Nanoparticles for Control of Biofilms of Acinetobacter Species
Abstract
:1. Introduction
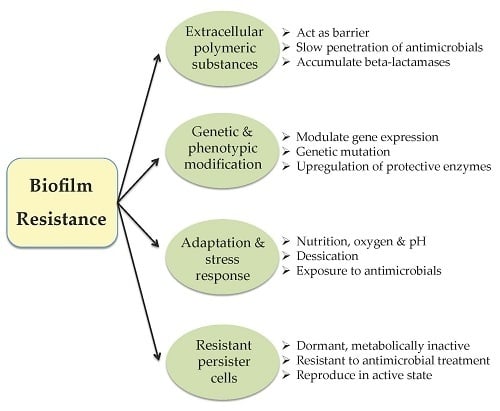
1.1. Acinetobacter: A Nosocomial Biofilm-Producing Pathogen
1.2. Treatment Therapies for Control of Acinetobacter Biofilm
2. Acinetobacter Biofilm Control through Nanomaterials
2.1. Organic Nanoparticles
2.1.1. Liposomes and Nanoemulsions
2.1.2. Polymeric Nanoparticles
2.2. Inorganic Nanoparticles
2.2.1. Silver Nanoparticles
2.2.2. Gold Nanoparticles
2.2.3. Selenium Nanoparticles
2.2.4. Nitric-Oxide Releasing Nanoparticles
2.2.5. Multi-Metallic Nanoparticles
2.3. Nanoconjugates, Nanoalloys and Nanocomposites
2.4. Bacteriophages as Living Nanobullets
3. Resistance towards Nanoparticles
4. Future Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Branda, S.S.; Vik, A.; Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Barraud, N.; Kelso, M.J.; Rice, S.A.; Kjelleberg, S. Nitric oxide: A key mediator of biofilm dispersal with applications in infectious diseases. Curr. Pharm. Des. 2015, 21, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, K.K. What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 2004, 236, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilm formation: A clinically relevant microbiological process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health Guide: Research on Microbial Biofilms. Available online: http://grants.nih.gov/grants/guide/pa-files/PA-03-047.html (accessed on 9 May 2016).
- Potera, C. Forging a link between biofilms and disease. Science 1999, 283, 1837–1839. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; van de Mortel, M.; Nielsen, L.; de Guzman, G.N.; Li, X.; Halverson, L.J. Alginate production by Pseudomonas putida creates a hydrated microenvironment and contributes to biofilm architecture and stress tolerance under water-limiting conditions. J. Bacteriol. 2007, 189, 8290–8299. [Google Scholar] [CrossRef] [PubMed]
- Elasri, M.O.; Miller, R.V. Study of the response of a biofilm bacterial community to UV radiation. Appl. Environ. Microbiol. 1999, 65, 2025–2031. [Google Scholar] [PubMed]
- Goodman, S.D.; Obergfell, K.P.; Jurcisek, J.A.; Novotny, L.A.; Downey, J.S.; Ayala, E.; Tjokro, A.N.; Li, B.; Justice, S.S.; Bakaletz, L.O. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunol. 2011, 4, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Prabhakara, R.; Harro, J.M.; Leid, J.G.; Harris, M.; Shirtliff, M.E. Murine immune response to a chronic Staphylococcus aureus biofilm infection. Infect. Immun. 2011, 79, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Jagtap, S.; More, P.; Shete, U.J.; Maheshwari, N.O.; Rao, S.J.; Kitture, R.; Kale, S.; Bellare, J.; Patil, S.; et al. Dioscorea bulbifera mediated synthesis of novel Aucore-Agshell nanoparticles with potent antibiofilm and antileishmanial activity. J. Nanomater. 2015, 2015, 562938. [Google Scholar] [CrossRef]
- Salunke, G.R.; Ghosh, S.; Santoshkumar, R.J.; Khade, S.; Vashisth, P.; Kale, T.; Chopade, S.; Pruthi, V.; Kundu, G.; Bellare, J.R.; et al. Rapid efficient synthesis and characterization of silver, gold and bimetallic nanoparticles from the medicinal plant Plumbago zeylanica and their application in biofilm control. Int. J. Nanomedicine 2014, 9, 2635–2653. [Google Scholar] [PubMed]
- Sahu, P.K.; Iyer, P.S.; Oak, A.M.; Pardesi, K.R.; Chopade, B.A. Characterization of eDNA from the clinical strain Acinetobacter baumannii AIIMS 7 and its role in biofilm formation. Sci. World J. 2012, 2012, 973436. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Iyer, P.S.; Gaikwad, M.B.; Talreja, S.C.; Pardesi, K.R.; Chopade, B.A. An MFS transporter-like ORF from MDR Acinetobacter baumannii AIIMS 7 is associated with adherence and biofilm formation on biotic/abiotic surface. Int. J. Microbiol. 2012, 2012, 490647. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, M.; Serpooshan, V. Silver-coated engineered magnetic nanoparticles are promising for the success in the fight against antibacterial resistance threat. ACS Nano 2012, 6, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Halwani, M.; Hebert, S.; Suntres, Z.E.; Lafrenie, R.M.; Azghani, A.O.; Omri, A. Bismuth-thiolincorporation enhances biological activities of liposomal tobramycin against bacterial biofilm and quorum sensing molecules production by Pseudomonas aeruginosa. Int. J. Pharm. 2009, 373, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Dow, J.M.; Crossman, L.; Findlay, K.; He, Y.Q.; Feng, J.X.; Tang, J.L. Biofilm dispersal in Xanthomonas campestris is controlled by cell-cell signaling and is required for full virulence to plants. Proc. Natl. Acad. Sci. USA 2003, 100, 10995–11000. [Google Scholar] [CrossRef] [PubMed]
