Photocatalytic Properties of g-C3N4–TiO2 Heterojunctions under UV and Visible Light Conditions
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Nanomaterials
2.2.1. Preparation of Titanium Dioxide
2.2.2. Preparation of g-C3N4
2.2.3. Preparation of g-C3N4–TiO2 Materials
2.3. Characterization
2.4. Photocatalytic Study
3. Results and Discussion
3.1. X-ray Diffraction (XRD)
3.2. Raman Spectroscopy
3.3. Fourier Transform Infrared Spectroscopy (FT-IR)
3.4. X-ray Photoelectron Spectroscopy (XPS)
3.5. Diffuse Absorbance
3.6. Photoluminescence (PL)
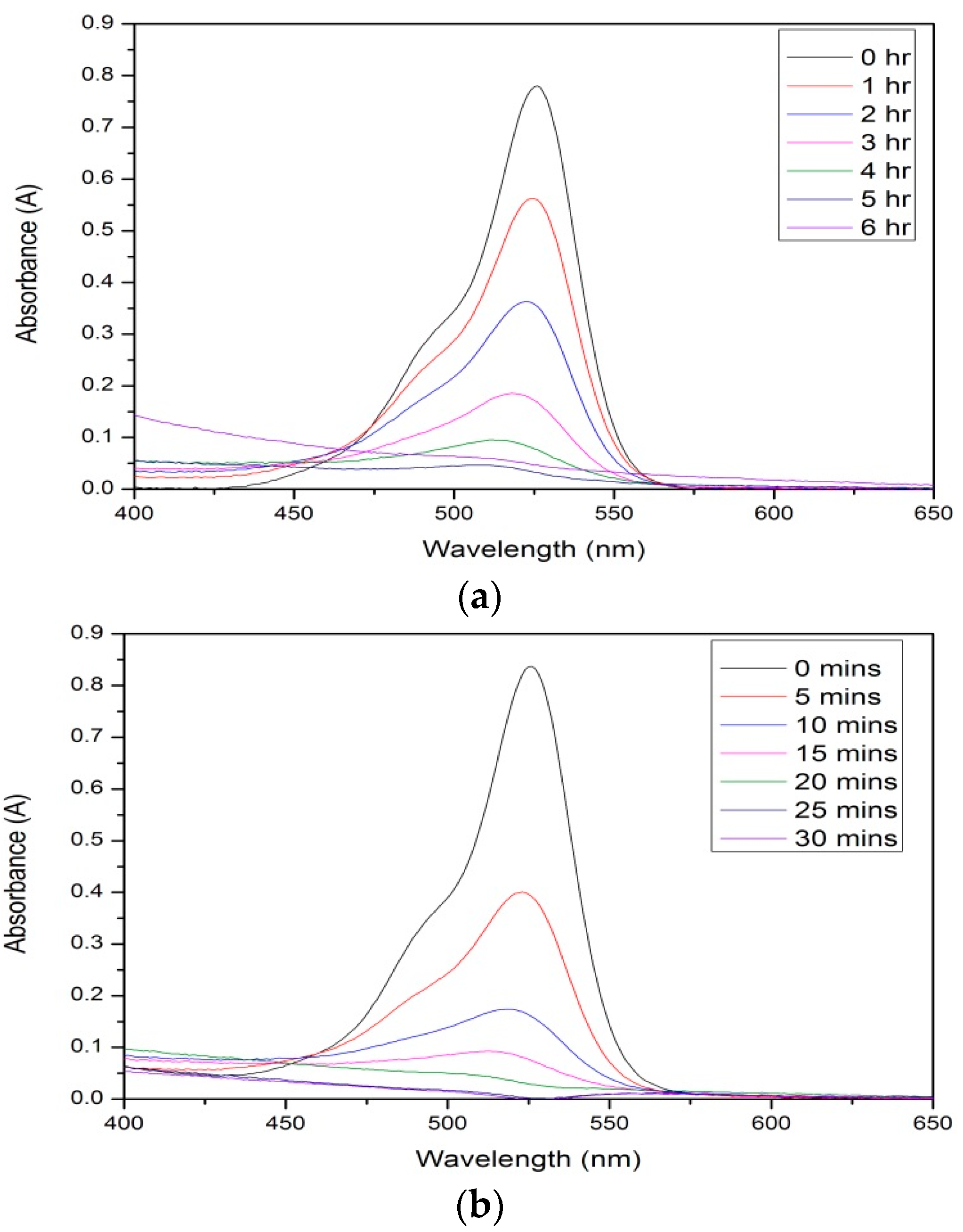
3.7. Photocatalysis
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Etacheri, V.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. Highly Visible Light Active TiO2−xNx Heterojunction Photocatalysts. Chem. Mater. 2010, 22, 3843–3853. [Google Scholar] [CrossRef]
- Etacheri, V.; Michlits, G.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. A Highly Efficient TiO2–xCx Nano-heterojunction Photocatalyst for Visible Light Induced Antibacterial Applications. ACS Appl. Mater. Interfaces 2013, 5, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Gong, Y.; Li, H.; Chen, Z.; Wang, Y. Selective oxidation of benzene to phenol by FeCl3/mpg-C3N4 hybrids. RSC Adv. 2013, 3, 5121–5126. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Miranda, C.; Mansilla, H.; Yáñez, J.; Obregón, S.; Colón, G. Improved photocatalytic activity of g-C3N4/TiO2 composites prepared by a simple impregnation method. J. Photochem. Photobiol. A Chem. 2013, 253, 16–21. [Google Scholar] [CrossRef]
- Sridharan, K.; Jang, E.; Park, T.J. Novel visible light active graphitic C3N4–TiO2 composite photocatalyst: Synergistic synthesis, growth and photocatalytic treatment of hazardous pollutants. Appl. Catal. B Environ. 2013, 142–143, 718–728. [Google Scholar] [CrossRef]
