Forming Spacers in Situ by Photolithography to Mechanically Stabilize Electrofluidic-Based Switchable Optical Elements
Abstract
:1. Introduction
2. Results and Discussion
2.1. Swelling of PEGDA Hydrogels
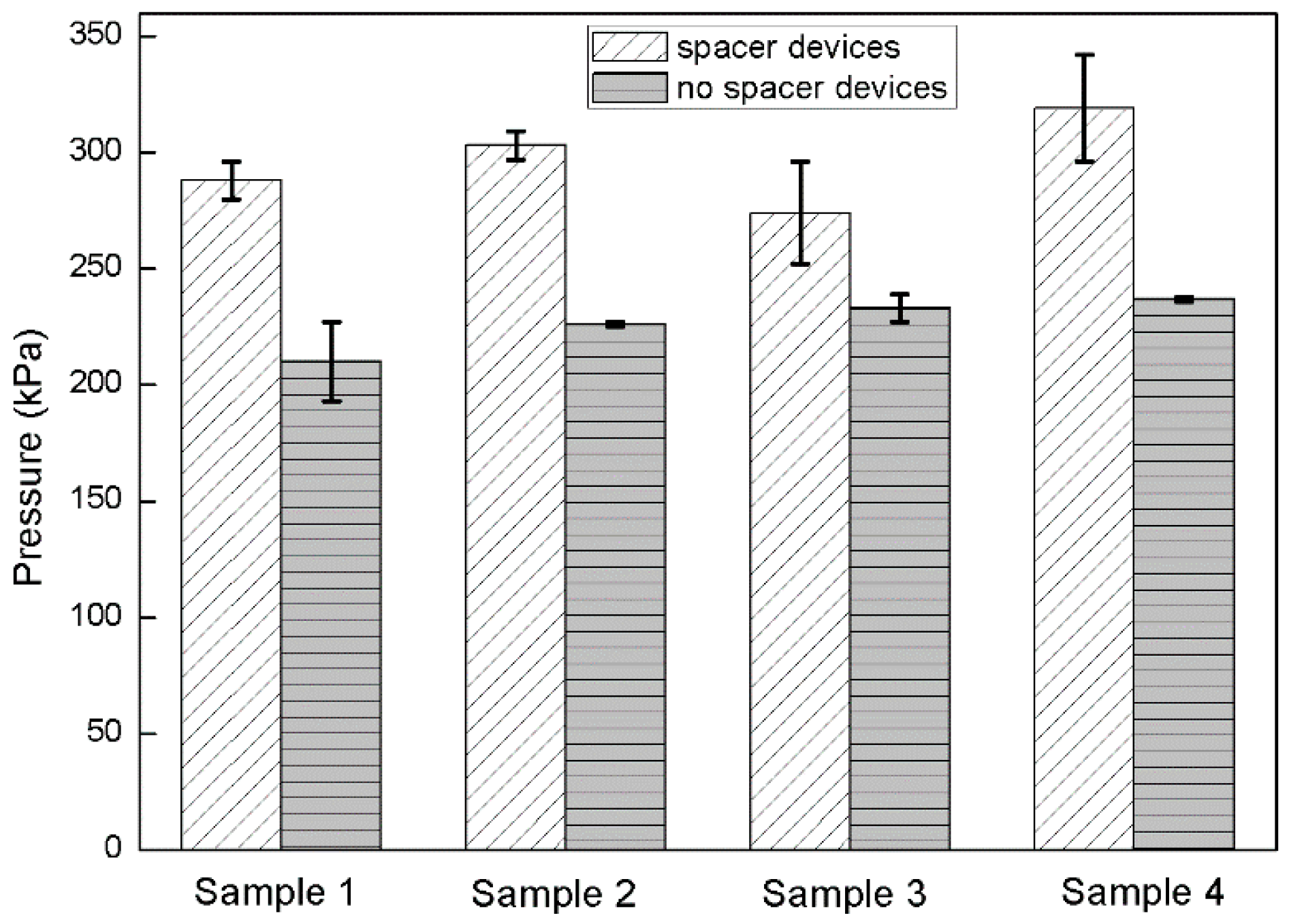
2.2. Mechanical Properties
2.3. Influence of Exposure Time on the Polymerization
2.4. Influence of Aperture Size on the Polymerization
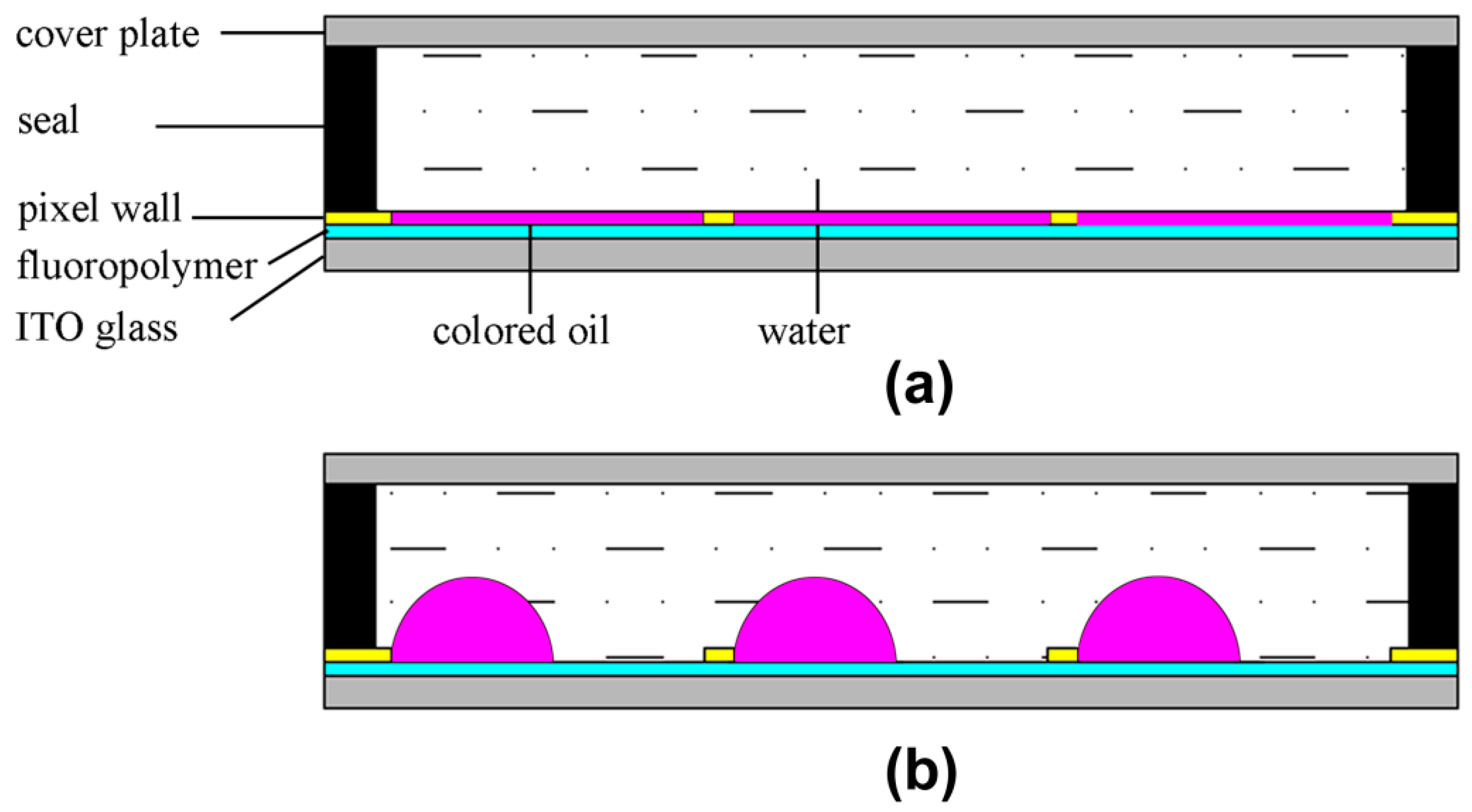
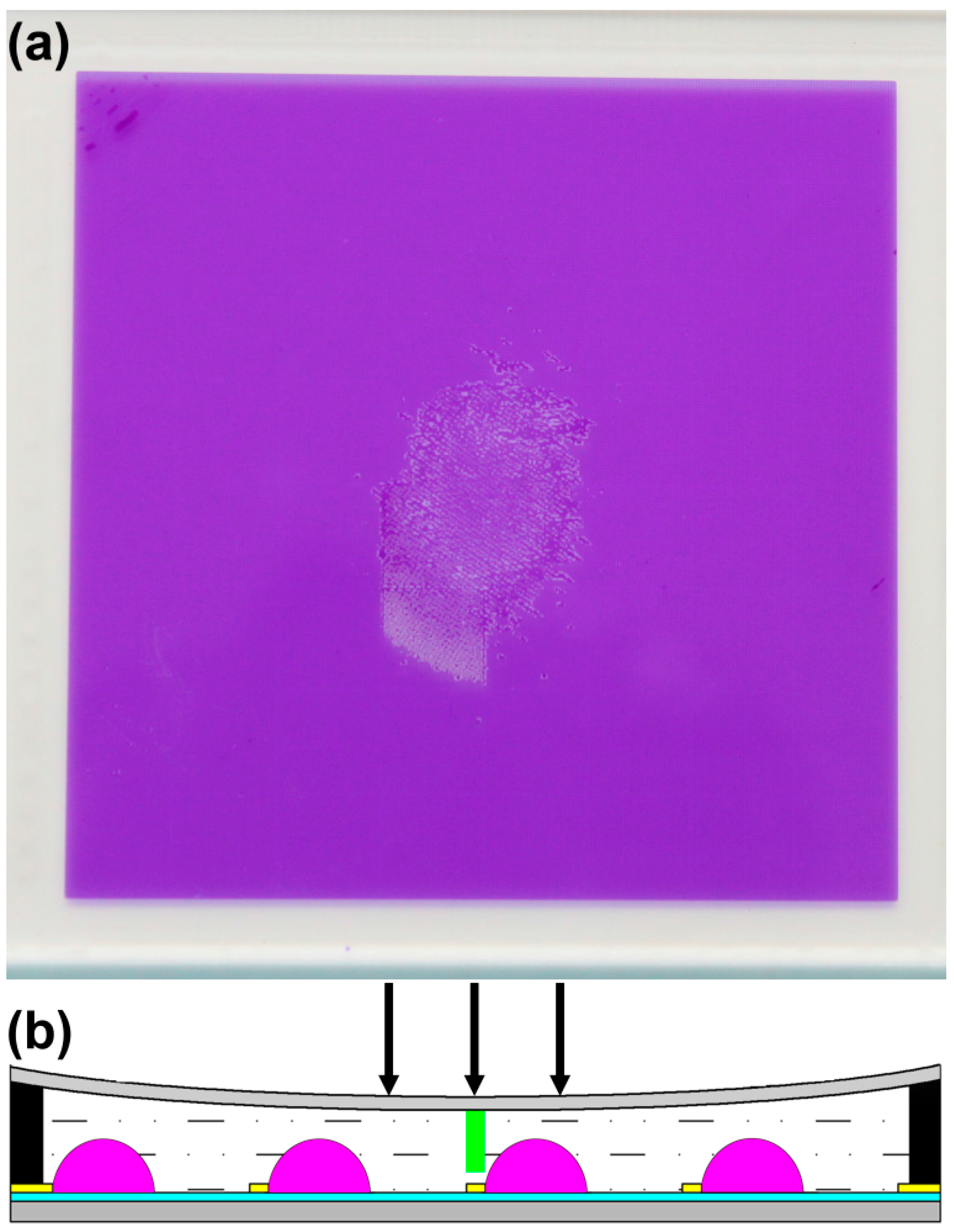
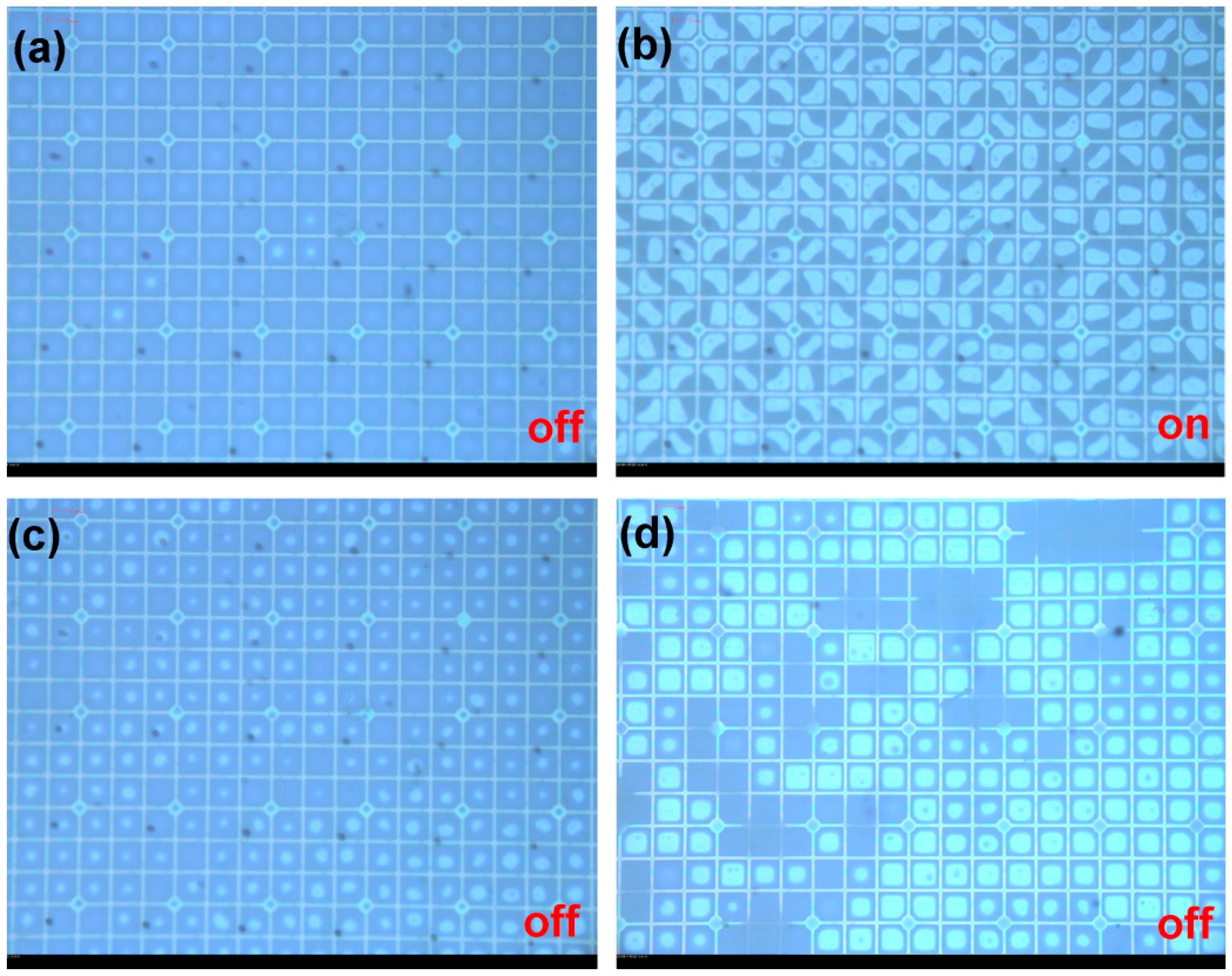
3. Application in EFD Devices
4. Experimental
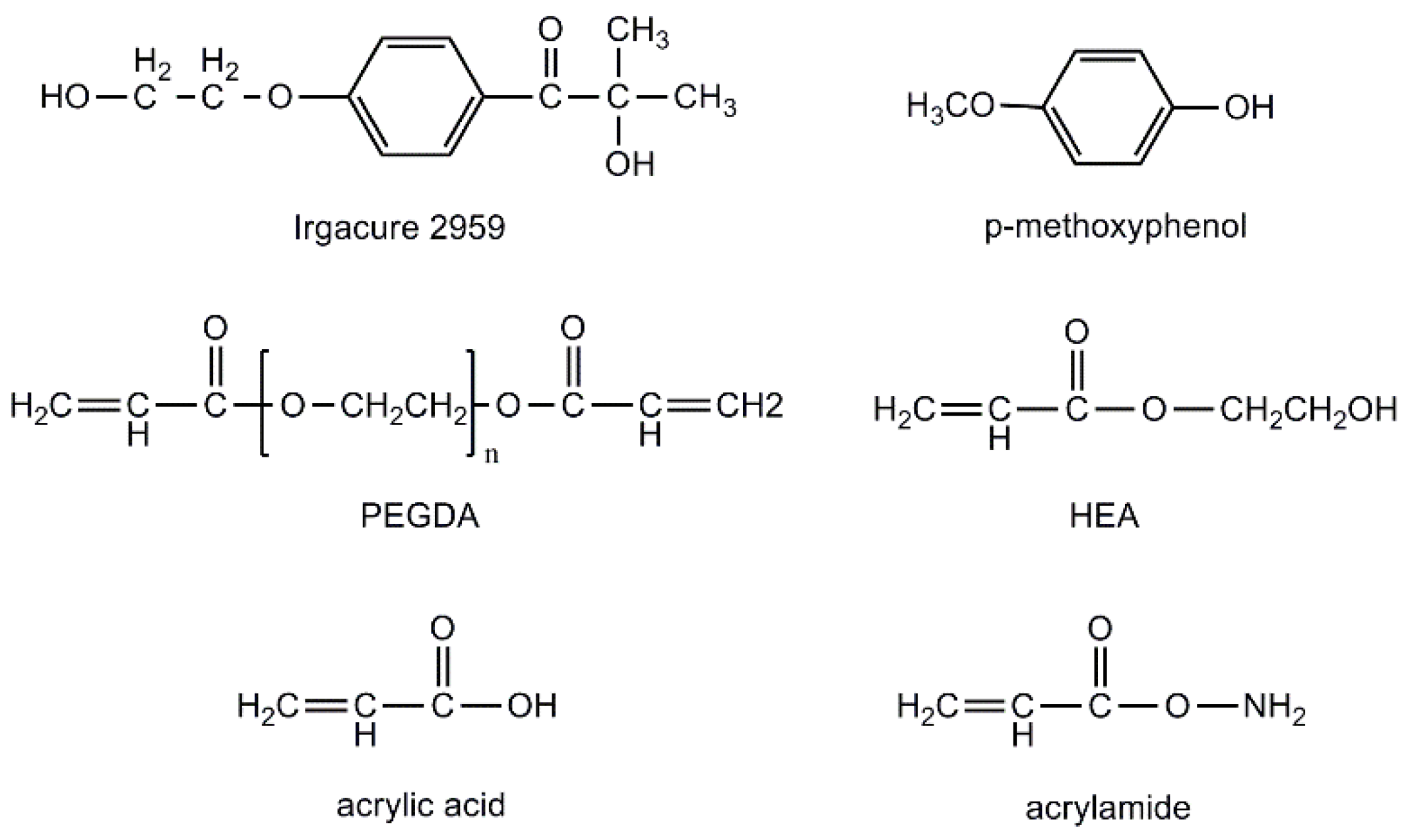
4.1. Materials and Instruments
4.2. Preparation of Samples
4.3. In Situ Fabrication Spacer in EFD Cell
4.4. Equilibrium Swelling Behavior
4.5. Mechanical Properties
4.6. Influence of Exposure Time on the Polymerization
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| EFD | electro-fluidic display |
| LCD | liquid crystal display |
| ITO | indium tin oxide |
| PEGDA | polyethylene glycol diacrylate |
| HEA | 2-hydroxyethyl acrylate |
| EWOD | electrowetting on dielectric |
| MEMS | microelectromechanical systems |
| EWD | electrowetting display |
| PDLC | polymer dispersed liquid crystal |
| SEM | scanning electron microscope |
| SR | swelling ratio |
References
- Lippmann, G. Relation entre les phénomènes électriques et capillaires. Ann. Chim. Phys. 1875, 5, 494–549. [Google Scholar]
