A Novel MgO-CaO-SiO2 System for Fabricating Bone Scaffolds with Improved Overall Performance
Abstract
:1. Introduction
2. Results and Discussion
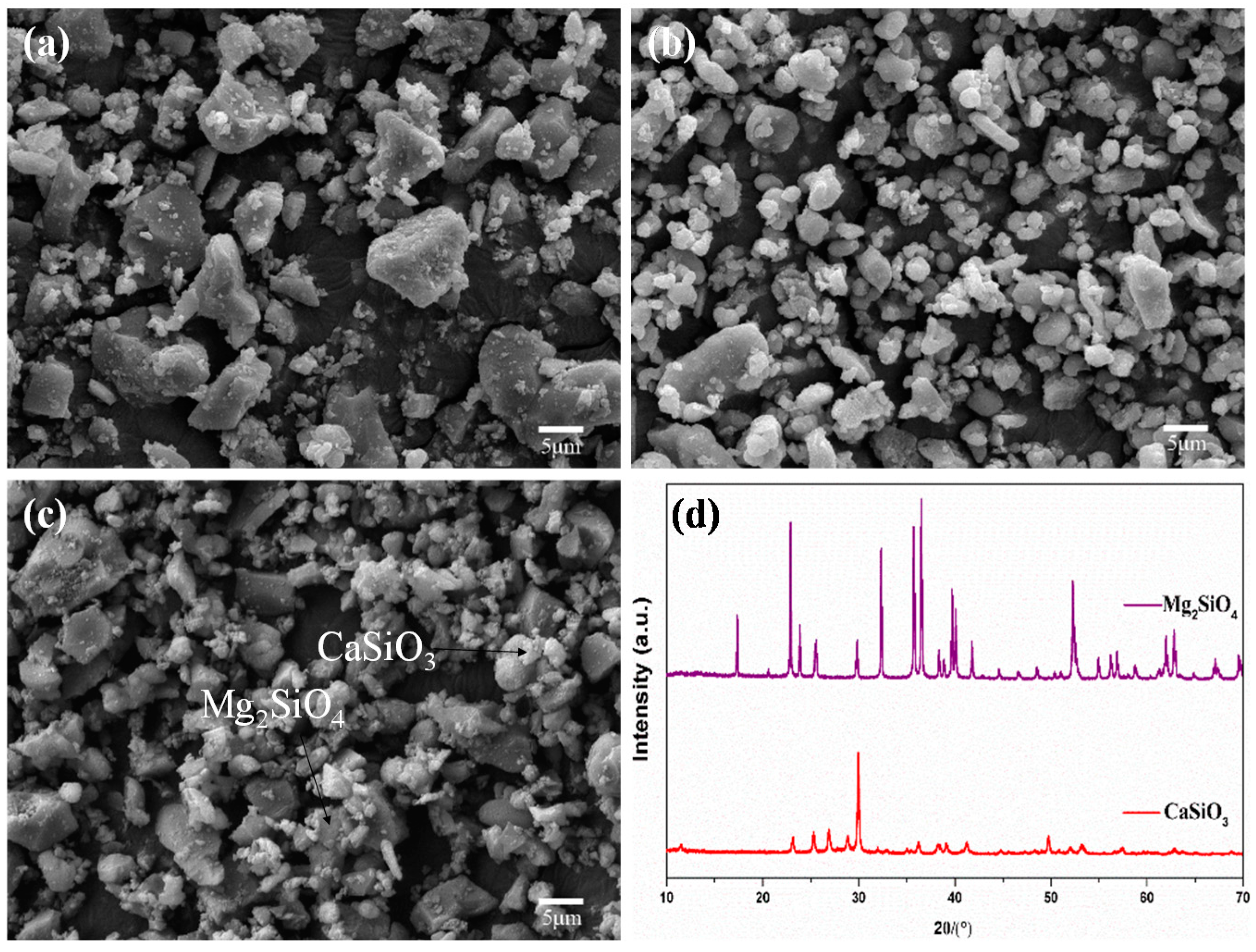
2.1. Mixed Powders
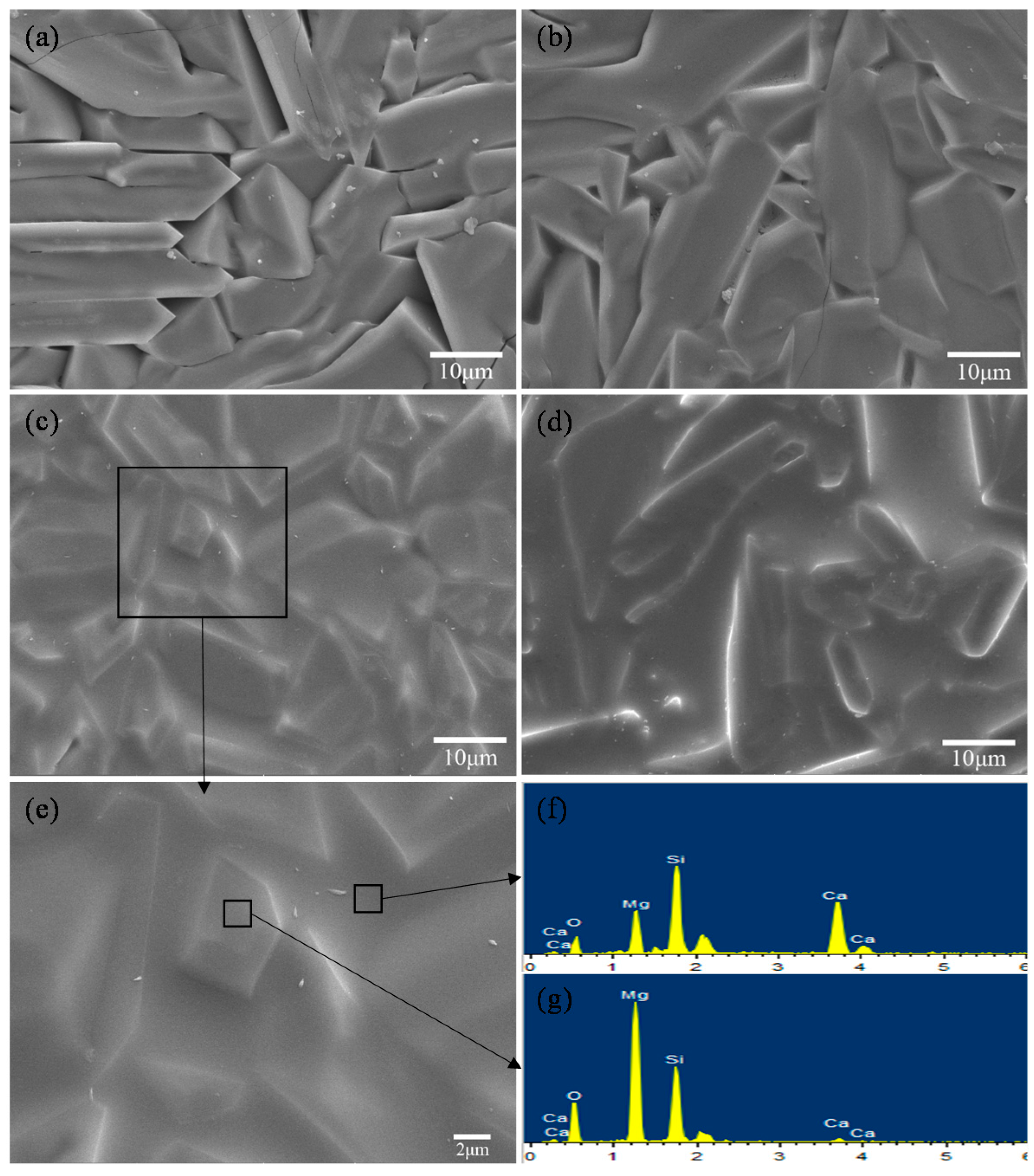
2.2. Scaffolds
2.3. Degradability
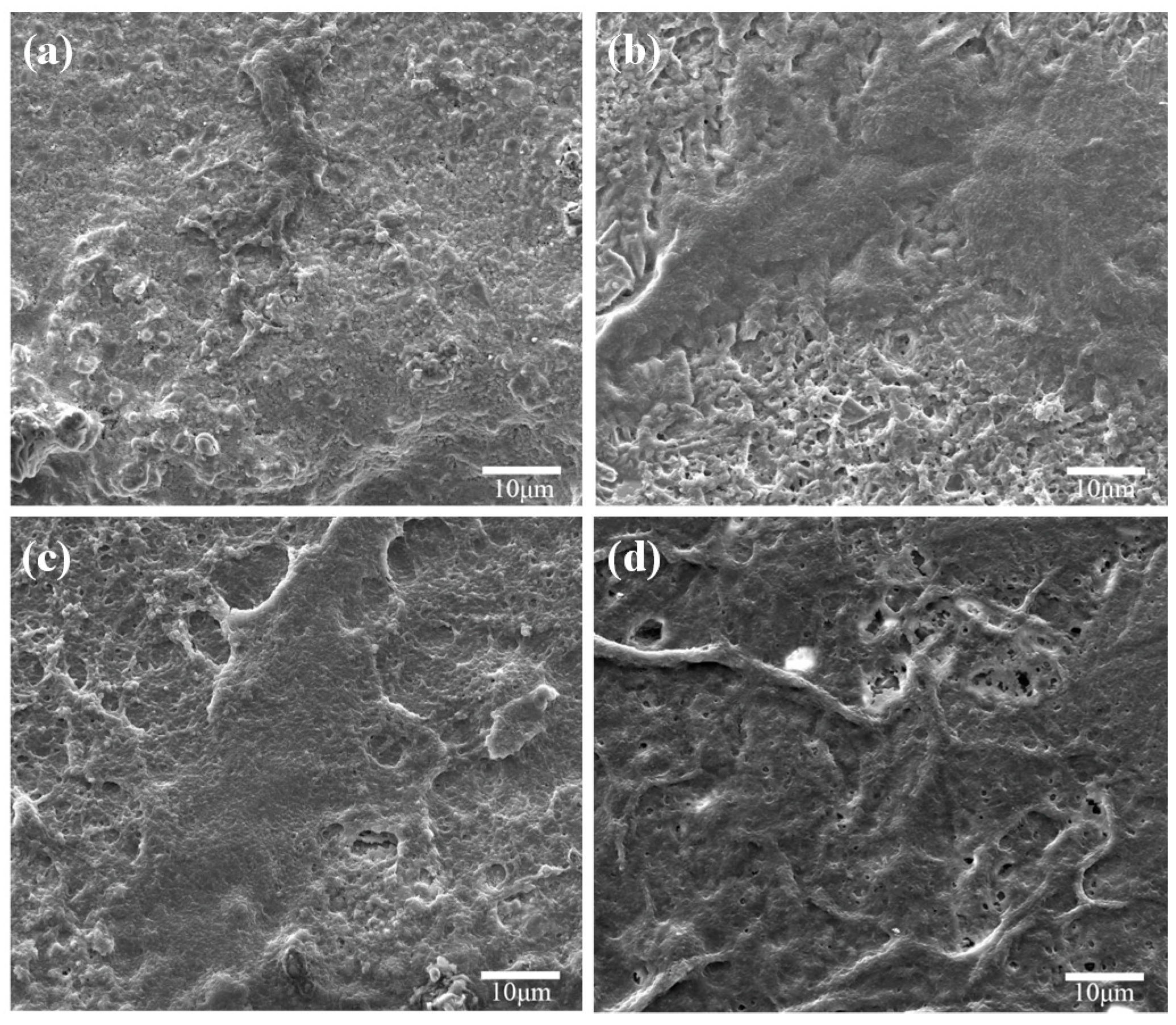
2.4. Bioactivity
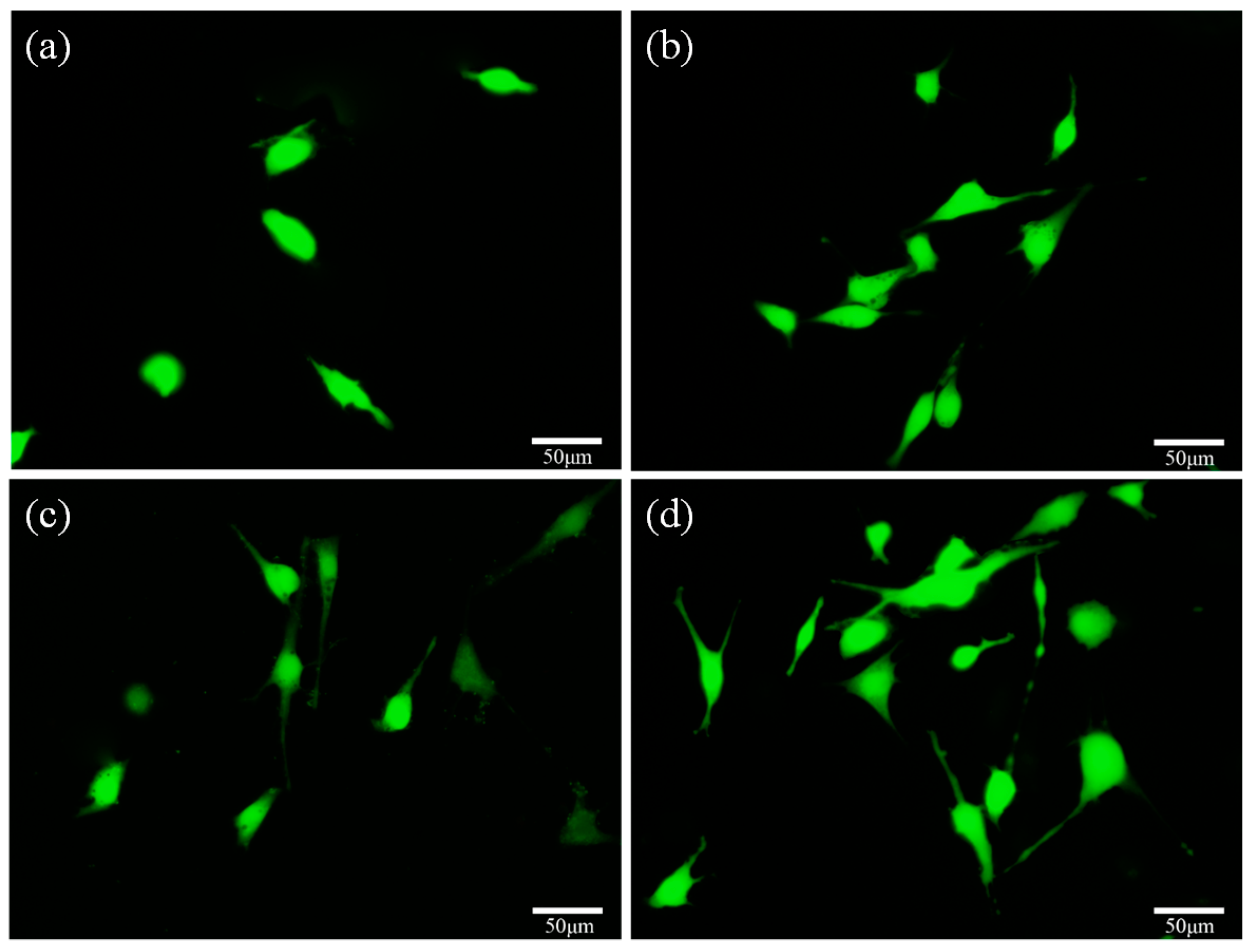
2.5. Cell Behavior
3. Materials and Methods
3.1. Scaffold Fabrication
3.2. Scaffold Characterization
3.3. Degradation Behavior
3.4. Bioactivity
3.5. Cell Culture
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Cao, Y.; Shuai, C.; Peng, S. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int. J. Mol. Sci. 2014, 15, 4714–4732. [Google Scholar] [CrossRef] [PubMed]
- Pilia, M.; Guda, T.; Appleford, M. Development of composite scaffolds for load-bearing segmental bone defects. BioMed Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Pighinelli, L.; Kucharska, M. Chitosan-hydroxyapatite composites. Carbohydr. Polym. 2013, 93, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Encinas-Romero, M.A.; Aguayo-Salinas, S.; Valenzuela-García, J.L.; Payán, S.R.; Castillón-Barraza, F.F. Mechanical and bioactive behavior of hydroxyapatite-wollastonite sintered composites. Int. J. Appl. Ceram. Technol. 2010, 7, 164–177. [Google Scholar] [CrossRef]
- Saidi, R.; Fathi, M.H.; Salimijazi, H. Fabrication and characterization of hydroxyapatite-coated forsterite scaffold for tissue regeneration applications. Bull. Mater. Sci. 2015, 38, 1367–1374. [Google Scholar] [CrossRef]
