Abstract
Synthetic nerve conduits have emerged as an alternative to guide axonal regeneration in peripheral nerve gap injuries. Migration of Schwann cells (SC) from nerve stumps has been demonstrated as one essential factor for nerve regeneration in nerve defects. In this experiment, SC viability and migration were investigated for various materials to determine the optimal conditions for nerve regeneration. Cell viability and SC migration assays were conducted for collagen I, laminin, fibronectin, lysine and ornithine. The highest values for cell viability were detected for collagen I, whereas fibronectin was most stimulatory for SC migration. At this time, clinically approved conduits are based on single-material structures. In contrast, the results of this experiment suggest that material compounds such as collagen I in conjunction with fibronectin should be considered for optimal nerve healing.
1. Introduction
Transsection of peripheral nerves is a frequent injury that causes severe impairment of activities of daily living in affected patients [1,2]. Tensionless coaptation of nerve stumps is known to be inevitable for successful nerve healing [3,4]. However, the recoil of nerve stumps due to debridement or delayed surgical care causes nerve gaps which frequently impede this tensionless nerve repair and remain a great challenge in clinical practice [5]. The gold standard for the treatment of these nerve gaps is the interposition of an autologous nerve segment to allow axonal outgrowth towards the distal nerve stump [6]. The limited availability of donor nerves of adequate diameter and quality has led to nerve conduits as an alternative to guide axonal regeneration. Nerve conduits are cylindrically shaped grafts made of synthetic or autologous materials that are sutured microscopically in between the proximal and distal nerve end to allow axonal regrowth towards the distal nerve stump [7,8,9]. Various materials have been investigated for their suitability as nerve guidance conduits, and there is an ongoing debate on which material is most optimal for allowing axonal regrowth [10].
Schwann cells (SC) are known to dedifferentiate into an activated SC phenotype during the course of nerve injury and, hence, fundamentally contribute by secretion of growth factors in the complex process of nerve regeneration [11]. Further, SCs have been shown to migrate from the proximal as well as from the distal nerve stump to form Bands of Büngner to direct axonal regrowth [12]. Thus, the lack of SCs in the scenario of long distance nerve gaps might be one key factor for the poor regeneration of these injuries.
Despite this crucial role of SC in peripheral nerve regeneration, optimal materials for the migration of SCs have found little attention in the field of nerve conduit engineering. Various extracellular matrix (ECM) materials have been suggested to be essential for nerve recovery [10,13,14,15]. To elucidate what specific ECM might provide the most optimal conditions for the induction of nerve recovery, different biocompatible compounds were investigated for their quality in inducing SC migration and viability.
2. Results
2.1. Viability Assay
SCs cultivated on poly-l-lysine or poly-l-ornithine showed no difference in cell viability when compared with the cells cultured on uncoated polystyrene. Whereas collagen I and fibronectin had a positive effect on cell viability, cells cultured on laminin showed a decreased viability after two days. However, five days after the cells were seeded onto the differently coated surfaces, no statistically significant difference in cell vitality could be observed for collagen I, poly-l-lysine, or poly-l-ornithine. Moreover, the viability-stimulating effect of fibronectin that was observed after two days changed to a viability-decreasing effect after five days (Figure 1).
