vanadium pentoxide nanostructures: An effective control of morphology and crystal structure in hydrothermal conditions. Cryst. Growth Des. 2009, 9, 3626–3631. [Google Scholar] [CrossRef]
- Xiong, C.; Aliev, A.E.; Gnade, B.; Balkus, K.J., Jr. Fabrication of silver vanadium oxide and V2O5 nanowires for electrochromics. ACS Nano 2008, 2, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Lewis, M.B.; Blau, P.J.; Mansur, L.K. Improved surface properties of polymer materials by multiple ion beam treatment. J. Mater. Res. 1991, 6, 610–628. [Google Scholar] [CrossRef]
- Kim, B.H.; Hong, W.G.; Moon, H.R.; Lee, S.M.; Kim, J.M.; Kang, S.; Jun, Y.; Kimet, H.J. Investigation on the existence of optimum interlayer distance for H 2 uptake using pillared-graphene oxide. Int. J. Hydrog. Energy 2012, 37, 14217–14222. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, Q.; Tang, L.A.L.; Zhong, Y.; Loh, K.P. Hydrothermal dehydration for the “Green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties. Chem. Mater. 2009, 21, 2950–2956. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Isobe, M.; Ueda, Y. Magnetic susceptibility of quasi-one-dimensional compound α′- NaV2O5 –possible spin-peierls compound with high critical temperature of 34 K–. J. Phys. Soc. Jpn. 1996, 65, 1178–1181. [Google Scholar] [CrossRef]