- Shuai, C.; Deng, J.; Li, P.; Peng, S. Novel forsterite scaffolds for bone tissue engineering: Selective laser sintering fabrication and characterisation. Mater. Res. Innov. 2014, 18, S2:74–S2:78. [Google Scholar] [CrossRef]
- Mohammadi, H.; Hafezi, M.; Nezafati, N.; Heasarki, S.; Nadernezhad, A.; Ghazanfari, S.; Sepantafar, M. Bioinorganics in bioactive calcium silicate ceramics for bone tissue repair: Bioactivity and biological properties. J. Ceram. Sci. Technol. 2014, 5, 1–12. [Google Scholar]
- Ghomi, H.; Jaberzadeh, M.; Fathi, M. Novel fabrication of forsterite scaffold with improved mechanical properties. J. Alloy. Compd. 2011, 509, L63–L68. [Google Scholar] [CrossRef]
- Zhai, W.; Lu, H.; Wu, C.; Chen, L.; Lin, X.; Naoki, K.; Chen, G.; Chang, J. Stimulatory effects of the ionic products from Ca–Mg–Si bioceramics on both osteogenesis and angiogenesis in vitro. Acta Biomater. 2013, 9, 8004–8014. [Google Scholar] [CrossRef] [PubMed]
- Kharaziha, M.; Fathi, M. Improvement of mechanical properties and biocompatibility of forsterite bioceramic addressed to bone tissue engineering materials. J. Mech. Behav. Biomed. Mater. 2010, 3, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Tavangarian, F.; Emadi, R. Improving degradation rate and apatite formation ability of nanostructure forsterite. Ceram. Int. 2011, 37, 2275–2280. [Google Scholar] [CrossRef]
