Molecular Dynamics Study of Self-Assembly of Aqueous Solutions of Poly[9,9-bis(4-Sulfonylbutoxyphenylphenyl) Fluorene-2,7-diyl-2,2’-Bithiophene] (PBS-PF2T) in the Presence of Pentaethylene Glycol Monododecyl Ether (C12E5)
Abstract
:1. Introduction
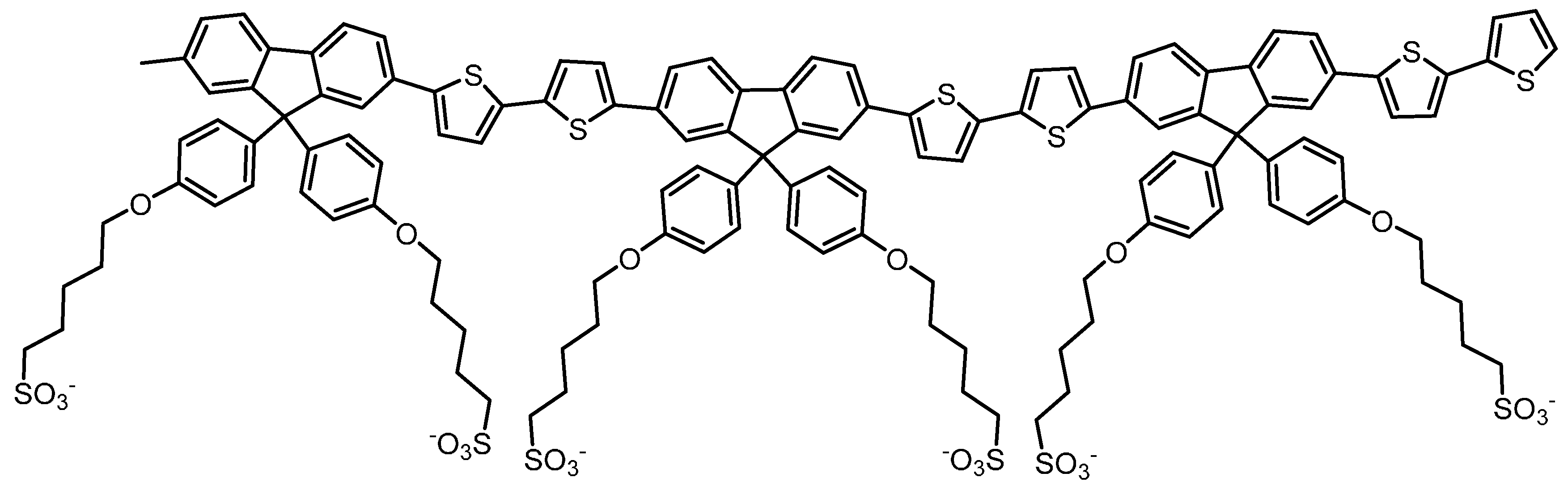
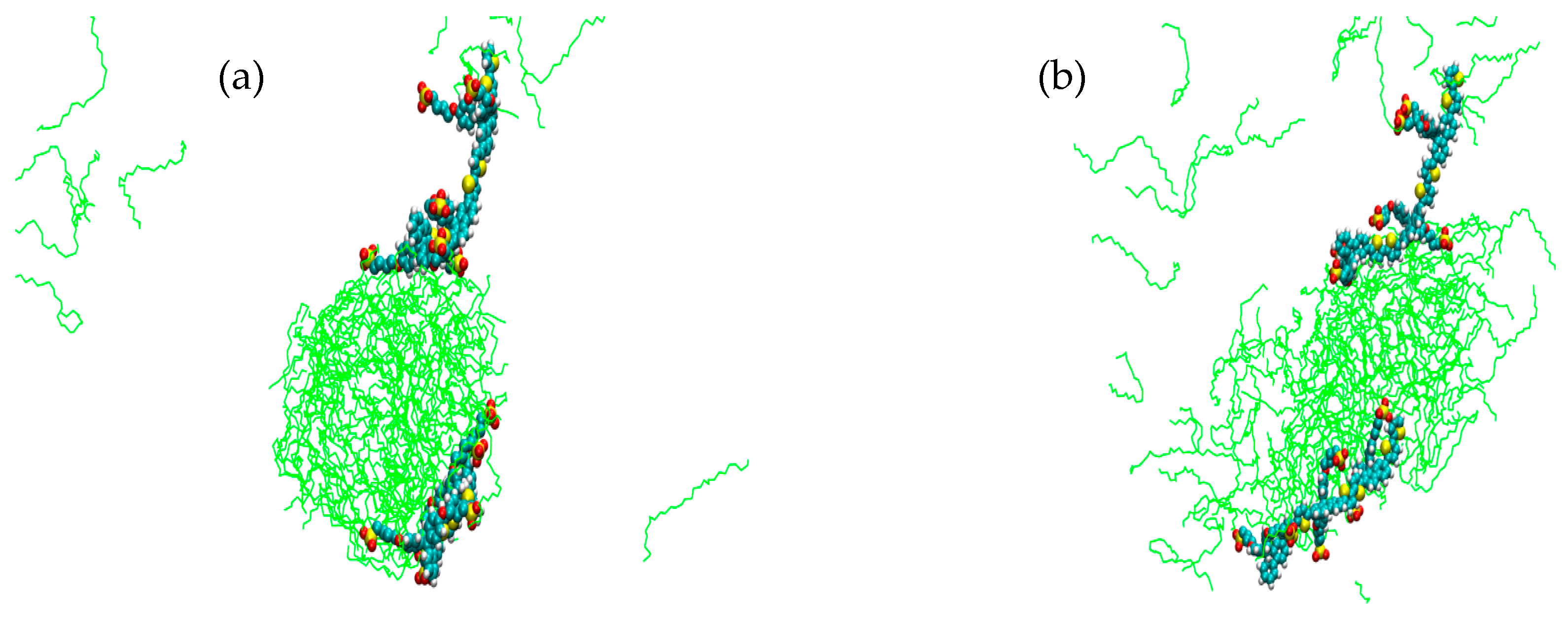
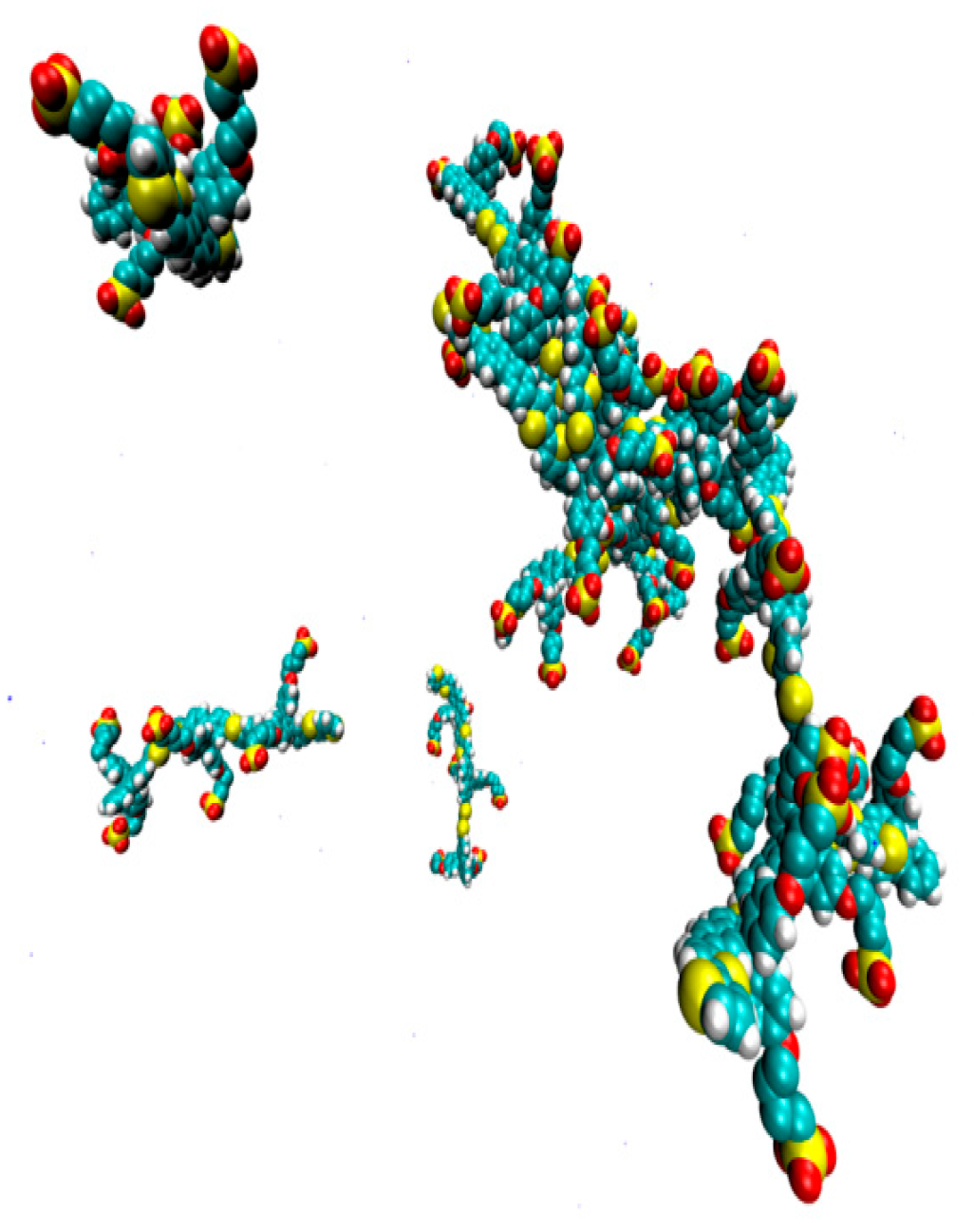
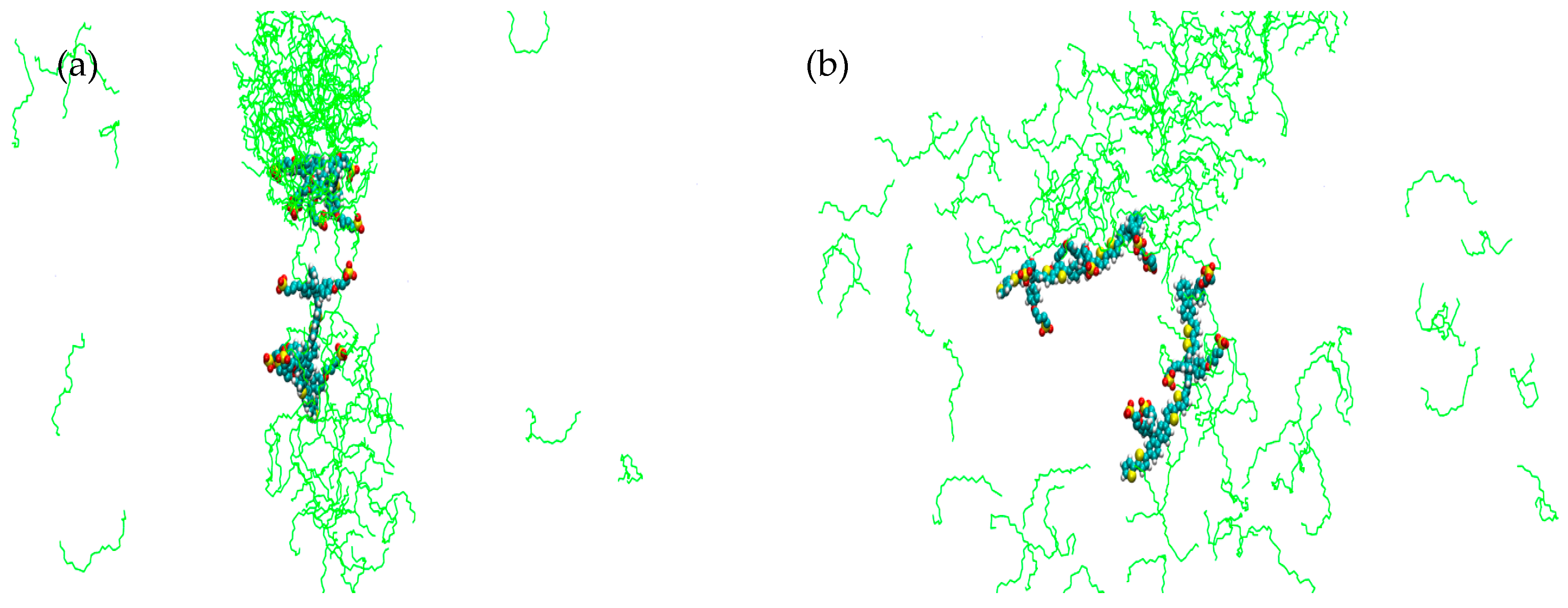
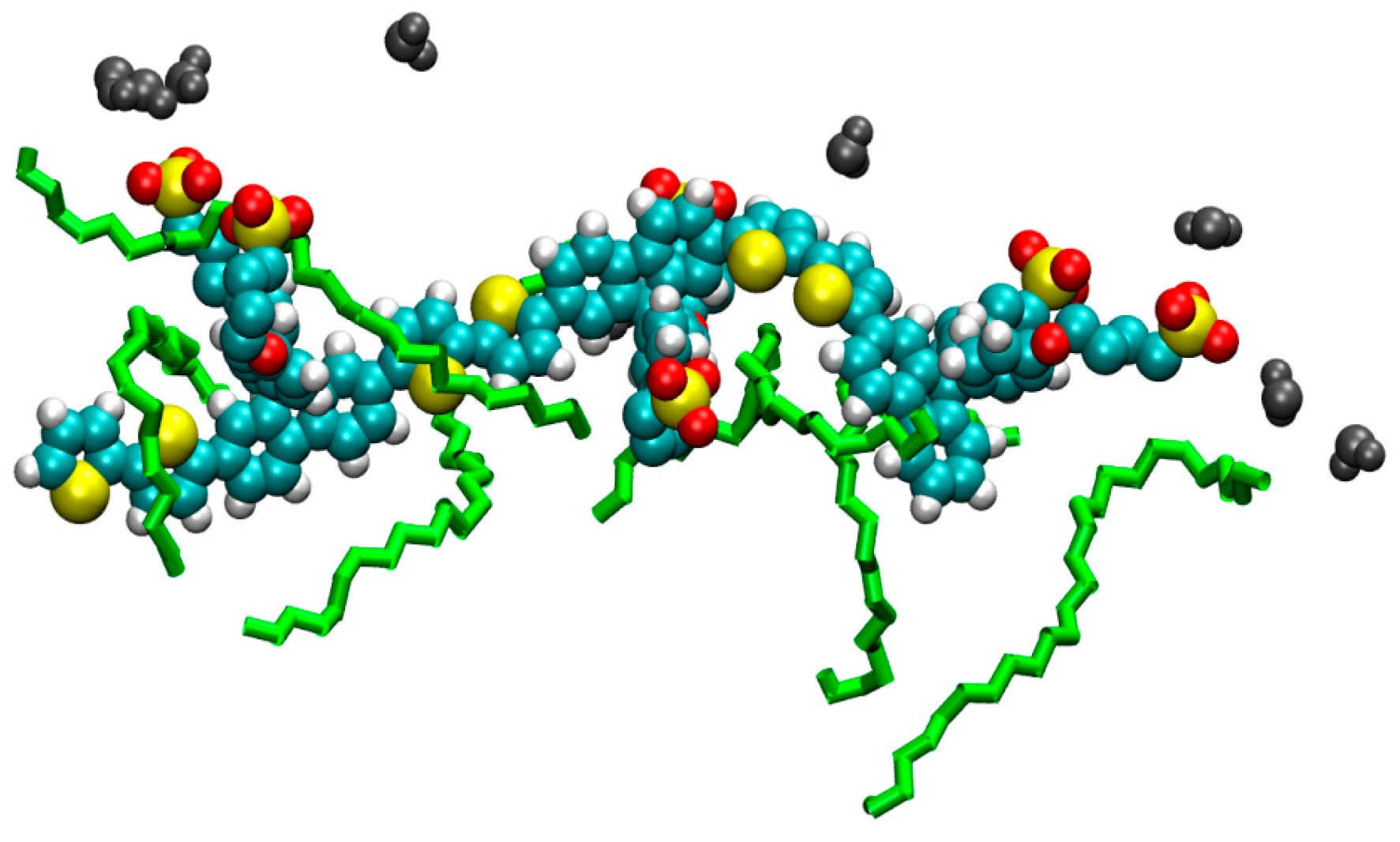
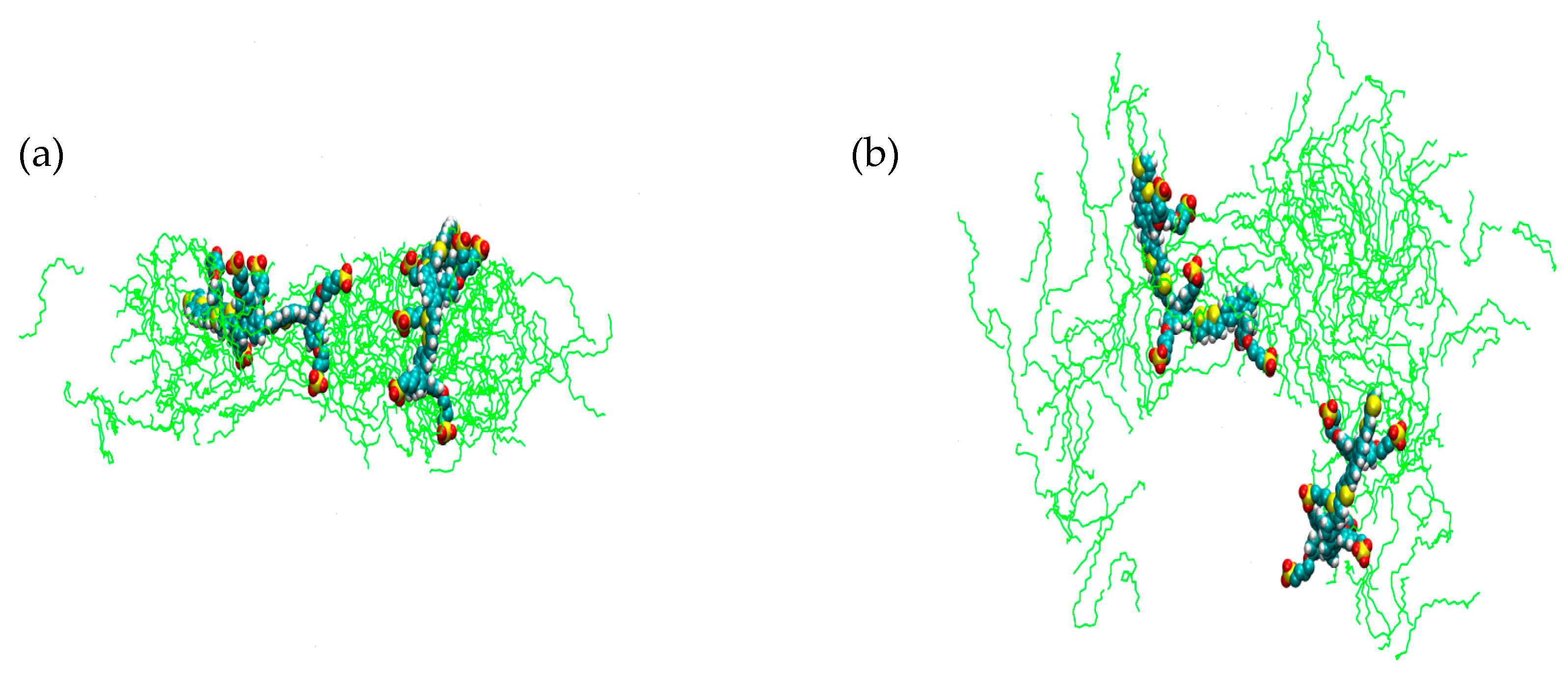
2. Results
3. Discussion
4. Materials and Methods
4.1. Molecular Dynamics Simulations
4.2. Computational Details
4.3. Molecular Dynamics Details
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MDS | Molecular Dynamics Simulations |
| PBS-PF2T | poly[9,9-bis(4-sulfonylbutoxyphenylphenyl) fluorene-2,7-diyl-2,2’-bithiophene] |
| C12E5 | pentaethylene glycol monododecyl ether |
| C12E4 | tetraethylene glycol monododecyl ether (C12E4) |
| GISAXS | grazing incidence small angle X-ray scattering |
| SAXS | small angle X-ray scattering |
References
- Bin, L.; Bazan, G.C. Conjugated Polyelectrolytes—Fundamentals and Applications; Wiley-VCH: Weinheim Germany, 2013. [Google Scholar]
- Hiang, H.; Taranekar, P.; Reynolds, J.R.; Schanze, K.S. Conjugated polyelectrolytes: Synthesis, photophysics, and applications. Angew. Chem. Int. Ed. 2009, 48, 4300–4316. [Google Scholar]