- Visca, P.; Seifert, H.; Towner, K.J. Acinetobacter infection- an emerging threat to human health. IUBMB Life 2011, 63, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Rokhbakhsh-Zamin, F.; Sachdev, D.; Kazemi-Pour, N.; Engineer, A.; Pardesi, K.R.; Zinjarde, S.; Dhakephalkar, P.K.; Chopade, B.A. Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J. Microbiol. Biotechnol. 2011, 21, 556–566. [Google Scholar] [PubMed]
- Sachdev, D.; Nema, P.; Dhakephalkar, P.; Zinjarde, S.; Chopade, B. Assessment of 16S rRNA gene-based phylogenetic diversity and promising plant growth-promoting traits of Acinetobacter community from the rhizosphere of wheat. Microbiol. Res. 2010, 165, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Huddedar, S.B.; Shete, A.M.; Tilekar, J.N.; Gore, S.D.; Dhavale, D.D.; Chopade, B.A. Isolation, characterization and plasmid pUPI126-mediated indole-3-acetic acid production in Acinetobacter strains from rhizosphere of wheat. Appl. Biochem. Biotechnol. 2002, 103, 21–39. [Google Scholar] [CrossRef]
- Deshpande, L.M.; Kapadnis, B.P.; Chopade, B.A. Metal resistance in Acinetobacter and its relation to beta-lactamse production. Biometals 1993, 6, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.C.; Chopade, B.A. Effect of food preservatives on Acinetobacter genospecies isolated from meat. J. Food Sci. Technol. 2002, 39, 26–32. [Google Scholar]
- Saha, S.C.; Chopade, B.A. Studies on occurrence and distribution of Acinetobacter spp. and other gram-negative bacterial from meat. J. Food Sci. Technol. 2001, 38, 17–22. [Google Scholar]
- Yavankar, S.P.; Pardesi, K.R.; Chopade, B.A. Species distribution and physiological characterization of Acinetobacter genospecies from healthy human skin of tribal population in India. Indian J. Med. Microbiol. 2007, 25, 336–345. [Google Scholar] [PubMed]
- Jagtap, S.; Gore, S.; Yavankar, S.; Pardesi, K.; Chopade, B. Optimization of medium for lipase production by Acinetobacter haemolyticus from healthy human skin. Indian J. Exp. Biol. 2010, 48, 936–941. [Google Scholar] [PubMed]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Shakibaie, M.R.; Dhakephalkar, P.K.; Kapadnis, B.P.; Chopade, B.A. Silver resistance in Acinetobacter baumannii BL54 occurs through binding to a Ag-binding protein. Iran. J. Biotechnol. 2003, 1, 41–46. [Google Scholar]
- Pour, N.K.; Dusane, D.H.; Dhakephalkar, P.K.; Zamin, F.R.; Zinjarde, S.S.; Chopade, B.A. Biofilm formation by Acinetobacter baumannii strains isolated from urinary tract infection and urinary catheters. FEMS Immunol. Med. Microbiol. 2011, 62, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, R.B.; Dhakephalkar, P.K.; Niphadkar, K.B.; Chopade, B.A. A study on nosocomial pathogens in ICU with special reference to multiresistant Acinetobacter baumannii harbouring multiple plasmids. Indian J. Med. Res. 2008, 128, 178–187. [Google Scholar] [PubMed]
- Bergogne-Berezin, E.; Towner, K.J. Acinetobacter spp. as nosocomial pathogens: Microbiological, clinical and epidemiological features. Clin. Microbiol. Rev. 1996, 9, 148–165. [Google Scholar] [PubMed]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.R.; Jog, N.R.; Chopade, B.A. Isolation and characterization of Acinetobacter spp. from upper respiratory tract of healthy humans and demonstration of lectin activity. Indian J. Med. Microbiol. 2001, 19, 30–35. [Google Scholar]
- Patil, J.R.; Chopade, B.A. Distribution and in vitro antimicrobial susceptibility of Acinetobacter species on the skin of healthy humans. Natl. Med. J. India 2001, 14, 204–208. [Google Scholar] [PubMed]