- Boonprakob, N.; Wetchakun, N.; Phanichphant, S.; Waxler, D.; Sherrell, P.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Enhanced visible-light photocatalytic activity of g-C3N4/TiO2 films. J. Colloid Interface Sci. 2014, 417, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, J.; Xie, H.; Qu, A. Synthesis of g-C3N4/TiO2 with enhanced photocatalytic activity for H2 evolution by a simple method. Int. J. Hydrog. Energy 2014, 39, 6354–6363. [Google Scholar] [CrossRef]
- Su, F.; Mathew, S.C.; Lipner, G.; Fu, X.; Antonietti, M.; Blechert, S.; Wang, X. mpg-C3N4-Catalyzed Selective Oxidation of Alcohols Using O2 and Visible Light. J. Am. Chem. Soc. 2010, 132, 16299–16301. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Q.; Cui, D. Preparation and Photocatalytic Properties of g-C3N4/TiO2 Hybrid Composite. J. Mater. Sci. Technol. 2010, 26, 925–930. [Google Scholar] [CrossRef]
- Yan, H.; Yang, H. TiO2–g-C3N4 composite materials for photocatalytic H2 evolution under visible light irradiation. J. Alloys Compd. 2011, 509, L26–L29. [Google Scholar] [CrossRef]
- Gu, L.; Wang, J.; Zou, Z.; Han, X. Graphitic-C3N4-hybridized TiO2 nanosheets with reactive {0 0 1} facets to enhance the UV- and visible-light photocatalytic activity. J. Hazard. Mater. 2014, 268, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Han, D.; Zhou, M.; Sun, H.; Wang, L.; Dong, X.; Niu, L. Ultrathin g-C3N4/TiO2 composites as photoelectrochemical elements for the real-time evaluation of global antioxidant capacity. Chem. Sci. 2014, 5, 3946–3951. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, X.; Zheng, Z.; Ke, X.; Jaatinen, E.; Zhao, J.; Guo, C.; Xie, T.; Wang, D. Mechanism of supported gold nanoparticles as photocatalysts under ultraviolet and visible light irradiation. Chem. Commun. (Camb.) 2009, 48, 7524–7526. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, Y.; Li, J.; Wang, Y.; Jiang, G.; Zhao, Z.; Wang, D.; Duan, A.; Liu, J.; Wei, Y. Facile in situ synthesis of graphitic carbon nitride (g-C3N4)-N-TiO2 heterojunction as an efficient photocatalyst for the selective photoreduction of CO2 to CO. Appl. Catal. B Environ. 2014, 158–159, 20–29. [Google Scholar] [CrossRef]
- Dai, K.; Lu, L.; Liang, C.; Liu, Q.; Zhu, G. Heterojunction of facet coupled g-C3N4/surface-fluorinated TiO2 nanosheets for organic pollutants degradation under visible LED light irradiation. Appl. Catal. B Environ. 2014, 156–157, 331–340. [Google Scholar] [CrossRef]
- Kondo, K.; Murakami, N.; Ye, C.; Tsubota, T.; Ohno, T. Development of highly efficient sulfur-doped TiO2 photocatalysts hybridized with graphitic carbon nitride. Appl. Catal. B Environ. 2013, 142–143, 362–367. [Google Scholar] [CrossRef]
- Yang, M.; Hu, S.; Li, F.; Fan, Z.; Wang, F.; Liu, D.; Gui, J. The influence of preparation method on the photocatalytic performance of g-C3N4/WO3 composite photocatalyst. Ceram. Int. 2014, 40, 11963–11969. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.; Meng, S.; Fu, X. Study on the separation mechanisms of photogenerated electrons and holes for composite photocatalysts g-C3N4-WO3. Appl. Catal. B Environ. 2014, 150–151, 564–573. [Google Scholar] [CrossRef]
- Jin, Z.; Murakami, N.; Tsubota, T.; Ohno, T. Complete oxidation of acetaldehyde over a composite photocatalyst of graphitic carbon nitride and tungsten(VI) oxide under visible-light irradiation. Appl. Catal. B Environ. 2014, 150–151, 479–485. [Google Scholar] [CrossRef]
- Ge, L.; Han, C. Synthesis of MWNTs/g-C3N4 composite photocatalysts with efficient visible light photocatalytic hydrogen evolution activity. Appl. Catal. B Environ. 2012, 117–118, 268–274. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Hu, W.; Cai, J.; Zhang, L.; Dong, L.; Zhao, L.; He, Y. Synthesis and photocatalytic activity of SiO2/g-C3N4 composite photocatalyst. Mater. Lett. 2014, 115, 53–56. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Liu, J.; Li, Y. Enhanced visible light photocatalytic activity of novel polymeric g-C3N4 loaded with Ag nanoparticles. Appl. Catal. A Gen. 2011, 409–410, 215–222. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, W.; Guo, Y.; Zhao, Y.; Yuan, X.; Guo, Y. Fabrication of Z-scheme plasmonic photocatalyst Ag@AgBr/g-C3N4 with enhanced visible-light photocatalytic activity. J. Hazard. Mater. 2014, 271, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Tao, H.; Chen, L.; Sun, Y. Synthesis of Fe/g-C3N4 composites with improved visible light photocatalytic activity. Mater. Lett. 2014, 116, 265–267. [Google Scholar] [CrossRef]
- Hu, S.; Ma, L.; You, J.; Li, F.; Fan, Z.; Lu, G.; Liu, D.; Gui, J. Enhanced visible light photocatalytic performance of g-C3N4 photocatalysts co-doped with iron and phosphorus. Appl. Surf. Sci. 2014, 311, 164–171. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Wang, Y.; Yao, W.; Zhu, Y. Enhanced oxidation ability of g-C3N4 photocatalyst via C60 modification. Appl. Catal. B Environ. 2014, 152–153, 262–270. [Google Scholar] [CrossRef]
- Han, C.; Ge, L.; Chen, C.; Li, Y.; Xiao, X.; Zhang, Y.; Guo, L. Novel visible light induced Co3O4-g-C3N4 heterojunction photocatalysts for efficient degradation of methyl orange. Appl. Catal. B Environ. 2014, 147, 546–553. [Google Scholar] [CrossRef]
- Kato, H.; Hori, M.; Konta, R.; Shimodaira, Y.; Kudo, A. Construction of Z-scheme Type Heterogeneous Photocatalysis Systems for Water Splitting into H2 and O2 under Visible Light Irradiation. Chem. Lett. 2004, 33, 1348–1349. [Google Scholar] [CrossRef]