- Berge, U. Electrocapillarity and wetting of insulator films by water. C. R. Acad. Sci. Paris Ser. II 1993, 90, 157–163. [Google Scholar]
- Shamai, R.; Andelman, D.; Berge, B.; Hayes, R. Water, electricity, and between ... On electrowetting and its applications. Soft Matter 2008, 4, 38–45. [Google Scholar] [CrossRef]
- Mugele, F.; Baret, J.C. Electrowetting: From basics to applications. J. Phys. Condens. Matter. 2005, 17, R705–R774. [Google Scholar] [CrossRef]
- Wang, J.; Chatrathi, M.P.; Tian, B. Micromachined separation chips with a precolumn reactor and end-column electrochemical detector. Anal. Chem. 2000, 72, 5774–5778. [Google Scholar] [CrossRef] [PubMed]
- Ro, K.W.; Lim, K.; Kim, H.; Hahn, J.H. Poly(dimethylsiloxane) microchip for precolumn reaction and micellar electrokinetic chromatography of biogenic amines. Electrophoresis 2002, 23, 1129–1137. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, H.H.; Bachman, M.; Sims, C.E.; Li, G.P.; Allbritton, N.L. Covalent micropatterning of poly(dimethylsiloxane) by photografting through a mask. Anal. Chem. 2005, 77, 7539–7546. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Le, T.V.; Wirth, M.J. Surface modification of the channels of poly(dimethylsiloxane) microfluidic chips with polyacrylamide for fast electrophoretic separations of proteins. Anal. Chem. 2004, 76, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, K.; Jinno, N.; Nakajima, R. Development of a micro total analysis system incorporating chemiluminescence detection and application to detection of cancer markers. Anal. Chem. 2005, 77, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Luo, G.; Wang, L.; Gao, Y.; Lei, G.; Ren, K.; Chen, L.; Wang, Y.; Hu, Y.; Qiu, Y. A microfluidic device using a green organic light emitting diode as an integrated excitation source. Lab Chip 2005, 5, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.S.; Cho, I.J.; Bu, J.U.; Kim, C.J.; Yoon, E. A surface-tension driven micropump for low-voltage and low-power operations. J. Microelectromech. S. 2002, 11, 454–461. [Google Scholar]
- Junghoon, L.; Chang-Jin, K. Surface-tension-driven microactuation based on continuous electrowetting. J. Microelectromech. S. 2000, 9, 171–180. [Google Scholar] [CrossRef]
- Berge, B.; Peseux, J. Variable focal lens controlled by an external voltage: An application of electrowetting. Eur. Phys. J. E 2000, 3, 159–163. [Google Scholar] [CrossRef]
- Beni, G.; Hackwood, S.; Jackel, J.L. Continuous electrowetting effect. Appl. Phys. Lett. 1982, 40, 912–914. [Google Scholar] [CrossRef]
- Jackel, J.L.; Hackwood, S.; Veselka, J.J.; Beni, G. Electrowetting switch for multimode optical fibers. Appl. Opt. 1983, 22, 1765–1775. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.A.; Feenstra, B.J. Video-speed electronic paper based on electrowetting. Nature 2003, 425, 383–385. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Steckl, A.J. Three-color electrowetting display device for electronic paper. Appl. Phys. Lett. 2010, 97, 023514:1–023514:3. [Google Scholar] [CrossRef]
- Penterman, R.; Klink, S.I.; de Koning, H.; Nisato, G.; Broer, D.J. Single-substrate liquid-crystal displays by photo-enforced stratification. Nature 2002, 417, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Büyüktanir, E.A.; Gheorghiu, N.; West, J.L.; Mitrokhin, M.; Holter, B.; Glushchenko, A. Field-induced polymer wall formation in a bistable smectic A liquid crystal display. Appl. Phys. Lett. 2006, 89, 031101:1–031101:3. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Jang, S.-J.; Jung, J.-W.; Kim, H.-R.; Jin, M.Y.; Choi, Y.; Kim, J.-H. Mechanical stability of pixel-isolated liquid crystal mode for flexible display application. Mol. Cryst. Liq. Cryst. 2006, 458, 81–87. [Google Scholar] [CrossRef]
- Gheorghiu, N.; West, J.L.; Glushchenko, A.V.; Mitrokhin, M. Patterned field induced polymer walls for smectic A bistable flexible displays. Appl. Phys. Lett. 2006, 88, 263511:1–263511:3. [Google Scholar] [CrossRef]
