Preparation of Nanofibrous Structure of Mesoporous Bioactive Glass Microbeads for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
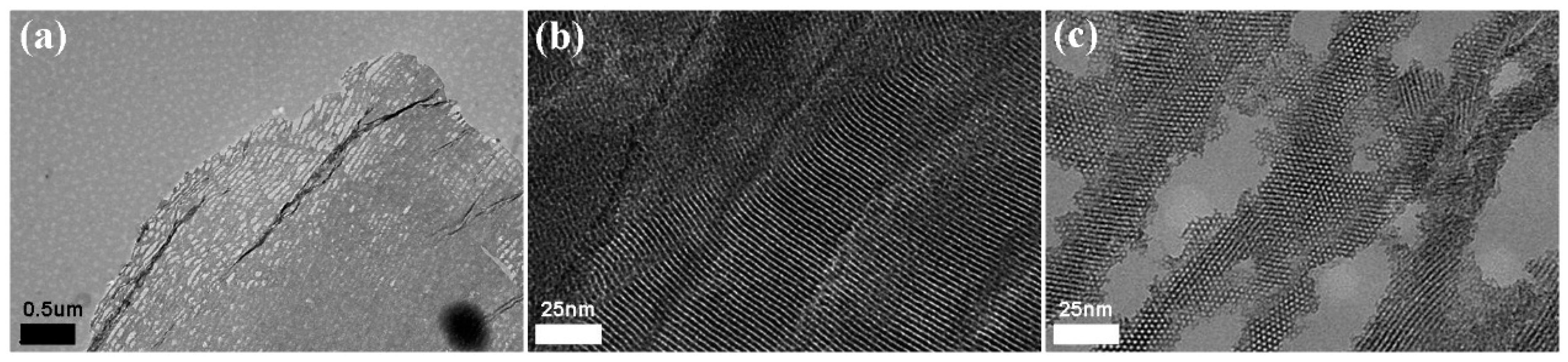
2.2. Synthesis of MBGNFs
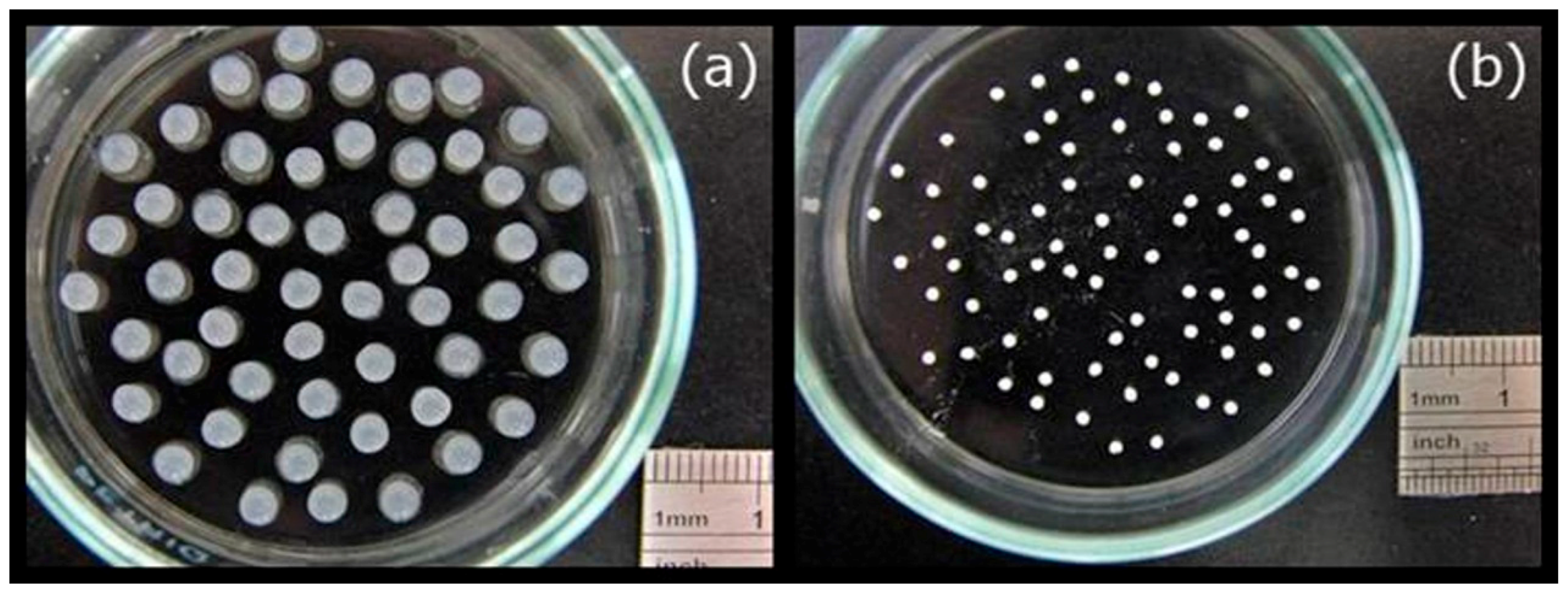
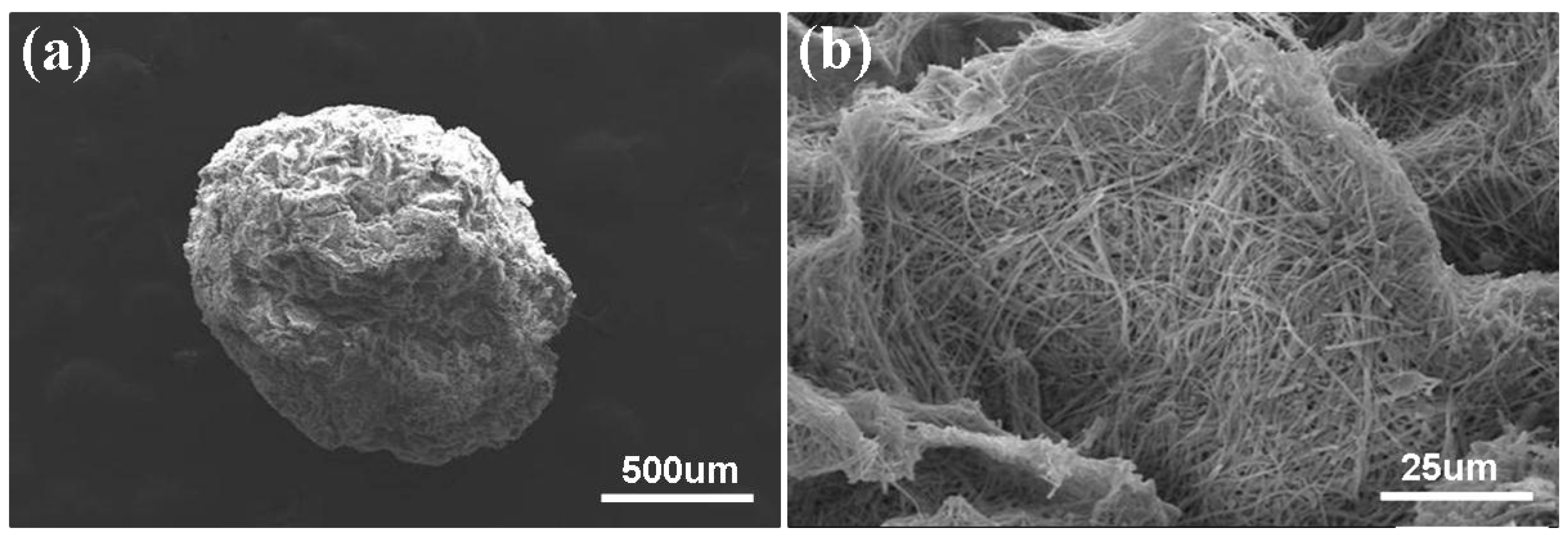
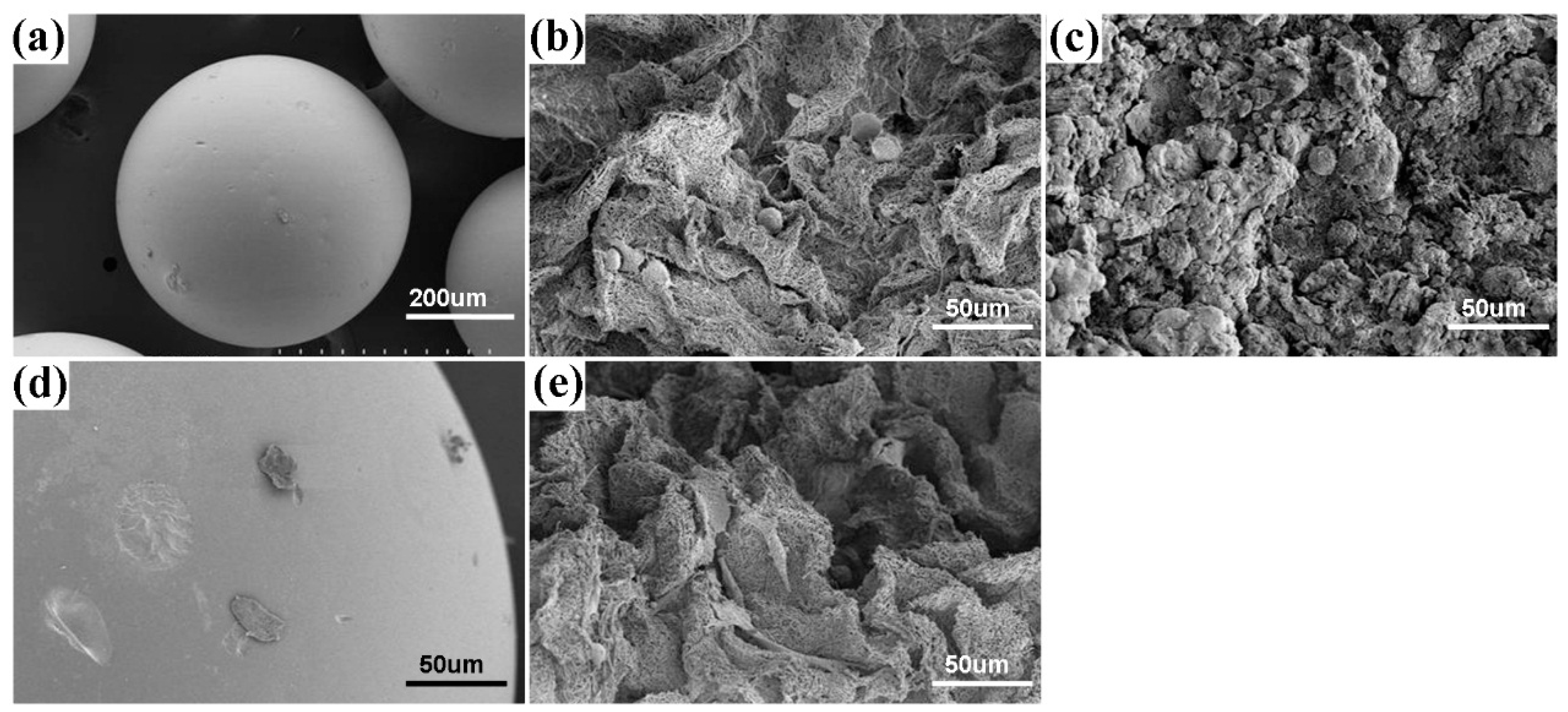
2.3. Preparation and Characterization of the MBGNF Microbeads
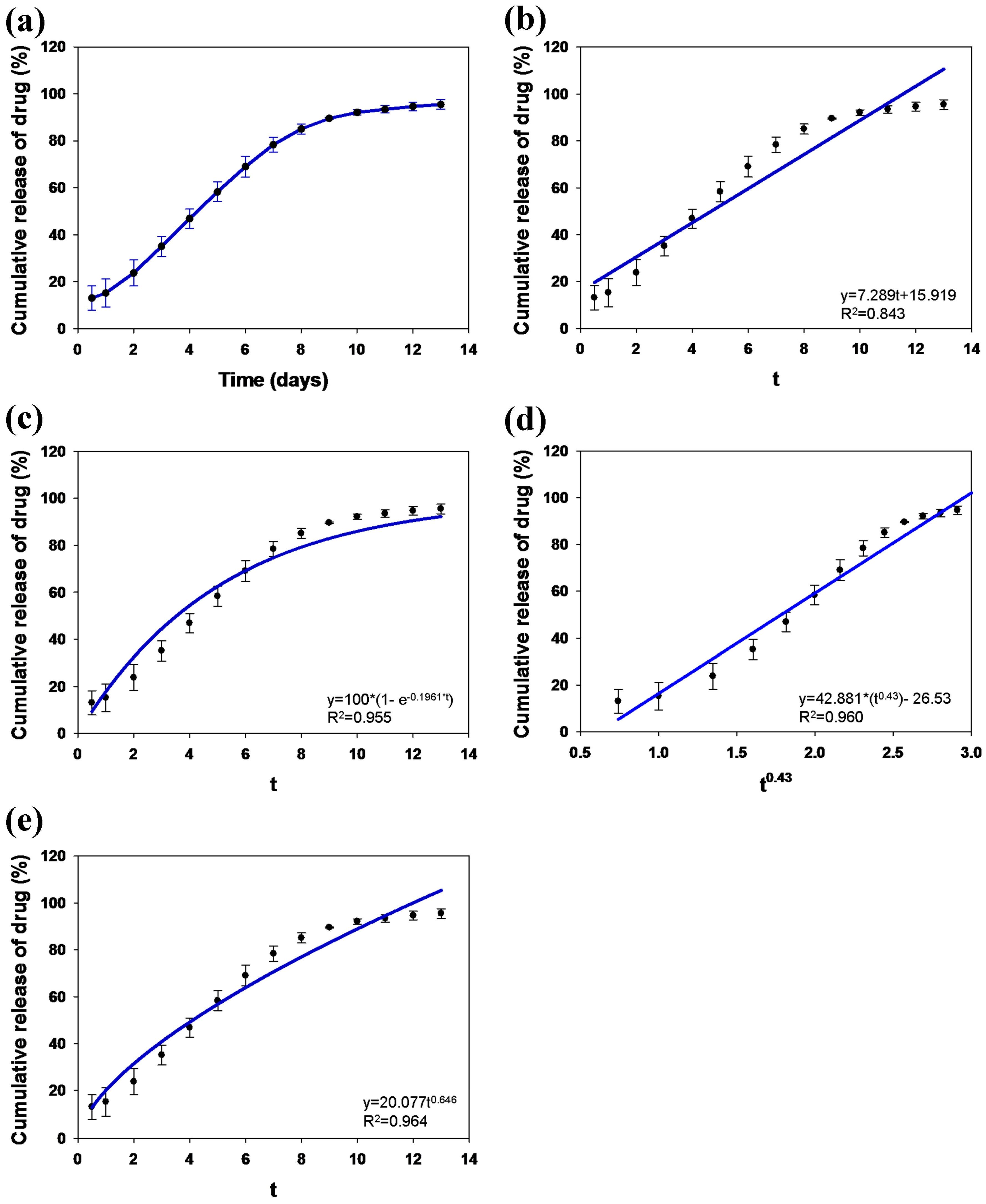
2.4. In Vitro Study of Drug Loading and Release
2.5. Cellular Adhesion on MFB
2.6. Cellular Morphology
2.7. Statistical Analyses
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hsu, F.Y.; Hung, Y.S.; Liou, H.M.; Shen, C.H. Electrospun hyaluronate-collagen nanofibrous matrix and the effects of varying the concentration of hyaluronate on the characteristics of foreskin fibroblast cells. Acta Biomater. 2010, 6, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Huang, C.C.; Rau, L.R.; Hsu, F.Y. Fabrication of aligned carbon nanotube/polycaprolactone/gelatin nanofibrous matrices for Schwann cell immobilization. J. Nanomater. 2014. [Google Scholar] [CrossRef]