- Dai, X.; Xie, M.; Meng, S.; Fu, X.; Chen, S. Coupled systems for selective oxidation of aromatic alcohols to aldehydes and reduction of nitrobenzene into aniline using CdS/g-C3N4 photocatalyst under visible light irradiation. Appl. Catal. B Environ. 2014, 158–159, 382–390. [Google Scholar] [CrossRef]
- Dong, F.; Sun, Y.; Wu, L.; Fu, M.; Wu, Z. Facile transformation of low cost thiourea into nitrogen-rich graphitic carbon nitride nanocatalyst with high visible light photocatalytic performance. Catal. Sci. Technol. 2012, 2, 1332–1335. [Google Scholar] [CrossRef]
- Dante, R.C.; Martín-Ramos, P.; Correa-Guimaraes, A.; Martín-Gil, J. Synthesis of graphitic carbon nitride by reaction of melamine and uric acid. Mater. Chem. Phys. 2011, 130, 1094–1102. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Y.; Xu, S. Synthesis of graphitic carbon nitride by directly heating sulfuric acid treated melamine for enhanced photocatalytic H2 production from water under visible light. Int. J. Hydrog. Energy 2012, 37, 125–133. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Preparation and Enhanced Visible-Light Photocatalytic H2-Production Activity of Graphene/C3N4 Composites. J. Phys. Chem. C 2011, 115, 7355–7363. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation Performance of g-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Fischer, F. Goettmann, Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Teter, D.M.; Hemley, R.J. Low-compressibility carbon nitrides. Science 1996, 271, 53–55. [Google Scholar] [CrossRef]
- Zhai, H.S.; Cao, L.; Xia, X.H. Synthesis of graphitic carbon nitride through pyrolysis of melamine and its electrocatalysis for oxygen reduction reaction. Chin. Chem. Lett. 2013, 24, 103–106. [Google Scholar] [CrossRef]
- Schreiner, W.; Jenkins, R. Profile Fitting for Quantitative Analysis in X-ray Diffraction. Adv. X-ray Anal. 1983, 26, 141. [Google Scholar]