- Popovic, Z.; Konstantinovic, M.J.; Gajic, R.; Ueda, Y. Optical phonons in spin-Peierls compound Na2V2O5. J. Condens. Matt. 1998, 10, L513–L519. [Google Scholar] [CrossRef]
- Clauws, P.; Broeckx, J.; Vennik, J. Lattice Vibrations of V2O5. Calculation of normal vibrations in a urey-bradley force field. Phys. Status Solidi B 1985, 131, 459–473. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Hermann, K.; Druzinic, R.; Witko, M.; Wagner, F.; Petersen, M. Geometric and electronic structure of vanadium pentoxide: A density functional bulk and surface study. Phys. Rev. B 1998, 59, 10583–10586. [Google Scholar] [CrossRef]
- Gupta, S.; Saxena, A. Nanocarbon materials: Probing the curvature and topology effects using phonon spectra. J. Raman Spectrosc. 2009, 40, 1127–1137. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Eklund, P.C. Phonons in carbon nanotubes. Adv. Phys. 2000, 49, 705–815. [Google Scholar] [CrossRef]
- Perera, S.D.; Liyanage, A.D.; Nijem, N.; Ferraris, J.P.; Chabal, Y.J.; Balkus, K.J., Jr. Vanadium oxide nanowire—Graphene binder free nanocomposite paper electrodes for supercapacitors: A facile green approach. J. Power Sources 2013, 230, 130–140. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Marschilok, A.C.; Subramanian, A.; Takeuchi, K.J.; Takeuchi, E.S. Synthesis and characterization of sodium vanadium oxide gels: The effects of water (n) and sodium (x) content on the electrochemistry of NaxV2O5.nH2O. Phys. Chem. Chem. Phys. 2011, 13, 18047–18054. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, J.W.; Wu, X.D.; Han, Q.F.; Wang, X. Graphene Oxide−MnO2 nanocomposites for supercapacitors. ACS Nano 2010, 4, 2822–2830. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.S.; Lin, Y.P.; Lin, C.H.; Lee, J.T. Formation of nano-scaled crevices and spacers in NiO-attached graphene oxide nanosheets for supercapacitors. J. Mater. Chem. 2012, 22, 2442. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Y.X.; Yao, Z.Y.; Liu, A.R.; Shi, G.Q. Supercapacitors based on flexible graphene/polyaniline nanofiber composite films. ACS Nano 2010, 4, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Celzard, A.; Collas, F.; Marêché, J.F.; Furdin, G.; Rey, I. Porous electrodes-based double-layer supercapacitors: Pore structure versus series resistance. J. Power Sources 2002, 108, 153–162. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Constant-phase-element behavior caused by resistivity distributions in films I. Theory. J. Electrochim. Soc. 2010, 157, C452–C457. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Jorcin, J.-B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Zhou, Y. Synthesis of mesoporous polythiophene/MnO2 nanocomposite and its enhanced pseudocapacitive properties. J. Power Sources 2011, 196, 4088–4094. [Google Scholar] [CrossRef]
- Tüken, T.; Yazici, B.; Erbil, M. A new multilayer coating for mild steel protection. Prog. Org. Coat. 2004, 50, 115–122. [Google Scholar] [CrossRef]
- Grover, F.W. Inductance Calculations; Dover Publications: Schenectady, NY, USA, 2009. [Google Scholar]
- Martin, R.M. Electronic Structure: Basic Theory, Practical Methods; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]

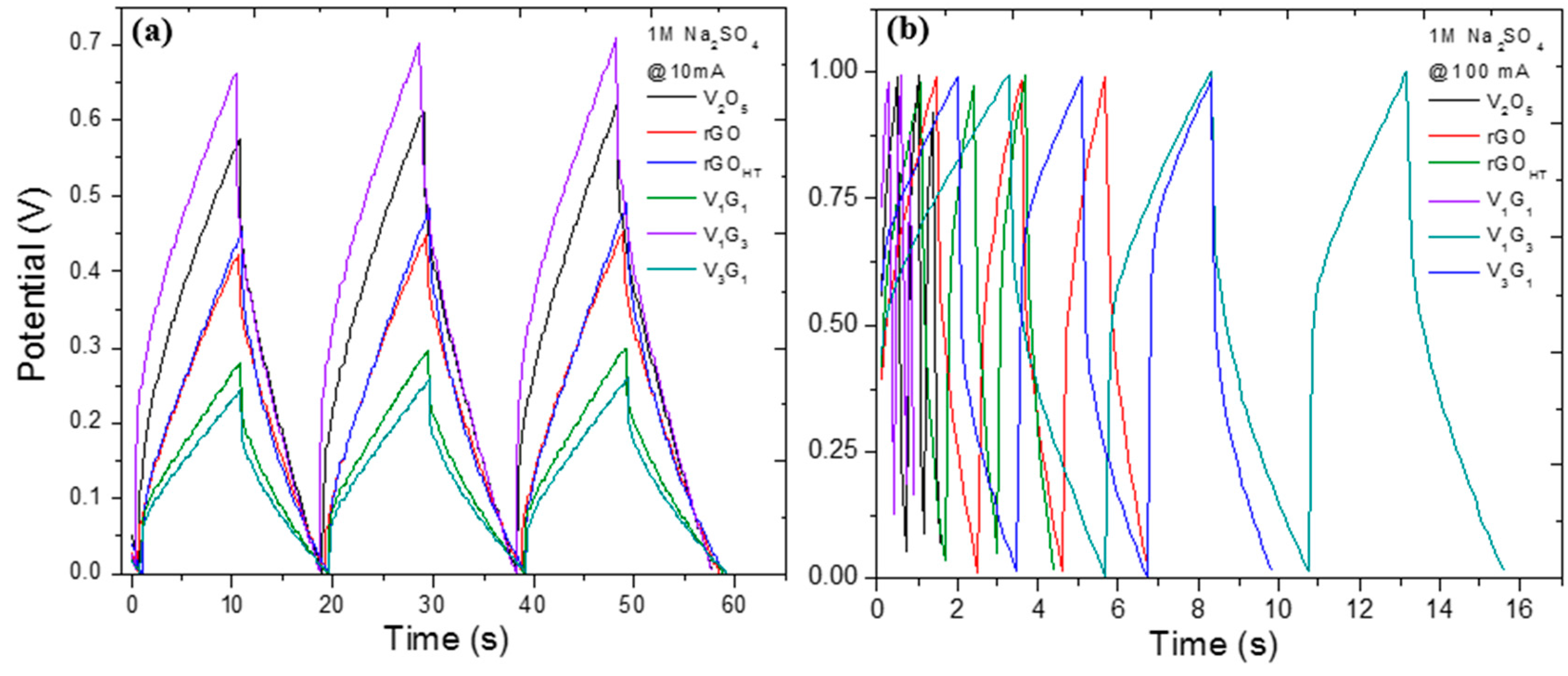
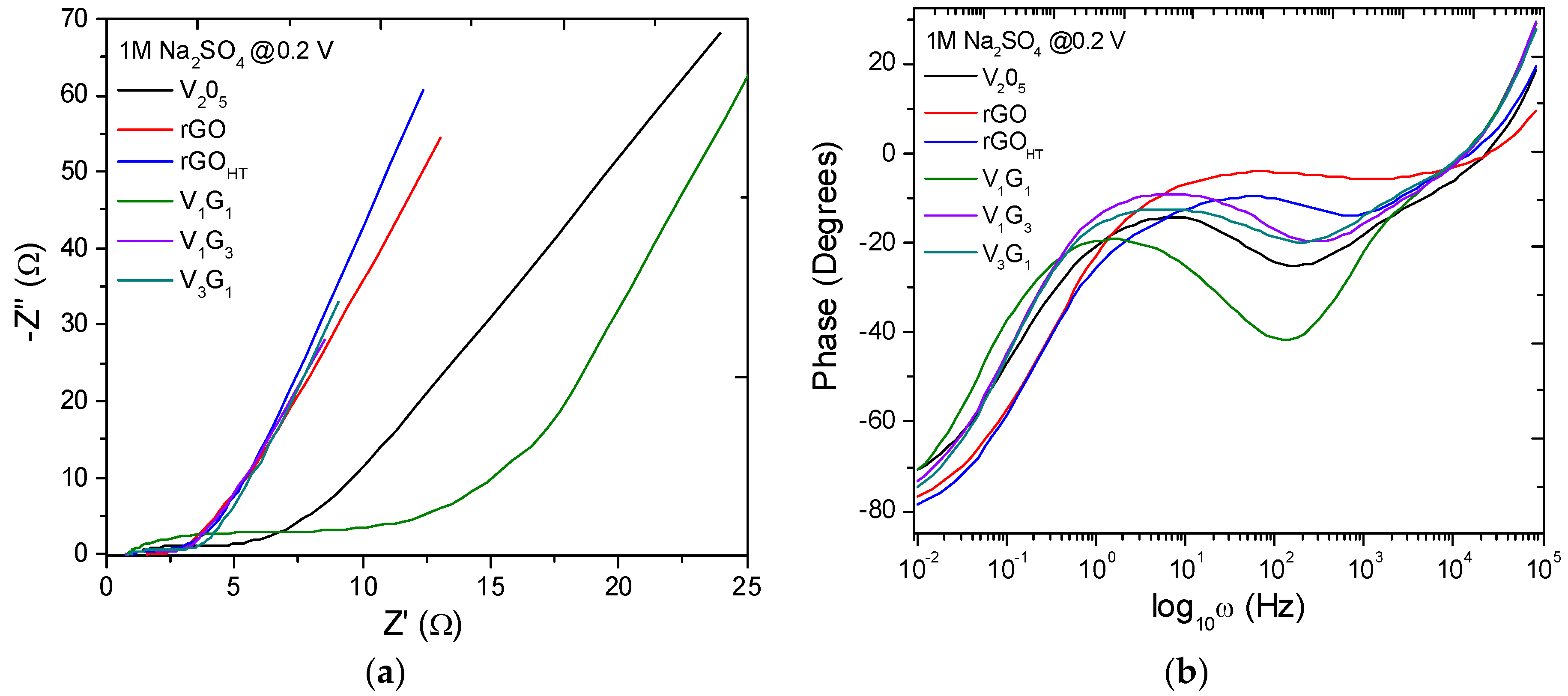
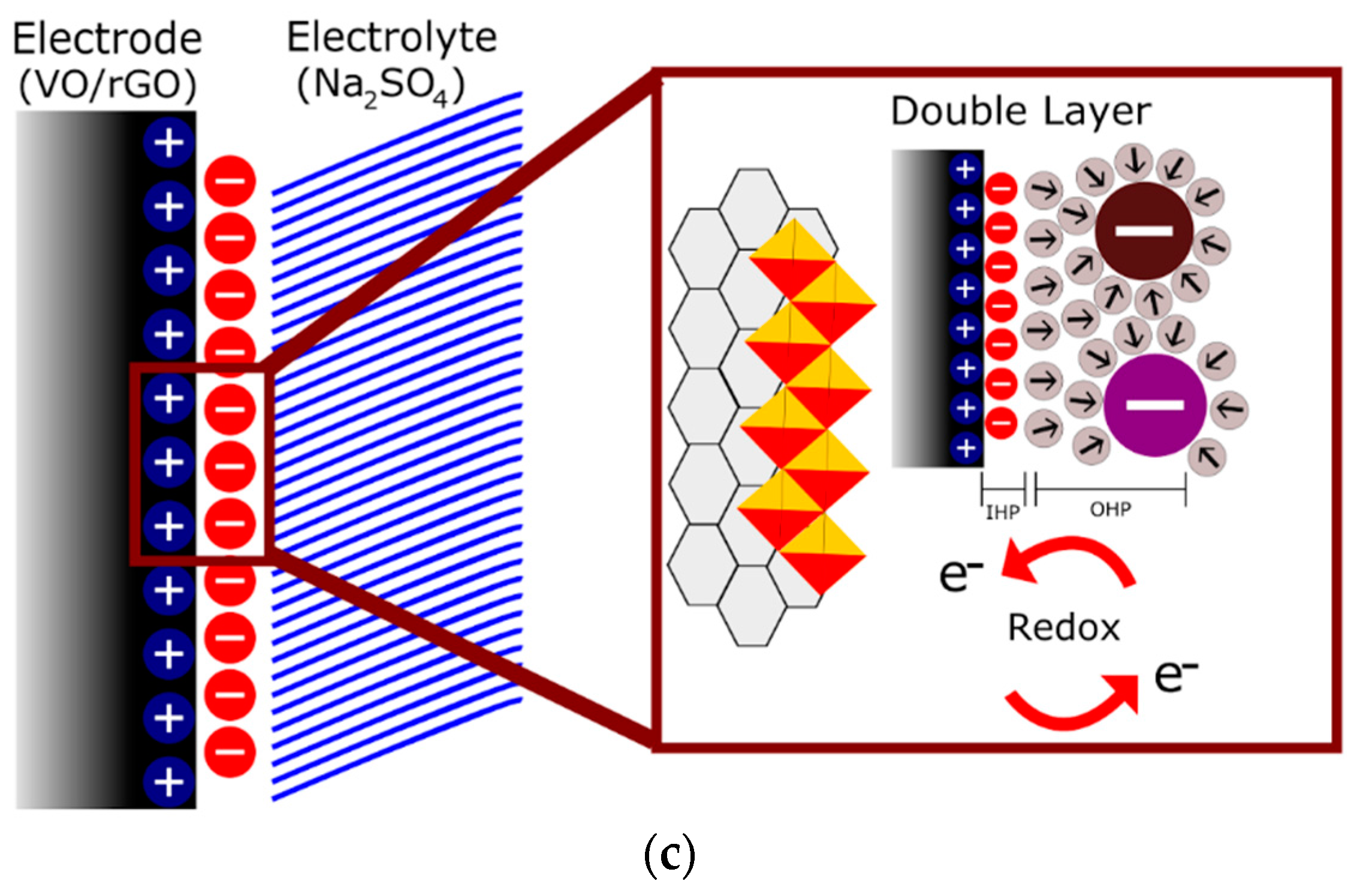
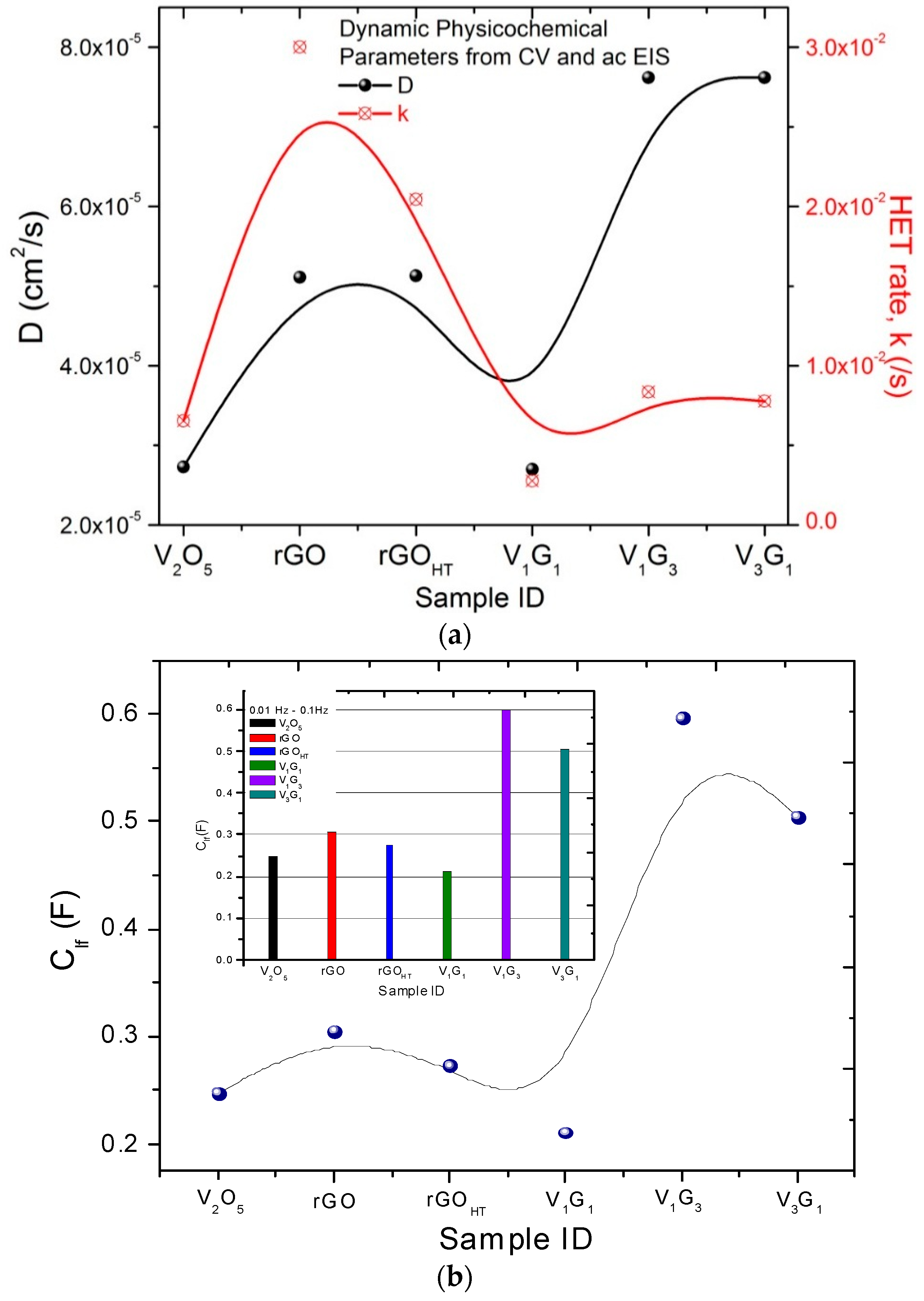


| Electrode Material | V2O5 Mass (mg) | GO Mass (mg) | Total Mass (mg) | Temperature (°C)/Time (h) | Concentration (mg/mL) |
|---|---|---|---|---|---|
| rGOHT | 0 | 20 | 20 | 180/6 | 0.5 |
| V1G1 | 20 | 20 | 40 | 120/24 | 1 |
| V1G3 | 10 | 30 | 40 | 120/24 | 1 |
| V3G1 | 30 | 10 | 40 | 120/24 | 1 |
| Electrode Material | Rs (Ω) | Cdl (F) | Rct (Ω) | ZW (Ω) | D (cm2·s−1) | k (s−1) | Error (%) |