- Liou, H.M.; Rau, L.R.; Huang, C.C.; Lu, M.R.; Hsu, F.Y. Electrospun hyaluronan-gelatin nanofibrous matrix for nerve tissue engineering. J. Nanomater. 2013. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, T.; Wang, X.P.; Fang, Q.F. Electrospun submicron bioactive glass fibers for bone tissue scaffold. J. Mater. Sci. Mater. Med. 2009, 20, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, H.; Zhao, S.; Zhou, N.; Li, L.; Huang, W.; Wang, D.; Zhang, C. In vivo and in vitro studies of borate based glass micro-fibers for dermal repairing. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Durgalakshmi, D.; Balakumar, S. Phase separation induced shell thickness variations in electrospun hollow Bioglass 45S5 fiber mats for drug delivery applications. Phys. Chem. Chem. Phys. 2015, 17, 15316–15323. [Google Scholar] [CrossRef] [PubMed]
- Oréfice, R.L.; Hench, L.L.; Clark, A.E.; Brennan, A.B. Novel sol-gel bioactive fibers. J. Biomed. Mater. Res. 2001, 55, 460–467. [Google Scholar] [CrossRef]
- Clupper, D.C.; Gough, J.E.; Hall, M.M.; Clare, A.G.; LaCourse, W.C.; Hench, L.L. In vitro bioactivity of S520 glass fibers and initial assessment of osteoblast attachment. J. Biomed. Mater. Res. A 2003, 67, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kim, H.E.; Knowles, J.C. Production and potential of bioactive glass nanofibers as a next-generation biomaterial. Adv. Funct. Mater. 2006, 16, 1529–1535. [Google Scholar] [CrossRef]