- Fujikake, H.; Sato, H.; Murashige, T. Polymer-stabilized ferroelectric liquid crystal for flexible displays. Displays 2004, 25, 3–8. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yamamoto, H.; Sato, H.; Kawakita, M.; Takizawa, K.; Fujikake, H. Formation of polymer-wall-stabilized bend-mode liquid crystal cells. J. Photopolym. Sci. Technol. 2003, 16, 181–186. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.K.; Hong, J.W. Investigation of the photopolymerization-induced phase separation process in polymer dispersed liquid crystal. Polym. Test. 2010, 29, 886–893. [Google Scholar] [CrossRef]
- Vaz, N.A.; Smith, G.W.; Montgomery, G.P. A light control film composed of liquid crystal droplets dispersed in a UV-curable polymer. Mol. Cryst. Liq. Cryst. 1987, 146, 1–15. [Google Scholar] [CrossRef]
- West, J.L. Phase separation of liquid crystals in polymers. Mol. Cryst. Liq. Cryst. 1988, 157, 427–441. [Google Scholar] [CrossRef]
- Duran, H.; Meng, S.; Kim, N.; Hu, J.; Kyu, T.; Natarajan, L.V.; Tondiglia, V.P.; Bunning, T.J. Kinetics of photopolymerization-induced phase separation and morphology development in mixtures of a nematic liquid crystal and multifunctional acrylate. Polymer 2008, 49, 534–545. [Google Scholar] [CrossRef]
- Qian, T.; Kim, J.H.; Kumar, S.; Taylor, P.L. Phase-separated composite films: Experiment and theory. Phys. Rev. E 2000, 61, 4007–4010. [Google Scholar] [CrossRef]
- Cho, J.D.; Lee, S.S.; Kim, Y.B.; Hong, J.W. Photocure kinetics and control of lc microdroplets for acrylate-based polymer dispersed liquid crystal with different methacrylate contents. Macromol. Res. 2013, 21, 1297–1304. [Google Scholar] [CrossRef]