- Wu, C.; Fan, W.; Zhou, Y.; Luo, Y.; Gelinsky, M.; Chang, J.; Xiao, Y. 3D-printing of highly uniform CaSiO3 ceramic scaffolds: Preparation, characterization and in vivo osteogenesis. J. Mater. Chem. 2012, 22, 12288–12295. [Google Scholar] [CrossRef]
- Shuai, C.; Mao, Z.; Han, Z.; Peng, S.; Li, Z. Fabrication and characterization of calcium silicate scaffolds for tissue engineering. J. Mech. Med. Biol. 2014, 14, 1450049. [Google Scholar] [CrossRef]
- Wu, C.; Ramaswamy, Y.; Boughton, P.; Zreiqat, H. Improvement of mechanical and biological properties of porous CaSiO3 scaffolds by poly(d, l-lactic acid) modification. Acta Biomater. 2008, 4, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.K.; Gervaso, F.; Carrozzo, M.; Scalera, F.; Sannino, A.; Licciulli, A. Wollastonite/hydroxyapatite scaffolds with improved mechanical, bioactive and biodegradable properties for bone tissue engineering. Ceram. Int. 2013, 39, 619–627. [Google Scholar] [CrossRef]
- Sathiyakumar, M.; Gnanam, F. Role of wollastonite additive on density, microstructure and mechanical properties of alumina. Ceram. Int. 2003, 29, 869–873. [Google Scholar] [CrossRef]
- Sainz, M.; Pena, P.; Serena, S.; Caballero, A. Influence of design on bioactivity of novel CaSiO3–CaMg(SiO3)2 bioceramics: In vitro simulated body fluid test and thermodynamic simulation. Acta Biomater. 2010, 6, 2797–2807. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhang, M.; Zhai, W.; Qu, H.; Chang, J. Fabrication and characterization of hydroxyapatite/wollastonite composite bioceramics with controllable properties for hard tissue repair. J. Am. Ceram. Soc. 2011, 94, 99–105. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Krishnan, U.M.; Sethuraman, S. Polymeric scaffold aided stem cell therapeutics for cardiac muscle repair and regeneration. Macromol. Biosci. 2013, 13, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Chang, J. In vitro degradation, bioactivity, and cytocompatibility of calcium silicate, dimagnesium silicate, and tricalcium phosphate bioceramics. J. Biomater. Appl. 2009, 24, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Kamalian, R.; Yazdanpanah, A.; Moztarzadeh, F.; Ravarian, R.; Moztarzadeh, Z.; Tahmasbi, M.; Mozafari, M. Synthesis and characterisation of bioactive glass/forsterite nanocomposites for bone and dental implants. Ceram. Silik. 2012, 56, 331–340. [Google Scholar]
- Naghiu, M.; Gorea, M.; Mutch, E.; Kristaly, F.; Tomoaia-Cotisel, M. Forsterite nanopowder: Structural characterization and biocompatibility evaluation. J. Mater. Sci. Technol. 2013, 29, 628–632. [Google Scholar] [CrossRef]
- Deng, J.; Li, P.; Gao, C.; Feng, P.; Shuai, C.; Peng, S. Bioactivity improvement of forsterite-based scaffolds with nano-58S bioactive glass. Mater. Manuf. Process. 2014, 29, 877–884. [Google Scholar] [CrossRef]