- Baino, F.; Fiorilli, S.; Mortera, R.; Onida, B.; Saino, E.; Visai, L.; Verné, E.; Vitale-Brovarone, C. Mesoporous bioactive glass as a multifunctional system for bone regeneration and controlled drug release. J. Appl. Biomater. Funct. Mater. 2012, 10, 12–21. [Google Scholar] [PubMed]
- Tallia, F.; Gallo, M.; Pontiroli, L.; Baino, F.; Fiorilli, S.; Onida, B.; Anselmetti, G.C.; Manca, A.; Vitale-Brovarone, C. Zirconia-containing radiopaque mesoporous bioactive glasses. Mater. Lett. 2014, 130, 281–284. [Google Scholar] [CrossRef]
- Yi, J.; Wei, G.; Huang, X.; Zhao, L.; Zhang, O.; Yu, C. Sol-gel derived mesoporous bioactive glass fibers as tissue-engineering scaffolds. J. Sol-Gel Sci. Technol. 2008, 45, 115–119. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, X.; Jing, X.; Fan, H.; Gu, Z.; Zhang, X. Fabrication and drug delivery of ultrathin mesoporous bioactive glass hollow fibers. Adv. Funct. Mater. 2010, 20, 1503–1510. [Google Scholar] [CrossRef]