|---|---|---|---|---|---|---|---|
| VO | 0.593 | 0.163 | 2.60 | 0.017 | 2.73 × 10−5 | 6.55 × 10−4 | 8 |
| rGO | 0.780 | 0.204 | 1.50 | 0.023 | 5.11 × 10−5 | 30.0 × 10−3 | 4 |
| rGOHT | 0.002 | 0.178 | 1.52 | 0.023 | 5.13 × 10−5 | 20.45 × 10−3 | 7 |
| V1G1 | 0.270 | 0.082 | 0.110 | 0.120 | 2.70 × 10−5 | 27.73 × 10−3 | 11 |
| V1G3 | 0.091 | 0.311 | 0.870 | 0.107 | 7.62 × 10−5 | 8.36 × 10−3 | 11 |
| V3G1 | 0.145 | 0.270 | 0.780 | 0.054 | 7.62 × 10−5 | 7.77 × 10−3 | 10 |
| Electrode Material | Rs (Ω) | Ce (F) | Rct (Ω) | Qo (Ω/s) | n | Yo (Ω−1) | ZW (Ω) | Error (%) |
|---|---|---|---|---|---|---|---|---|
| VO | 0.955 | 0. 304 | 4.170 | 0.0060 | 0.623 | 0.170 | 4.17 | 0.017 |
| rGO | 1.519 | 0.345 | 0.678 | 0.0035 | 0.650 | 0.311 | 2.27 | 0.023 |
| rGOHT | 0.937 | 0.323 | 1.199 | 0.006 | 0.653 | 0.259 | 2.73 | 0.023 |
| V1G1 | 0.702 | 0.257 | 10.40 | 0.0057 | 0.665 | 0.160 | 4.43 | 0.120 |
| V1G3 | 0.766 | 0.692 | 1.756 | 0.0057 | 0.687 | 0.527 | 1.34 | 0.107 |
| V3G1 | 0.777 | 0.581 | 2.156 | 0.1145 | 0.626 | 0.462 | 1.53 | 0.054 |
| Structure | Charge Changes | ||
|---|---|---|---|
| Adsorbate Orbital Populations Per Atom | Overall Charge Transferred to Graphene Support | ||
| O/Graphene 1 | - | O-2s = 1.92 e | −0.56 e |