- Gopi, D.; Murugan, N.; Ramya, S.; Kavitha, L. Electrodeposition of a porous strontium-substituted hydroxyapatite/zinc oxide duplex layer on AZ91 magnesium alloy for orthopedic applications. J. Mater. Chem. B 2014, 2, 5531–5540. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Kamalian, R.; Moztarzadeh, F.; Mozafari, M.; Ravarian, R.; Tayebi, L. Enhancement of fracture toughness in bioactive glass-based nanocomposites with nanocrystalline forsterite as advanced biomaterials for bone tissue engineering applications. Ceram. Int. 2012, 38, 5007–5014. [Google Scholar] [CrossRef]
- Haberko, K.; Bućko, M.M.; Brzezińska-Miecznik, J.; Haberko, M.; Mozgawa, W.; Panz, T.; Pyda, A.; Zarębski, J. Natural hydroxyapatite—Its behaviour during heat treatment. J. Eur. Ceram. Soc. 2006, 26, 537–542. [Google Scholar] [CrossRef]
- Diba, M.; Tapia, F.; Boccaccini, A.R.; Strobel, L.A. Magnesium-containing bioactive glasses for biomedical applications. Int. J. Appl. Glass Sci. 2012, 3, 221–253. [Google Scholar] [CrossRef]
- Magallanes-Perdomo, M.; Luklinska, Z.; De Aza, A.; Carrodeguas, R.; De Aza, S.; Pena, P. Bone-like forming ability of apatite-wollastonite glass ceramic. J. Eur. Ceram. Soc. 2011, 31, 1549–1561. [Google Scholar] [CrossRef]
- Shuai, C.; Nie, Y.; Gao, C.; Feng, P.; Zhuang, J.; Zhou, Y.; Peng, S. The microstructure evolution of nanohydroxapatite powder sintered for bone tissue engineering. J. Exp. Nanosci. 2013, 8, 762–773. [Google Scholar] [CrossRef]