- Baek, J.I.; Shin, J.H.; Oh, M.C.; Kim, J.C.; Yoon, T.H. Pixel-isolation walls of liquid crystal display formed by fluorinated uv-curable polymers. Appl. Phys. Lett. 2006, 88, 161104:1–161104:3. [Google Scholar] [CrossRef]
- Sung, S.J.; Jung, E.A.; Sim, K.; Kim, D.H.; Cho, K.Y. Structure control of lattice-patterned liquid crystals-polymer composites prepared by polarization-selective uv-curing through the addition of a fluorinated acrylate monomer. Microelectron. Eng. 2013, 103, 42–48. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, D.; Alexander, P.G.; Yang, G.; Tan, J.; Cheng, A.W.; Tuan, R.S. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 2013, 34, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Sokic, S.; Papavasiliou, G. Controlled proteolytic cleavage site presentation in biomimetic pegda hydrogels enhances neovascularization in vitro. Tissue Eng. Part A 2012, 18, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Haryanto; Kim, S.; Kim, J.H.; Kim, J.O.; Ku, S.; Cho, H.; Han, D.H.; Huh, P. Fabrication of poly(ethylene oxide) hydrogels for wound dressing application using e-beam. Macromol. Res. 2014, 22, 131–138. [Google Scholar] [CrossRef]
- Shu, X.Z.; Liu, Y.C.; Palumbo, F.S.; Lu, Y.; Prestwich, G.D. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials 2004, 25, 1339–1348. [Google Scholar]