- Manchanda, V.; Sinha, S.; Singh, N.P. Multidrug Resistant Acinetobacter. J. Glob. Infect. Dis. 2010, 2, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Litzler, P.Y.; Benard, L.; Barbier-Frebourg, N.; Vilain, S.; Jouenne, T.; Beucher, E.; Bunel, C.; Lemeland, J.F.; Bessou, J.P. Biofilm formation on pyrolytic carbon heart valves: Influence of surface free energy, roughness, and bacterial species. J. Thorac. Cardiovasc. Surg. 2007, 134, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Dallo, S.F.; Weitao, T. Insights into Acinetobacter war-wound infections, biofilms and control. Adv. Skin Wound Care 2010, 23, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Tien, H.C.; Battad, A.; Bryce, E.A. Multi-drug resistant Acinetobacter infections in critically injured Canadian forces soldiers. BMC Infect. Dis. 2007, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Aronson, N.E.; Sanders, J.W.; Moran, K.A. In harm’s way: Infections in deployed American military forces. Clin. Infect. Dis. 2006, 43, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Charnot-Katsikas, A.; Dorafshar, A.H.; Aycock, J.K.; David, M.Z.; Weber, S.G.; Frank, K.M. Two cases of necrotizing fasciitis due to Acinetobacter baumannii. J. Clin. Microbiol. 2009, 47, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Karveli, E.A.; Kelesidis, I.; Kelesidis, T. Community acquired Acinetobacter infections. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Koh, Y.M.; Kim, J.; Lee, J.C.; Lee, Y.C.; Seol, S.Y.; Cho, D.T.; Kim, J. Capacity of multidrug-resistant clinical isolates of Acinetobacter baumannii to form biofilm and adhere to epithelial cell surfaces. Clin. Microbiol. Infect. 2008, 14, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Gaidhani, S.V.; Raskar, A.V.; Poddar, S.; Gosavi, S.; Sahu, P.K.; Pardesi, K.R.; Bhide, S.V.; Chopade, B.A. Time dependent enhanced resistance against antibiotics and metal salts by planktonic and biofilm form of Acinetobacter haemolyticus MMC 8 clinical isolate. Indian J. Med. Res. 2014, 140, 665–671. [Google Scholar] [PubMed]
- Wong, H.S.; Townsend, K.M.; Fenwick, S.G.; Trengove, R.D.; O’Handley, R.M. Comparative susceptibility of planktonic and 3-day-old Salmonella typhimurium biofilms to disinfectants. J. Appl. Microbiol. 2010, 108, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Langsrud, S.; Sidhu, M.S.; Heir, E.; Holck, A.L. Bacterial disinfectant resistance—A challenge for the food industry. Int. Biodeterior. Biodegr. 2003, 51, 283–290. [Google Scholar] [CrossRef]
- Russell, A.D. Similarities and differences in the responses of microorganisms to biocides. J. Antimicrob. Chemother. 2003, 52, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.; O’Toole, G.A. Microbial Biofilms; ASM Press: Washington, DC, USA, 2004. [Google Scholar]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef] [PubMed]
- Tomaras, A.P.; Dorsey, C.W.; Edelmann, R.E.; Actis, L.A. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: Involvement of a novel chaperone-usher pili assembly system. Microbiology 2003, 149, 3473–3484. [Google Scholar] [CrossRef] [PubMed]
- Loehfelm, T.W.; Luke, N.R.; Campagnari, A.A. Identification and characterization of an Acinetobacter baumannii biofilm—Associated protein. J. Bacteriol. 2008, 190, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Iyer, P.S.; Barage, S.H.; Sonawane, K.D.; Chopade, B.A. Characterization of the algC gene expression pattern in the multidrug resistant Acinetobacter baumannii AIIMS 7 and correlation with biofilm development on abiotic surface. Sci. World J. 2014, 2014, 593546. [Google Scholar] [CrossRef] [PubMed]
- Schleheck, D.; Barraud, N.; Klebensberger, J.; Webb, J.S.; McDougald, D.; Rice, S.A.; Kjelleberg, S. Pseudomonas aeruginosa PAO1 preferentially grows as aggregates in liquid batch cultures and disperses upon starvation. PLoS ONE 2009, 4, e5513. [Google Scholar] [CrossRef] [PubMed]