| - | O-2p = 4.64 e | - | |
| VO2/Graphene 2 | V-4s = 0.05 e | - | 0.19 e |
| V-4p = 0.29 e | O-2s = 1.96 e | - | |
| V-3d = 3.48 e | O-2p = 4.47 e | - | |
| V2O5/Graphene 2 | V-4s = 0.06 e | - | −0.07 e |
| V-4p = 0.21 e | O-2s = 1.95 e | - | |
| V-3d = 3.33 e | O-2p = 4.57 e | - | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Aberg, B.; Carrizosa, S.B.; Dimakis, N. Vanadium Pentoxide Nanobelt-Reduced Graphene Oxide Nanosheet Composites as High-Performance Pseudocapacitive Electrodes: ac Impedance Spectroscopy Data Modeling and Theoretical Calculations. Materials 2016, 9, 615. https://doi.org/10.3390/ma9080615
Gupta S, Aberg B, Carrizosa SB, Dimakis N. Vanadium Pentoxide Nanobelt-Reduced Graphene Oxide Nanosheet Composites as High-Performance Pseudocapacitive Electrodes: ac Impedance Spectroscopy Data Modeling and Theoretical Calculations. Materials. 2016; 9(8):615. https://doi.org/10.3390/ma9080615
Chicago/Turabian StyleGupta, Sanju, Bryce Aberg, Sara B. Carrizosa, and Nicholas Dimakis. 2016. "Vanadium Pentoxide Nanobelt-Reduced Graphene Oxide Nanosheet Composites as High-Performance Pseudocapacitive Electrodes: ac Impedance Spectroscopy Data Modeling and Theoretical Calculations" Materials 9, no. 8: 615. https://doi.org/10.3390/ma9080615