- Cruise, G.M.; Scharp, D.S.; Hubbell, J.A. Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials 1998, 19, 1287–1294. [Google Scholar] [CrossRef]
- Nemir, S.; Hayenga, H.N.; West, J.L. Pegda hydrogels with patterned elasticity: Novel tools for the study of cell response to substrate rigidity. Biotechnol. Bioeng. 2010, 105, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Patel, A.; Gaharwar, A.K.; Mihaila, S.M.; Iviglia, G.; Mukundan, S.; Bae, H.; Yang, H.; Khademhosseini, A. Hyperbranched polyester hydrogels with controlled drug release and cell adhesion properties. Biomacromolecules 2013, 14, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Perelaer, J.; Hermans, K.; Bastiaansen, C.W.M.; Broer, D.J.; Schubert, U.S. Photo-embossed surface relief structures with an increased aspect ratio by addition of kinetic interfering compounds. J. Photopolym. Sci. Technol. 2009, 22, 667–670. [Google Scholar] [CrossRef]
- Hermans, K.; Wolf, F.K.; Perelaer, J.; Janssen, R.A.J.; Schubert, U.S.; Bastiaansen, C.W.M.; Broer, D.J. High aspect ratio surface relief structures by photoembossing. Appl. Phys. Lett. 2007, 91, 174103:1–174103:3. [Google Scholar] [CrossRef]