- Musk, D.J.; Banko, D.A.; Hergenrother, P.J. Iron salts perturb biofilm formation and disrupt existing biofilms of Pseudomonas aeruginosa. Chem. Biol. 2005, 12, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Kolodkin-Gal, I.; Romero, D.; Cao, S.; Clardy, J.; Kolter, R.; Losick, R. D-amino acids trigger biofilm disassembly. Science 2010, 328, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.G.; Marques, C.N. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol. 2009, 191, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Ragas, X.; Dai, T.; Tegos, G.P.; Agut, M.; Nonell, S.; Hamblin, M.R. Photodynamic inactivation of Acinetobacter baumannii using phenothiazinium dyes: In vitro and in vivo studies. Lasers Surg. Med. 2010, 42, 384–390. [Google Scholar] [CrossRef] [PubMed]
- López-Rojas, R.; Docobo-Pérez, F.; Pachón-Ibáñez, M.E.; de la Torre, B.G.; Fernández-Reyes, M.; March, C.; Bengoechea, J.A.; Andreu, D.; Rivas, L.; Pachón, J. Efficacy of cecropin A–melittin peptides on a sepsis model of infection by pan-resistant Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Fattahian, Y.; Rasooli, I.; MousaviGargari, S.L.; Rahbar, M.R.; Darvish Alipour Astaneh, S.; Amani, J. Protection against Acinetobacter baumannii infection via its functional deprivation of biofilm associated protein (Bap). Microb. Pathog. 2011, 51, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Lin, L.; Ibrahim, A.S.; Baquir, B.; Pantapalangkoor, P.; Bonomo, R.A.; Doi, Y.; Adams, M.D.; Russo, T.A.; Spellberg, B. Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PLoS ONE 2012, 7, e29446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentancor, L.V.; O’Malley, J.M.; Bozkurt-Guzel, C.; Pier, G.B.; Maira-Litan, T. Poly-N-acetyl-beta-(1-6)-glucosamine is a target for protective immunity against Acinetobacter baumannii infections. Infect. Immun. 2012, 80, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Bentancor, L.V.; Routray, A.; Bozkurt-Guzel, C.; Camacho-Peiro, A.; Pier, G.B.; Maira-Litan, T. Evaluation of the trimeric auto-transporter Ata as a vaccine candidate against Acinetobacter baumannii infections. Infect. Immun. 2012, 80, 3381–3388. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Ahmed, E.; Codamine, E. Antimicrobial properties of brevinin-2-related peptide and its analogs: Efficacy against multidrug-resistant Acinetobacter baumannii. Chem. Biol. Drug Des. 2009, 74, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Mechkarska, M.; Arafat, K.; Attoub, S.; Sonnevend, A. Analogues of the frog skin peptide alyteserin-2a with enhanced antimicrobial activities against Gram-negative bacteria. J. Pept. Sci. 2012, 18, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Sonnevend, A.; Pal, T.; Vila-Farres, X. Efficacy of six frog skin-derived antimicrobial peptides against colistin-resistant strains of the Acinetobacter baumannii group. Int. J. Antimicrob. Agents 2012, 39, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed]
- García-Quintanilla, M.; Pulido, M.R.; López-Rojas, R.; Pachón, J.; McConnell, M.J. Emerging therapies for multidrug resistant Acinetobacter baumannii. Trends Microbiol. 2013, 21, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S.; Clond, M.A.; Vogt, A. Medical biofilms- nanotechnology approaches. J. Biomed. Nanotech 2014, 10, 1–22. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Patel, R.B.; Parikh, J.R.; Solanki, A.B.; Patel, B.G. Investigating effect of microemulsion components: In vitro permeation of ketoconazole. Pharm. Dev. Technol. 2011, 16, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Sun, H.; Bradley, M.A. Novel antibacterial nanofibrous PLLA scaffolds. J. Control. Release 2010, 146, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.M.V.; Crusz, S.A.; Kolomiets, E.; Buts, L.; Kadam, R.U.; Cacciarini, M.; Bartels, K.M.; Diggle, S.P.; Camara, M.; Williams, P.; et al. Inhibition and dispersion of Pseudomonas aeruginosa biofilms by glycopeptides dendrimers targeting the fucose-specific lectin LecB. Chem. Biol. 2008, 15, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; More, P.; Nitnavre, R.; Jagtap, S.; Chippalkatti, R.; Derle, A.; Kitture, R.; Asok, A.; Kale, S.; Singh, S.; et al. Antidiabetic and antioxidant properties of copper nanoparticles synthesized by medicinal plant Dioscorea bulbifera. J. Nanomed. Nanotechnol. 2015, S6, 007. [Google Scholar]