- Spurr, R.A.; Myers, H. Quantitative Analysis of Anatase-Rutile Mixtures with an X-ray Diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, Y.; Xu, H.; Wang, H.; Da, Z.; Huang, S.; Ji, H.; Li, H. In situ oxidation synthesis of visible-light-driven plasmonic photocatalyst Ag/AgCl/g-C3N4 and its activity. Ceram. Int. 2014, 40, 9293–9301. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; Zhang, G.; Huang, J.; Liu, P.; Antonietti, M.; Wang, X. Synthesis of bulk and nanoporous carbon nitride polymers from ammonium thiocyanate for photocatalytic hydrogen evolution. J. Mater. Chem. 2011, 21, 13032–13039. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Kumar, K.N.P.; Keizer, K.; Burggraaf, A.J.; Okubo, T.; Nagamoto, H.; Morooka, S. Densification of nanostructured titania assisted by a phase transformation. Nature 1992, 358, 48–51. [Google Scholar] [CrossRef]
- Kumar, S.R.; Pillai, S.C.; Hareesh, U.; Mukundan, P.; Warrier, K.G. Synthesis of thermally stable, high surface area anatase–alumina mixed oxides. Mater. Lett. 2000, 43, 286–290. [Google Scholar] [CrossRef]
- Pillai, S.C.; Periyat, P.; George, R.; McCormack, D.E.; Seery, M.K.; Hayden, H.; Colreavy, J.; Corr, D.; Hinder, S.J. Synthesis of high-temperature stable anatase TiO2 photocatalyst. J. Phys. Chem. C 2007, 111, 1605–1611. [Google Scholar] [CrossRef] [Green Version]
- Xiong, L.B.; Li, J.L.; Yang, B.; Yu, Y. Ti3+ in the surface of titanium dioxide: Generation, properties and photocatalytic applications. J. Nanomater. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Liu, H.-B.; Gu, G.B. Preparation of nanometer crystalline TiO2 with high photo-catalytic activity by pyrolysis of titanyl organic compounds and photo-catalytic mechanism. Mater. Chem. Phys. 2005, 91, 317–324. [Google Scholar]