- Buxton, A.N.; Zhu, J.; Marchant, R.; West, J.L.; Yoo, J.U.; Johnstone, B. Design and characterization of poly(ethylene glycol) photopolymerizable semi-interpenetrating networks for chondrogenesis of human mesenchymal stem cells. Tissue Eng. 2007, 13, 2549–2560. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Liu, F. Uv-radiation curing of simultaneous interpenetrating polymer network hydrogels for enhanced heavy metal ion removal. Mater. Sci. Eng. B-Adv. 2012, 177, 1633–1640. [Google Scholar] [CrossRef]
- Guo, B.L.; Finne-Wistrand, A.; Albertsson, A.C. Versatile functionalization of polyester hydrogels with electroactive aniline oligomers. J. Polym. Sci. Pol. Chem. 2011, 49, 2097–2105. [Google Scholar] [CrossRef]







© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Guo, Y.; Hayes, R.A.; Liu, D.; Broer, D.J.; Zhou, G. Forming Spacers in Situ by Photolithography to Mechanically Stabilize Electrofluidic-Based Switchable Optical Elements. Materials 2016, 9, 250. https://doi.org/10.3390/ma9040250
Wang M, Guo Y, Hayes RA, Liu D, Broer DJ, Zhou G. Forming Spacers in Situ by Photolithography to Mechanically Stabilize Electrofluidic-Based Switchable Optical Elements. Materials. 2016; 9(4):250. https://doi.org/10.3390/ma9040250
Chicago/Turabian StyleWang, Meihong, Yuanyuan Guo, Robert A. Hayes, Danqing Liu, Dirk J. Broer, and Guofu Zhou. 2016. "Forming Spacers in Situ by Photolithography to Mechanically Stabilize Electrofluidic-Based Switchable Optical Elements" Materials 9, no. 4: 250. https://doi.org/10.3390/ma9040250