- Xu, C.; Inai, R.; Kotaki, M.; Ramakrishna, S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 2004, 10, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.Y.; Weng, R.C.; Lin, H.M.; Lin, Y.H.; Lu, M.R.; Yu, J.L.; Hsu, H.W. A biomimetic extracellular matrix composed of mesoporous bioactive glass as a bone graft material. Microporous Mesoporous Mater. 2015, 212, 56–65. [Google Scholar] [CrossRef]
- Kang, S.W.; Yang, H.S.; Seo, S.W.; Han, D.K.; Kim, B.S. Apatite-coated poly(lactic-co-glycolic acid) microspheres as an injectable scaffold for bone tissue engineering. J. Biomed. Mater. Res. A 2008, 85, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, Y.; Ke, X.; Xie, Y.; Zhu, H.; Crawford, R.; Xiao, Y. Bioactive mesopore-glass microspheres with controllable protein-delivery properties by biomimetic surface modification. J. Biomed. Mater. Res. A 2010, 95, 476–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, S.W.; Yu, D.S.; Tsao, S.W.; Hsu, F.Y. Hyaluronan-cisplatin conjugate nanoparticles embedded in Eudragit S100-coated pectin/alginate microbeads for colon drug delivery. Int. J. Nanomed. 2013, 8, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Zamani, Z.; Alipour, D.; Moghimi, H.R.; Mortazavi, S.A.; Saffary, M. Development and evaluation of thymol microparticles using cellulose derivatives as controlled release dosage form. Iran J. Pharm. Res. 2015, 14, 1031–1040. [Google Scholar] [PubMed]