- Joly, G.D.; Geiger, L.; Kooi, S.E.; Swager, T.M. Highly effective water-soluble fluorescence quenchers of conjugated polymer thin films in aqueous environments. Macromolecules 2006, 39, 7175–7177. [Google Scholar] [CrossRef]
- Nguyen, B.L.; Jeong, J.-E.; Jung, I.H.; Kim, B.; Le, V.S.; Kim, I.; Khym, K.; Woo, H.Y. Conjugated polyelectrolyte and aptamer based potassium assay via single- and two-step fluorescence energy transfer with a tunable dynamic detection range. Adv. Funct. Mater. 2014, 24, 1748–1757. [Google Scholar] [CrossRef]
- Wågberg, T.; Liu, B.; Orädd, G.; Eliasson, B.; Edman, L. Cationic polyfluorene: Conformation and aggregation in a “good” solvent. Eur. Polym. J. 2009, 45, 3230–3235. [Google Scholar] [CrossRef]
- Wang, H.; Lu, P.; Wang, B.; Qiu, S.; Liu, M.; Hanif, M.; Cheng, G.; Liu, S.; Ma, Y. A Water-Soluble π-conjugated polymer with up to 100 mg·mL−1 solubility. Macromol. Rapid Commun. 2007, 28, 1645–1650. [Google Scholar] [CrossRef]
- Hoven, C.; Yang, R.; Garcia, A.; Heeger, A.J.; Nguyen, T.-Q.; Bazan, G.C. Ion motion in conjugated polyelectrolyte electron transporting layers. J. Am. Chem. Soc. 2007, 129, 10976–10977. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Gutacker, A.; Sun, Y.; Wu, H.; Huang, F.; Cao, Y.; Scherf, U.; Heeger, A.J.; Bazan, G.C. Improved high-efficiency organic solar cells via incorporation of a conjugated polyelectrolyte interlayer. J. Am. Chem. Soc. 2011, 133, 8416–8419. [Google Scholar] [CrossRef] [PubMed]