- Sachiko, H.S.; Jun, S.; Makoto, S.; Hideaki, E. Determination of fracture toughness of human permanent and primary enamel using an indentation microfracture method. J. Mater. Sci. Mater. Med. 2012, 23, 2047–2054. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity. Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]







| Ion Type | Ion Concentration/(mmol/L) | ||||||
|---|---|---|---|---|---|---|---|
| Na+ | K+ | Mg2+ | Ca2+ | Cl− | |||
| Blood Plasma | 142.0 | 5.0 | 1.5 | 2.5 | 103.0 | 17.0 | 1.0 |
| SBF | 142.0 | 5.0 | 1.5 | 2.5 | 148.8 | 4.2 | 1.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; He, S.; Wu, P.; Gao, C.; Feng, P.; Xiao, T.; Deng, Y.; Shuai, C. A Novel MgO-CaO-SiO2 System for Fabricating Bone Scaffolds with Improved Overall Performance. Materials 2016, 9, 287. https://doi.org/10.3390/ma9040287
Sun H, He S, Wu P, Gao C, Feng P, Xiao T, Deng Y, Shuai C. A Novel MgO-CaO-SiO2 System for Fabricating Bone Scaffolds with Improved Overall Performance. Materials. 2016; 9(4):287. https://doi.org/10.3390/ma9040287
Chicago/Turabian StyleSun, Hang, Shiwei He, Ping Wu, Chengde Gao, Pei Feng, Tao Xiao, Youwen Deng, and Cijun Shuai. 2016. "A Novel MgO-CaO-SiO2 System for Fabricating Bone Scaffolds with Improved Overall Performance" Materials 9, no. 4: 287. https://doi.org/10.3390/ma9040287
APA StyleSun, H., He, S., Wu, P., Gao, C., Feng, P., Xiao, T., Deng, Y., & Shuai, C. (2016). A Novel MgO-CaO-SiO2 System for Fabricating Bone Scaffolds with Improved Overall Performance. Materials, 9(4), 287. https://doi.org/10.3390/ma9040287