- Shedbalkar, U.; Singh, R.; Wadhwani, S.; Gaidhani, S.; Chopade, B.A. Microbial synthesis of gold nanoparticles: Current status and future prospects. Adv. Colloid Interface Sci. 2014, 209, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Nawale, L.; Arkile, M.; Wadhwani, S.; Shedbalkar, U.; Chopade, S.; Sarkar, D.; Chopade, B.A. Phytogenic silver, gold and bimetallic nanoparticles as novel antitubercular agents. Int. J. Nanomed. 2016, 11, 1889–1897. [Google Scholar]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic silver nanoparticles: Synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Asok, A.; Ghosh, S.; More, P.A.; Chopade, B.A.; Gandhi, M.N.; Kulkarni, A.R. Surface defect rich ZnO quantum dots as antioxidants inhibiting α-amylase and α-glucosidase: A potential anti-diabetic nanomedicine. J. Mater. Chem. B 2015, 3, 4597–4606. [Google Scholar] [CrossRef]
- Kitture, R.; Chordiya, K.; Gaware, S.; Ghosh, S.; More, P.A.; Kulkarni, P.; Chopade, B.A.; Kale, S.N. ZnO nanoparticles-red sandalwood conjugate: A promising anti-diabetic agent. J. Nanosci. Nanotechnol. 2015, 15, 4046–4051. [Google Scholar] [CrossRef] [PubMed]
- Mallick, A.; More, P.; Ghosh, S.; Chippalkatti, R.; Chopade, B.A.; Lahiri, M.; Basu, S. Dual drug conjugated nanoparticle for simultaneous targeting of mitochondria and nucleus in cancer cells. ACS Appl. Mater. Interfaces 2015, 7, 7584–7598. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Dorval, C.; Suntres, Z.E.; Omri, A. Bismuth-ethanedithiol incorporated in a liposome loaded tobramycin formulation modulates the alginate levels in mucoid Pseudomonas aeruginosa. J. Pharm. Pharmacol. 2011, 63, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Montagu, A.; Saulnier, P.; Cassissa, V.; Rossines, E.; Eveillard, M.; Joly-Guillou, M.-L. Aromatic and terpenic compounds loaded in lipidic nanocapsules: Activity against multi-drug resistant Acinetobacter baumannii assessed in vitro and in a murine model of sepsis. J. Nanomed. Nanotechnol. 2014, 5, 3. [Google Scholar]
- Singh, R.; Wagh, P.; Wadhwani, S.; Gaidhani, S.; Kumbhar, A.; Bellare, J.; Chopade, B.A. Synthesis, optimization, and characterization of silver nanoparticles from Acinetobacter calcoaceticus and their enhanced antibacterial activity when combined with antibiotics. Int. J. Nanomed. 2013, 8, 4277–4290. [Google Scholar]
- Uppu, D.S.S.M.; Samaddar, S.; Ghosh, C.; Paramanandham, K.; Shome, B.R.; Haldar, J. Amide side chain amphiphilic polymers disrupt surface established bacterial biofilms and protect mice from chronic Acinetobacter baumannii infection. Biomaterials 2016, 74, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Ramya, S.; Shanmugasundaram, T.; Balagurunathan, R. Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J. Trace Elem. Med. Biol. 2015, 32, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.Y.; Ramalingam, K.; Bienek, D.R.; Lee, V.; You, T.; Alvarez, R. Antimicrobial activity of nano emulsion in combination with cetylpyridinium chloride in multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013, 57, 3568–3575. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gutierrez, F.; Boegli, L.; Agostinho, A.; Morales Sánchez, E.; Bach, H.; Ruiz, F.; James, G. Anti-biofilm activity of silver nanoparticles against different microorganisms. Biofouling 2013, 29, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Alzubaidi, A.N.A.; Alkozai, Z.M.F. Immunogenic properties of outer membrane protein of Acinetobacter baumannii that loaded on chitosan nanoparticles. Am. J. Biomed. 2015, 3, 59–74. [Google Scholar]
- Mihu, M.R.; Sandkovsky, U.; Han, G.; Friedman, J.M.; Nosanchuk, J.D.; Martinez, L.R. Nitric oxide releasing nanoparticles are therapeutic for Acinetobacter baumannii wound infections. Virulence 2010, 1, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Gaidhani, S.; Singh, R.; Singh, D.; Patel, U.; Shevade, K.; Yeshvekar, R.; Chopade, B.A. Biofilm disruption activity of silver nanoparticles synthesized by Acinetobacter calcoaceticus PUCM 1005. Mater. Lett. 2013, 108, 324–327. [Google Scholar] [CrossRef]
- Hendiani, S.; AhyaAbdi, A.; Mohammadi, P.; Kharrazi, S. Synthesis of silver nanoparticles and its synergistic effects in combination with imipenem and two biocides against biofilm producing Acinetobacter baumannii. Nanomed. J. 2015, 2, 291–298. [Google Scholar]