- McQuade, D.T.; Pullen, A.E.; Swager, T.M. Conjugated polymer-based chemical sensors. Chem. Rev. 2000, 100, 2537–2574. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.W., III; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Surin, M.; Janssen, P.G.A.; Lazzaroni, R.; Leclere, P.; Meijer, E.W.; Schenning, A.P.H.J. Supramolecular organization of ssDNA-templated π-conjugated oligomers via hydrogen bonding. Adv. Mater. 2009, 21, 1126–1130. [Google Scholar] [CrossRef]
- Wosnick, J.H.; Mello, C.M.; Swager, T.M. Synthesis and application of poly(phenylene ethynylene)s for bioconjugation: A conjugated polymer-based fluorogenic probe for proteases. J. Am. Chem. Soc. 2005, 127, 3400–3405. [Google Scholar] [CrossRef] [PubMed]
- Lukkari, J.; Salomäki, M.; Viinikanoja, A.; Ääritalo, T.; Paukkunen, J.; Kocjarova, N.; Kankare, J. Polyelectrolyte multilayers prepared from water-soluble poly(alkoxythiophene) derivatives. J. Am. Chem. Soc. 2001, 123, 6083–6091. [Google Scholar] [CrossRef] [PubMed]
- Lutkenhaus, J.L.; Hammond, P.T. Electrochemically enabled polyelectrolyte multilayer devices: From fuel cells to sensors. Soft Matter 2007, 3, 804–816. [Google Scholar] [CrossRef]
- Bolognesi, A.; Bajo, Z.; Geng, Z.; Porzio, W.; Speroni, F. Preparation of Langmuir-Blodgett multilayers with differently substituted poly(3-alkylthiophenes). Thin Solid Films 1994, 243, 683–686. [Google Scholar] [CrossRef]