- Li, Y.; Li, X.; Li, J.; Yin, J. TiO2-coated active carbon composities with increased photocatalytic activity prepared by a properly controlled sol-gel method. Mater. Lett. 2005, 59, 2659–2663. [Google Scholar]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’Shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New insights into the mechanism of visible light photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Haneda, H.; Labhsetwar, N.K.; Hishita, S.; Ohashi, N. Visible-light-driven photocatalysis on fluorine-doped TiO2 poweders by the creation of surface oxygen vacancies. Chem. Phys. Lett. 2005, 401, 579–584. [Google Scholar] [CrossRef]
- Li, D.; Ohashi, N.; Hishita, S.; Kolodiazhnyi, T.; Haneda, H. Origin of visible-light-driven photocatalysis: A comparative study on N/F-doped and N-F-codoped TiO2 powders by means of experimental characterizations and theoretical calculations. J. Solid State Chem. 2005, 178, 3293–3302. [Google Scholar] [CrossRef]
- Pelaez, M.; Falaras, P.; Likodimos, V.; Kontos, A.G.; de la Cruz, A.A.; O’Shea, K.; Dionysiou, D.D. Synthesis, structural characterization and evaluation of sol-gel-based NF-TiO2 films with visible light-photoactivation for the removal of microcystin-LR. Appl. Catal. B Environ. 2010, 99, 378–387. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, R.; Qiao, P.; Xiao, L.; Fan, J. TiO2 nanoparticles with increased surface hydroxyl groups and their improved photocatalytic activity. Catal. Commun. 2012, 19, 96–99. [Google Scholar] [CrossRef]
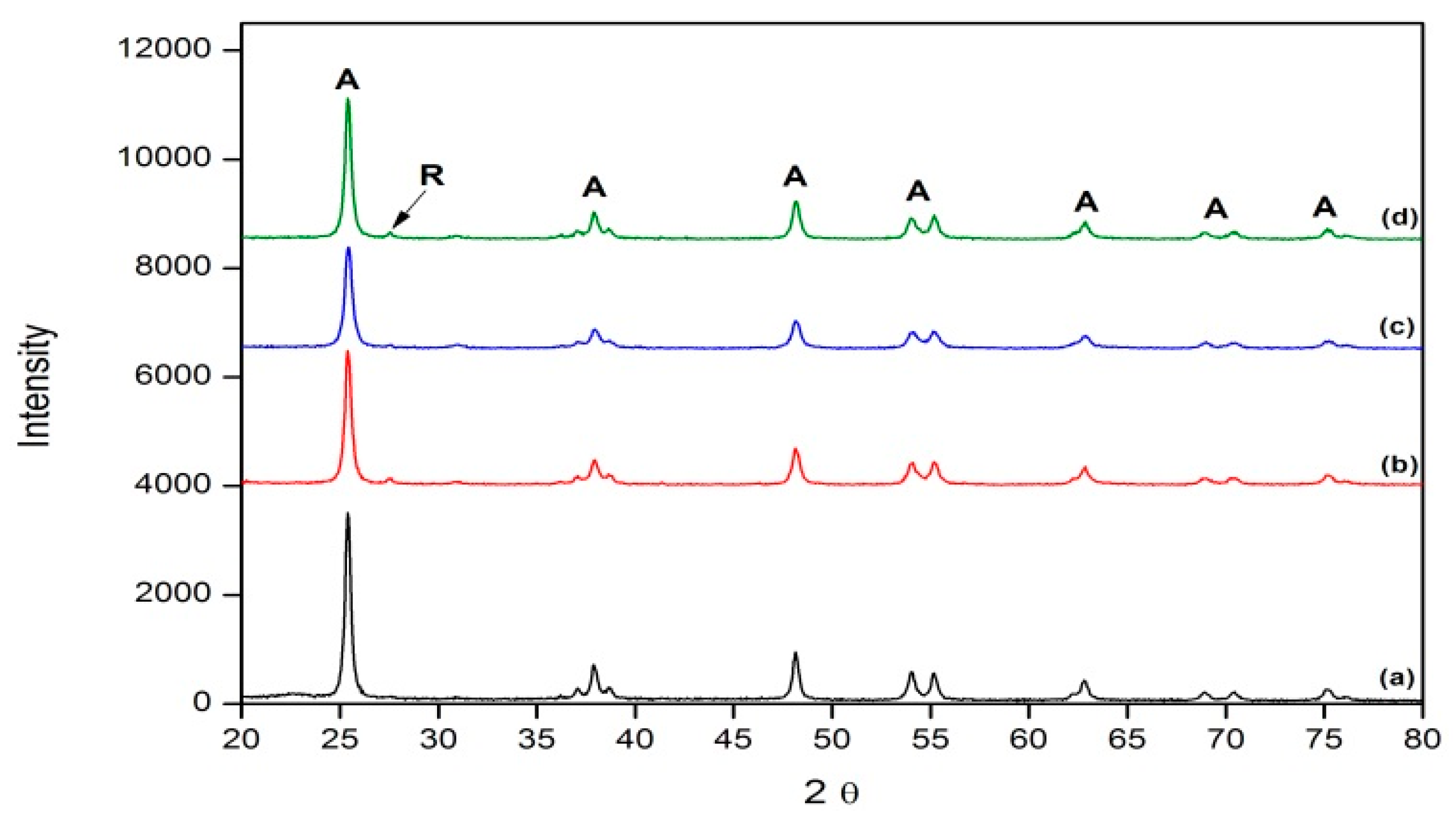
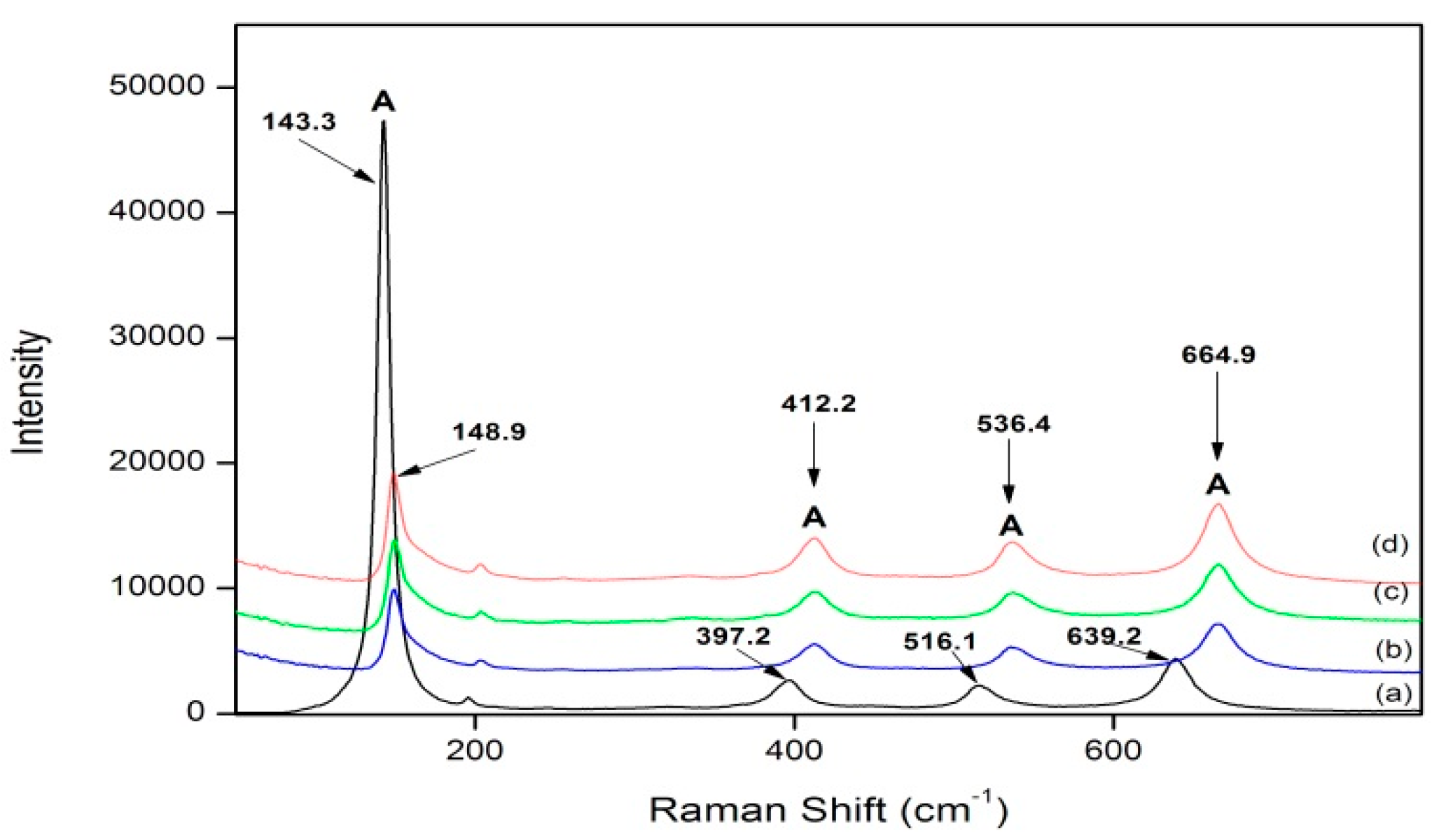
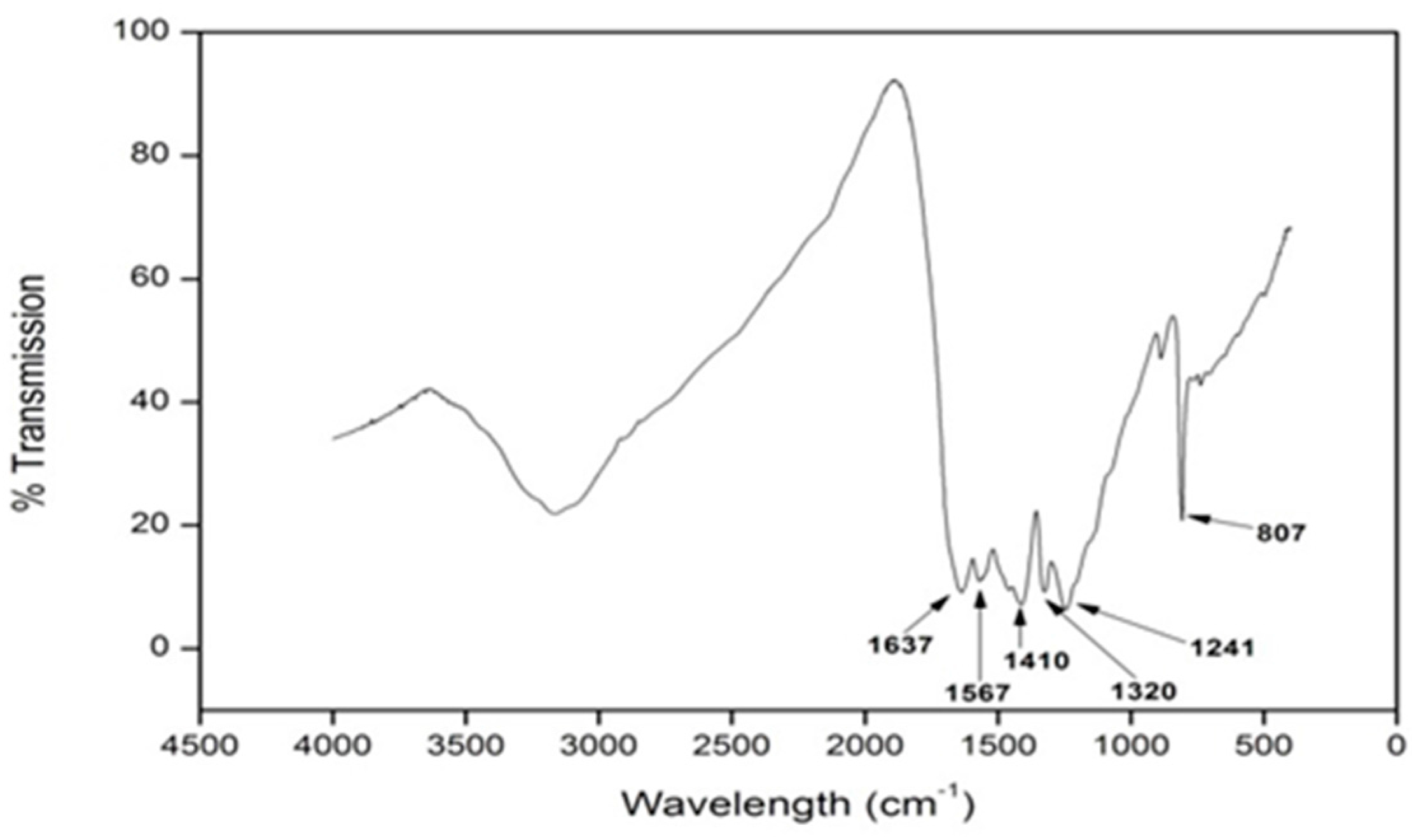

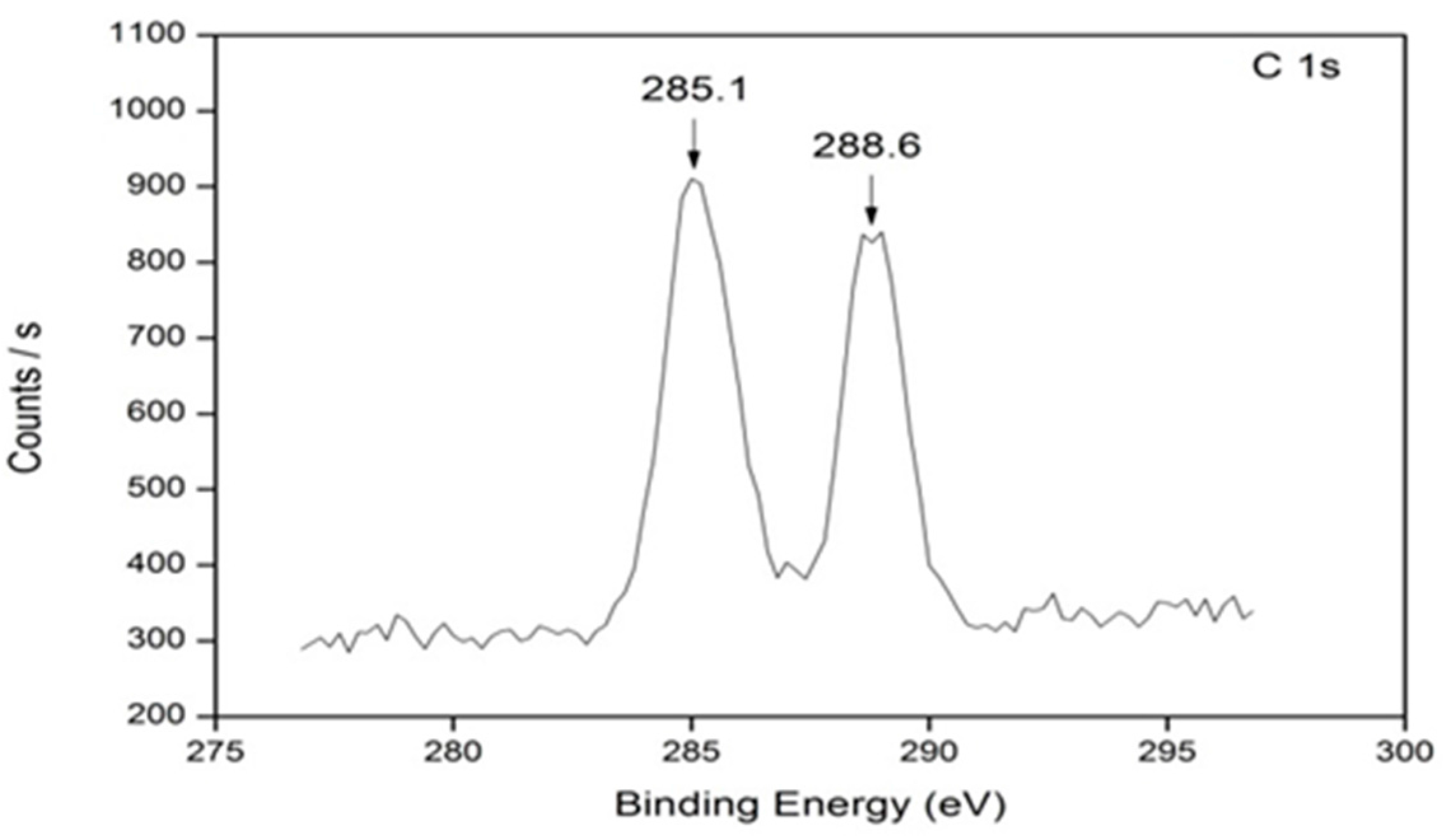

| Sample Name | Particle Size (nm) | |
|---|---|---|
| A | R | |
| Blank, TiO2 | 23.9 | 0 |
| 2% g-C3N4–TiO2 | 21.7 | 28.5 |
| 4% g-C3N4–TiO2 | 22.7 | 46.8 |
| 8% g-C3N4–TiO2 | 22.2 | 36.8 |
| Sample Name | Anatase Percentage (%) | Rutile Percentage (%) |
|---|---|---|
| Blank, TiO2 | 100 | 0 |
| 2% g-C3N4–TiO2 | 93.3 | 6.7 |
| 4% g-C3N4–TiO2 | 89.8 | 10.2 |