- Huang, W.C.; Tsai, P.-J.; Chen, Y.C. Functional gold nanoparticles as photothermal agents for selective-killing of pathogenic bacteria. Nanomedicine 2007, 2, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Cady, N.C.; Behnke, J.L.; Strickland, A.D. Copper-based nanostructured coatings on natural cellulose: Nanocomposites exhibiting rapid and efficient inhibition of a multi-drug resistant wound pathogen, A. baumannii, and mammalian cell biocompatibility in vitro. Adv. Eng. Mater. 2011, 21, 2506–2514. [Google Scholar] [CrossRef]
- Milenkovic, J.; Hrenovic, J.; Goic-Barisic, I.; Tomic, M.; Djonlagic, J.; Rajic, N. Synergistic anti-biofouling effect of Ag-exchanged zeolite and D-Tyrosine on PVC composite against the clinical isolate of Acinetobacter baumannii. Biofouling 2014, 30, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Yele, A.B.; Thawal, N.D.; Sahu, P.K.; Chopade, B.A. Novel lytic bacteriophage AB7-IBB1 of Acinetobacter baumannii: Isolation, characterization and its effect on biofilm. Arch. Virol. 2012, 157, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Thawal, N.D.; Yele, A.B.; Sahu, P.K.; Chopade, B.A. Effect of a novel podophage AB7-IBB2 on Acinetobacter baumannii biofilm. Curr. Microbiol. 2012, 65, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, U.S. Liposomes in drug delivery: Progress and limitations. Int. J. Pharm. 1997, 154, 123–140. [Google Scholar] [CrossRef]
- Rukholm, G.; Mugabe, C.; Azghani, A.O.; Omri, A. Antibacterial activity of liposomal gentamicin against Pseudomonas aeruginosa: A time-kill study. Int. J. Antimicrob. Agents 2006, 27, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Halwani, M.; Omri, A.; Suntres, Z.E. Antimicrobial effectiveness of liposomal polymyxin B against resistant Gram-negative bacterial strains. Int. J. Pharm. 2008, 355, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Lian, T.; Ho, R.J.Y. Trends and developments in liposome drug delivery systems. J. Pharm. Sci. 2001, 90, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, K.; Amaechi, B.T.; Ralph, R.H.; Lee, V.A. Antimicrobial activity of nanoemulsion on cariogenic planktonic and biofilm organisms. Arch. Oral Biol. 2012, 57, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Hemmila, M.R.; Mattar, A.; Taddonio, M.A.; Arbabi, S.; Hamouda, T.; Ward, P.A.; Wang, S.C.; Baker, J.R., Jr. Topical nano emulsion therapy reduces bacterial wound infection and inflammation after burn injury. Surgery 2010, 148, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Zhang, S.L.; Zhu, L.Y.; Xie, S.Y.; Dong, Z.; Wang, Y.; Zhou, W.Z. Enhancement of antibacterial activity of tilmicosin against Staphylococcus aureus by solid lipid nanoparticles in vitro and in vivo. Vet. J. 2012, 191, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lim, J.; Teoh, S.-H. Development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol. Adv. 2013, 31, 688–705. [Google Scholar] [CrossRef] [PubMed]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barrier. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Tessmar, J.K.; Göpferich, A.M. Matrices and scaffolds for protein delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Peng, L.; De Sousa, J.; Su, Z.; Novak, B.M.; Nevzorov, A.A.; Garland, E.R. Inhibition of Acinetobacter baumannii biofilm formation on a methacrylate polymer containing a 2-aminoimidazole subunit. Chem. Commun. 2011, 47, 4896–4898. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Chang, M.W.; Hadinoto, K. The roles of lipid in antibiofilm efficacy of lipid-polymer hybrid nanoparticles encapsulating antibiotics. Colloids Surf. A 2011, 389, 158–165. [Google Scholar] [CrossRef]
- Li, Y.; Fukushima, K.; Coady, D.J.; Engler, A.C.; Liu, S.; Huang, Y.; Cho, J.S.; Guo, Y.; Miller, L.S.; Tan, J.P.; et al. Broad- spectrum antimicrobial and biofilm-disrupting hydrogels: Stereo-Complex driven supramolecular assemblies. Angew. Chem. Int. Ed. Engl. 2013, 52, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, X.; Falk, S.P.; Masters, K.S.; Weisblum, B.; Gellman, S.H. Nylon-3-polymers active against drug-resistant Candida albicans biofilms. J. Am. Chem. Soc. 2015, 137, 2183–2186. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K.; Cameotra, S.S.; Bellare, J.; Dhavale, D.D.; Jabgunde, A.; et al. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed. 2012, 7, 483–496. [Google Scholar]