- Reitzel, N.; Greve, D.R.; Kjaer, K.; Howes, P.B.; Jayaraman, M.; Savoy, S.; McCullough, R.D.; McDevitt, J.T.; Bjørnholm, T. Self-assembly of conjugated polymers at the air/water interface. Structure and properties of Langmuir and Langmuir−Blodgett films of amphiphilic regioregular polythiophenes. J. Am. Chem. Soc. 2000, 122, 5788–5800. [Google Scholar] [CrossRef]
- Burrows, H.D.; Knaapila, M.; Fonseca, S.M.; Costa, T. Conjugated Polyelectrolytes—Fundamentals and Applications; Bin, L., Bazan, G.C., Eds.; Wiley-VCH: Weinham, Germany, 2013; pp. 127–168. [Google Scholar]
- McCullough, R.D.; Ewbank, P.C. Self-assembly and chemical response of conducting polymer superstructures. Synth. Met. 1997, 84, 311–312. [Google Scholar] [CrossRef]
- McCullough, R.D.; Ewbank, P.C.; Loewe, R.S. Self-assembly and disassembly of regioregular, water soluble polythiophenes: Chemoselective ionchromatic sensing in water. J. Am. Chem. Soc. 1997, 119, 633–634. [Google Scholar] [CrossRef]
- Lavigne, J.J.; Broughton, D.L.; Wilson, J.N.; Erdogan, B.; Bunz, U.H.F. “Surfactochromic” conjugated polymers: Surfactant effects on sugar-substituted PPEs. Macromolecules 2003, 36, 7409–7412. [Google Scholar] [CrossRef]
- Chen, L.-H.; Xu, S.; McBranch, D.; Whitten, D. Tuning the properties of conjugated polyelectrolytes through surfactant complexation. J. Am. Chem. Soc. 2000, 122, 9302–9303. [Google Scholar] [CrossRef]
- Burrows, H.D.; Lobo, V.M.M.; Pina, J.; Ramos, M.L.; de Melo, J.S.; Valente, A.J.M.; Tapia, M.J.; Pradhan, S.; Scherf, U. Fluorescence enhancement of the water-soluble poly{1,4-phenylene-[9,9-bis-(4-phenoxybutylsulfonate)]fluorene-2,7-diyl} copolymer in n-dodecylpentaoxyethylene glycol ether micelles. Macromolecules 2004, 37, 7425–7427. [Google Scholar] [CrossRef]