| 8% g-C3N4–TiO2 | 92.7 | 7.3 |
| Temperature (°C) | C 1s | C 1s | O 1s | Ti 2p | N 1s | |
|---|---|---|---|---|---|---|
| Binding energy (eV) | ||||||
| TiO2 | 600 | 284.9 | – | 534.0 | 458.5 | 401.8 |
| 2% | 600 | 285.0 | 288.6 | 529.8 | 458.6 | 399.8 |
| 4% | 600 | 285.1 | 288.6 | 529.9 | 458.7 | 399.8 |
| 8% | 600 | 285.1 | 288.6 | 529.9 | 458.7 | 399.4 |
| Atomic % | ||||||
| TiO2 | 600 | 10.6 | 65.2 | 23.9 | 0.2 | |
| 2% | 600 | 11.9 * | 64.9 | 22.9 | 0.3 | |
| 4% | 600 | 11.7 * | 64.8 | 23.4 | 0.2 | |
| 8% | 600 | 11.6* | 65.1 | 23.0 | 0.3 | |
| Sample | Temperature (°C) | Band Gap (eV) (± 0.1 eV) | |
|---|---|---|---|
| Absorption Edge (nm) | Band Gap (eV) | ||
| g-C3N4 | 600 | 439.8 | 2.82 |
| TiO2 | 600 | 393.7 | 3.15 |
| 2% g-C3N4–TiO2 | 600 | 404.8 | 3.06 |
| 4% g-C3N4–TiO2 | 600 | 400.8 | 3.09 |
| 8% g-C3N4–TiO2 | 600 | 398.7 | 3.11 |
| Sample Name | Temperature (°C) | k (min−1) | |
|---|---|---|---|
| UV | Visible | ||
| Undoped TiO2 | 600 | 0.130 | 0.012 |
| g-C3N4 | 600 | 0.034 | 0.005 |
| 2% g-C3N4–TiO2 | 600 | 0.141 | 0.009 |
| 4% g-C3N4–TiO2 | 600 | 0.187 | 0.009 |
| 8% g-C3N4–TiO2 | 600 | 0.103 | 0.011 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagan, R.; McCormack, D.E.; Hinder, S.J.; Pillai, S.C. Photocatalytic Properties of g-C3N4–TiO2 Heterojunctions under UV and Visible Light Conditions. Materials 2016, 9, 286. https://doi.org/10.3390/ma9040286
Fagan R, McCormack DE, Hinder SJ, Pillai SC. Photocatalytic Properties of g-C3N4–TiO2 Heterojunctions under UV and Visible Light Conditions. Materials. 2016; 9(4):286. https://doi.org/10.3390/ma9040286
Chicago/Turabian StyleFagan, Rachel, Declan E. McCormack, Steven J. Hinder, and Suresh C. Pillai. 2016. "Photocatalytic Properties of g-C3N4–TiO2 Heterojunctions under UV and Visible Light Conditions" Materials 9, no. 4: 286. https://doi.org/10.3390/ma9040286