- Singh, R.; Nawale, L.; Arkile, M.; Shedbalkar, U.U.; Wadhwani, S.A.; Sarkar, D.; Chopade, B.A. Chemical and biological metal nanoparticles as antimycobacterial agents: A comparative study. Int. J. Antimicrob. Agents 2015, 46, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, S.; Geo, S.; SukanyaPraseetha, P.K.; Dhanya, R.P. Biosynthesis of silver nanoparticles from Actinomycetes for therapeutic applications. Int. J. Nano Dimens. 2014, 5, 155–162. [Google Scholar]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Ip, M.; Lui, S.L.; Poon, V.K.; Lung, I.; Burd, A. Antimicrobial activities of silver dressings: An in vitro comparison. J. Med. Microbiol. 2006, 55, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Pradeep, T. Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol. Bioeng. 2005, 90, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; Taglietti, A.; Dacarro, G.; Diaz-Fernandez, Y.A.; Galli, M.; Grisoli, P.; Patrini, M.; De Magistris, G.S.; Zanoni, R. Self-Assembled monolayers of silver nanoparticles firmly grafted on glass surfaces: Low Ag+ release for an efficient antibacterial activity. J. Colloid Interface Sci. 2010, 350, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Karve, M.S.; Chopade, B.A. Novel polyhedral gold nanoparticles: Green synthesis, optimization and characterization by environmental isolate of Acinetobacter sp. SW30. World J. Microbiol. Biotechnol. 2014, 30, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
- Bindhu, M.R.; Umadevi, M. Antibacterial activities of green synthesized gold nanoparticles. Mater. Lett. 2014, 120, 122–125. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lu, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Bajaj, A.; Mout, R.; Rotello, V.M. Monolayer coated gold nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2012, 64, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Vigderman, L.; Zubarev, E.R. Therapeutic platforms based on gold nanoparticles and their covalent conjugates with drug molecules. Adv. Drug Deliv. Rev. 2013, 65, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Kong, Y.; Kundu, S.; Cirillo, J.D.; Liang, H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guerin. J. Nanobiotechnol. 2012, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.C. Perspectives series: Host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 1997, 99, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Cochis, A.; Azzimonti, B.; Della Valle, C.; de Giglio, E.; Bloise, N.; Visai, L.; Cometa, S.; Rimondini, L.; Chiesa, R. The effect of silver or gallium doped titanium against the multidrug resistant Acinetobacter baumannii. Biomaterials 2016, 80, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Quan, X.; Li, Q.; Wu, Y. Effects of D-amino acids and norspermidine on the disassembly of large, old-aged microbial aggregates. Water Res. 2014, 54, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, Y. D-Amino acid mitigated membrane biofouling and promoted biofilm detachment. J. Membr. Sci. 2011, 376, 266–274. [Google Scholar] [CrossRef]
- Lin, N.T.; Chiou, P.Y.; Chang, K.C.; Chen, L.K.; Lai, M.J. Isolation and characterization of phi AB2: A novel bacteriophage of Acinetobacter baumannii. Res. Microbiol. 2010, 161, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Duan, S.S.; Ouyang, Y.S.; Chen, Y.B. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Biometals 2011, 24, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar-Vemula, P.; Ajayan, P.M.; John, G. Silver nanoparticle embedded antimicrobial paints based on vegetable oil. Nat. Mater. 2008, 7, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Braydich-Stolle, L.; Hussain, S.; Schlager, J.J.; Hofmann, M.C. In vitro cytotoxicity of nanoparticles in mammalian germ-line stem cells. Toxicol. Sci. 2005, 88, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.L., Jr.; Tajkarimi, M.; Cunningham, Q.; Campbell, A.; Nonga, H.; Harrison, S.H.; Barrick, J.E. Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front. Genet. 2015, 6, 42. [Google Scholar] [CrossRef] [PubMed]