- Suzuki, H. Relations between Electronic absorption spectra and spatial configurations of conjugated systems. III. o,o′-bridged biphenyls. Bull. Chem. Soc. Jpn. 1959, 32, 1357–1361. [Google Scholar] [CrossRef]
- Charas, A.; Morgado, J.; Martinho, J.M.G.; Alcácer, L.; Lim, S.F.; Friend, R.H.; Cacialli, F. Synthesis and luminescence properties of three novel polyfluorene copolymers. Polymer 2003, 44, 1843–1850. [Google Scholar] [CrossRef]
- Surin, M.; Hennebicq, E.; Ego, E.; Marsitzky, D.; Grimsdale, A.C.; Müllen, K.; Brédas, J.-L.; Lazzaroni, R.; Leclère, P. Correlation between the Microscopic Morphology and the Solid-State Photoluminescence Properties in Fluorene-Based Polymers and Copolymers. Chem. Mater. 2004, 16, 994–1001. [Google Scholar] [CrossRef]
- Burrows, H.D.; Fonseca, S.M.; Silva, C.L.; Pais, A.A.C.C.; Tapia, M.J.; Pradhan, S.; Scherf, U. Aggregation of the hairy rod conjugated polyelectrolyte poly{1,4-phenylene-[9,9-bis(4-phenoxybutylsulfonate)]fluorene-2,7-diyl} in aqueous solution: An experimental and molecular modelling study. Phys. Chem. Chem. Phys. 2008, 10, 4420–4428. [Google Scholar] [CrossRef] [PubMed]
- Knaapila, M.; Fonseca, S.M.; Stewart, B.; Torkkeli, M.; Perlich, J.; Pradhan, S.; Sherf, U.; Castro, R.A.E.; Burrows, H.D. Nanostructuring of the conjugated polyelectrolyte poly[9,9-bis(4-sulfonylbutoxyphenylphenyl) fluorene-2,7-diyl-2,2’-bithiophene] in