- Wu, C.T.; Chang, J. Mesoporous bioactive glasses: Structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus 2012, 2, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Chime, S.A.; Onunkwo, G.C.; Onyishi, I.I. Kinetics and Mechanisms of Drug Release from Swellable and Non Swellable Matrices: A Review. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 97–103. [Google Scholar]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, S.-W.; Chang, Y.-H.; Yu, J.-L.; Hsu, H.-W.; Rau, L.-R.; Hsu, F.-Y. Preparation of Nanofibrous Structure of Mesoporous Bioactive Glass Microbeads for Biomedical Applications. Materials 2016, 9, 487. https://doi.org/10.3390/ma9060487
Tsai S-W, Chang Y-H, Yu J-L, Hsu H-W, Rau L-R, Hsu F-Y. Preparation of Nanofibrous Structure of Mesoporous Bioactive Glass Microbeads for Biomedical Applications. Materials. 2016; 9(6):487. https://doi.org/10.3390/ma9060487
Chicago/Turabian StyleTsai, Shiao-Wen, Yu-Han Chang, Jing-Lun Yu, Hsien-Wen Hsu, Lih-Rou Rau, and Fu-Yin Hsu. 2016. "Preparation of Nanofibrous Structure of Mesoporous Bioactive Glass Microbeads for Biomedical Applications" Materials 9, no. 6: 487. https://doi.org/10.3390/ma9060487