| NPs | Composition and Surface Property | Size (nm) | Acinetobacter Strain | Applied Dosage of NPs | Remarks | Ref. |
|---|---|---|---|---|---|---|
| Lipid-based NPs | ||||||
| Lipidic nanocapsules | (1) carvacol, eugenol and cinnamaldehyde (0.96% w/w) (2) carvacol (0.34% w/w), eugenol (1.83% w/w), cinnamaldehyde (0.39% w/w) and β-caryophyllene (0.32% w/w) | 85–95 62–70 | A. baumannii | 40 mg/kg | Increased survival in sepsis murine model | [86] |
| Nanoemulsion of CPC | CPC (1% w/v), triton X-100 (10% v/v) and soyabean oil (25% v/v) | 213.9 | A. baumannii ATCC BAA-1605 | ~5–25 μg/mL CPC | Loss in metabolic activity; complete biofilm disruption | [90] |
| Polymer-based NPs | ||||||
| Chitosan NPs | OMP loaded on NPs | - | A. baumannii | 533 + 170 μg/mL (OMP + chitosan) 1st and 3rd week: 0.5 mL; 5th week: 1 mL | Modulate cytokine profile; trigger immune response; act as nano-vaccine | [92] |
| Inorganic NPs | ||||||
| AgNPs | 12.05 | A. baumannii SRMC 27; A. haemolyticus MMC 8 | 2000 μg/mL | 80%–92% biofilm inhibition and disruption | [94] | |
| 21–29 | A. baumannii ATCC BAA-1605 | 250–1000 mg/mL | Biofilm disruption on polycarbonate membrane; ~4-log reduction in cell load at highest concentration | [91] | ||
| Combined with imipenem | - | A. baumannii | 0.0003–0.8 μg/mL | Synergistic action; reduced MBIC and MBEC | [95] | |
| 60 | A. baumannii AIIMS 7 | 1024 μg/200 μL well | 96%–99% biofilm inhibition; 88% eradication; change in cell morphology | [15] | ||
| AuNPs | Vancomycin bound | - | A. baumannii | - | Hyperthermic bactericidal action via NIR irradiation | [96] |
| Silver-gold bimetallic NPs | 90 | A. baumannii AIIMS 7 | 1024 μg/200 μL well | 93%–98% biofilm inhibition; 61%–77% eradication; cell lysis | [15] | |
| Au (core) and Ag (shell) | 13–19 | A. baumannii | 100 μg/mL | 83% biofilm inhibition | [14] | |
| SeNPs | - | 100–250 | Acinetobacter sp. (4117, 1677, 2030, 674, 2020, 1370) | 1.2–3.6 μg/mL | Dose-dependent anti-biofilm activity; 75% reduction | [89] |
| Nitric oxide-releasing NPs | Composite matrix of TMO, PEG, chitosan and glucose with sodium nitrite | 10 | A. baumannii 0057 | 5 mg | Reduced wound healing time in vivo; reduced inflammatory response; inhibited collagen degradation; induced cytokine expression | [93] |
| Nanocomposites | ||||||
| Cu1-based NPs in natural cellulose | Bare metal or metal oxide coating | <5 | A. baumannii | ~30 μg Cu in liquid culture | Bactericidal action without cytotoxicity | [97] |
| Ag1-based NPs in natural cellulose | Bare metal or metal oxide coating | - | A. baumannii | ~12 μg Ag in liquid culture | Bactericidal activity; toxic to NIH 3T3 cell line | [97] |
| Ag-exchanged zeolite | Coated with D-tyrosine | 500–1500 | A. baumannii ST145 | - | Complete bactericidal activity towards immobilized cells; 6.9-log cell reduction | [98] |
| Bacteriophages | ||||||
| AB7-IBB1 | Siphoviridae family | 50 (head); 240 × 10 (tail) | A. baumannii AIIMS 7 | MOI 105 with 102 CFU 1/well | Lyse 23 of 39 clinical isolates of A. baumannii; affected biofilm formation on biotic and abiotic surface; 75% eradication of biofilm | [99] |
| AB7-IBB2 | Podoviridae family | 35 (head); 7 (tail) | A. baumannii AIIMS 7 | MOI 105 and 103 with 102 and 104 CFU/well, respectively | Lyse 19 of 39 clinical isolates of A. baumannii; affected biofilm formation on biotic and abiotic surface; 80% eradication of biofilm | [100] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.; Nadhe, S.; Wadhwani, S.; Shedbalkar, U.; Chopade, B.A. Nanoparticles for Control of Biofilms of Acinetobacter Species. Materials 2016, 9, 383. https://doi.org/10.3390/ma9050383
Singh R, Nadhe S, Wadhwani S, Shedbalkar U, Chopade BA. Nanoparticles for Control of Biofilms of Acinetobacter Species. Materials. 2016; 9(5):383. https://doi.org/10.3390/ma9050383
Chicago/Turabian StyleSingh, Richa, Shradhda Nadhe, Sweety Wadhwani, Utkarsha Shedbalkar, and Balu Ananda Chopade. 2016. "Nanoparticles for Control of Biofilms of Acinetobacter Species" Materials 9, no. 5: 383. https://doi.org/10.3390/ma9050383