liquid crystalline C12E4 in bulk water and aligned thin films. Soft Matter 2014, 10, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, K.; Jönsson, B.; Kronberg, B.; Lindman, B. Surfactants and Polymers in Aqueous Solutions; Wiley: Chichester, UK, 2003. [Google Scholar]
- Kim, B.-G.; Jeong, E.J.; Chung, J.W.; Seo, S.; Koo, B.; Kim, J. A molecular design principle of lyotropic liquid-crystalline conjugated polymers with directed alignment capability for plastic electronics. Nat. Mater. 2013, 12, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.M.; Eusebio, M.E.; Castro, R.; Burrows, H.D.; Tapia, M.J.; Olsson, U. Interactions between hairy rod anionic conjugated polyelectrolytes and nonionic alkyloxyethylene surfactants in aqueous solution: Observations from cloud point behaviour. J. Colloid Interface Sci. 2007, 315, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Sirringhaus, H. Optical absorptions of polyfluorene transistors. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 72, 045207. [Google Scholar] [CrossRef]
- Burrows, H.D.; Tapia, M.J.; Fonseca, S.M.; Valente, A.J.M.; Lobo, V.M.M.; Justino, L.L.G.; Qiu, S.; Pradhan, S.; Scherf, U.; Chattopadhyay, N.; et al. Aqueous solution behavior of anionic fluorene-co-thiophene-based conjugated polyelectrolytes. ACS Appl. Mater. Interfaces 2009, 1, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Knaapila, M.; Almásy, L.; Garamus, V.M.; Pradhan, S.; Pearson, C.; Petty, M.C.; Scherf, U.; Burrows, H.D.; Monkman, A.P. Solubilization of polyelectrolytic hairy-rod polyfluorene in aqueous solutions of nonionic surfactant. J. Phys. Chem. B 2006, 110, 10248–10257. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.J.; Tiddy, G.J.T.; Waring, L.; Bostock, T.; McDonald, M.P. Phase behaviour of polyoxyethylene surfactants with water. Mesophase structures and partial miscibility (cloud points). J. Chem. Soc. Faraday Trans. 1983, 79, 975–1000. [Google Scholar] [CrossRef]
- Strey, R.; Schomäcker, R.; Roux, D.; Nallet, F.; Olsson, U. Dilute lamellar and L3 phases in the binary water–C12E5 system. J. Chem. Soc. Faraday Trans. 1990, 86, 2253–2261. [Google Scholar] [CrossRef]
- Olsson, U.; Wennerström, H. Globular and bicontinuous phases of nonionic surfactant films. Adv. Colloid Interface Sci. 1994, 49, 113–146. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; van der Spoel, D.; van Druren, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 41, 43–45. [Google Scholar] [CrossRef]
- Lindahl, E.; Hess, B.; van der Spoel, D. GROMACS 3.0: A package for molecular simulation and trajectory analysis. J. Mol. Model. 2001, 7, 306–317. [Google Scholar]
- Oostenbrink, C.; Villa, A.; Mark, A.E.; van Gunsteren, W.F. A biomolecular force field based on the free enthalpy of hydration and solvation: The GROMOS force-field parameter sets 53A5 and 53A6. J. Comp. Chem. 2004, 25, 1656–1676. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchinson, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminf. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oosterbrink, C.; Mark, A.E. An automated force field topology builder (ATB) and repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; Hermans, J. Intermolecular Forces; Bullman, B., Ed.; Reidel Publishing Company: Dordrecht, The Netherlands, 1981; pp. 331–3342. [Google Scholar]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 6, 33–38. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stewart, B.; Burrows, H.D. Molecular Dynamics Study of Self-Assembly of Aqueous Solutions of Poly[9,9-bis(4-Sulfonylbutoxyphenylphenyl) Fluorene-2,7-diyl-2,2’-Bithiophene] (PBS-PF2T) in the Presence of Pentaethylene Glycol Monododecyl Ether (C12E5). Materials 2016, 9, 379. https://doi.org/10.3390/ma9050379
Stewart B, Burrows HD. Molecular Dynamics Study of Self-Assembly of Aqueous Solutions of Poly[9,9-bis(4-Sulfonylbutoxyphenylphenyl) Fluorene-2,7-diyl-2,2’-Bithiophene] (PBS-PF2T) in the Presence of Pentaethylene Glycol Monododecyl Ether (C12E5). Materials. 2016; 9(5):379. https://doi.org/10.3390/ma9050379
Chicago/Turabian StyleStewart, Beverly, and Hugh Douglas Burrows. 2016. "Molecular Dynamics Study of Self-Assembly of Aqueous Solutions of Poly[9,9-bis(4-Sulfonylbutoxyphenylphenyl) Fluorene-2,7-diyl-2,2’-Bithiophene] (PBS-PF2T) in the Presence of Pentaethylene Glycol Monododecyl Ether (C12E5)" Materials 9, no. 5: 379. https://doi.org/10.3390/ma9050379