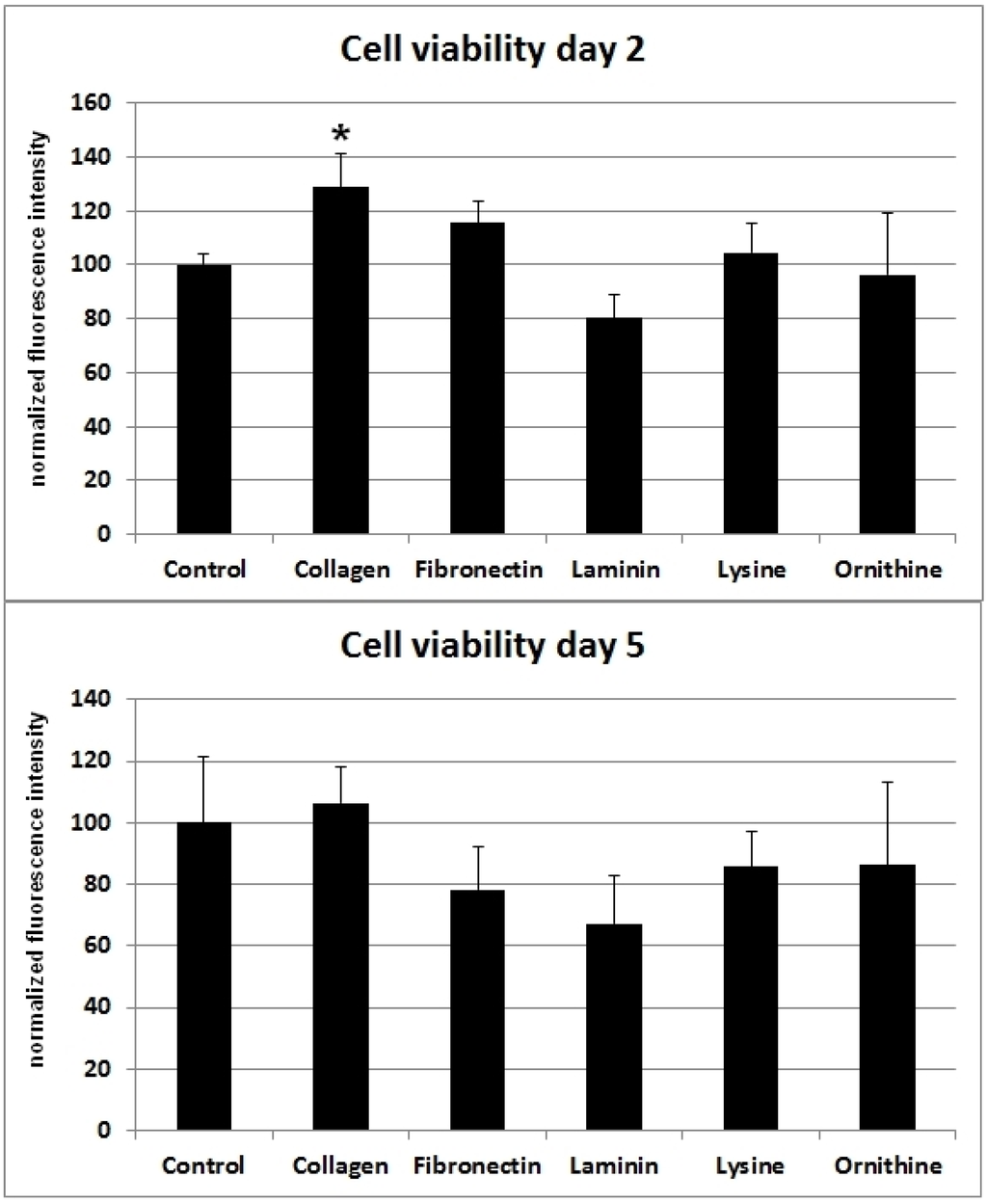
Figure 1.
Different ECMs and corresponding cell viability of SCs after two and five days. Ordinate indicates viability as percentage values of fluorescence intensity in relation to day of seeding. Viability was assessed using a resazurin assay. Significant (*: p < 0.05) results are in comparison to the control.
2.2. Migration Assay
Collagen I and fibronectin showed a migration-stimulating effect on SCs. On the uncoated polystyrene surface, the gap between the cell edges was 75.2% of the original area (t0) after 24 h and 54.4% after 48 h. On the collagen I–coated surface, the remaining cell-free area was 61.3% (t24) and 47.5% (t48), respectively. After 24 h, only 34.9% of the original scratch area remained on fibronectin-coated surfaces. Moreover, 48 h after the scratch was induced, no gap was detectable in any fibronectin-coated well. Laminin, poly-l-lysine, and poly-l-ornithine had no migration-stimulating effect compared to the polystyrene control. After 24 h, 70.7%, 79.3%, and 88.9%, respectively, of the original gap size were still without detectable cell migration. Remaining cell-free areas after 48 h were 57.5%, 67.5%, and 52.4%, respectively (Figure 2). Microscopic images of typical wells are shown in Figure 3.
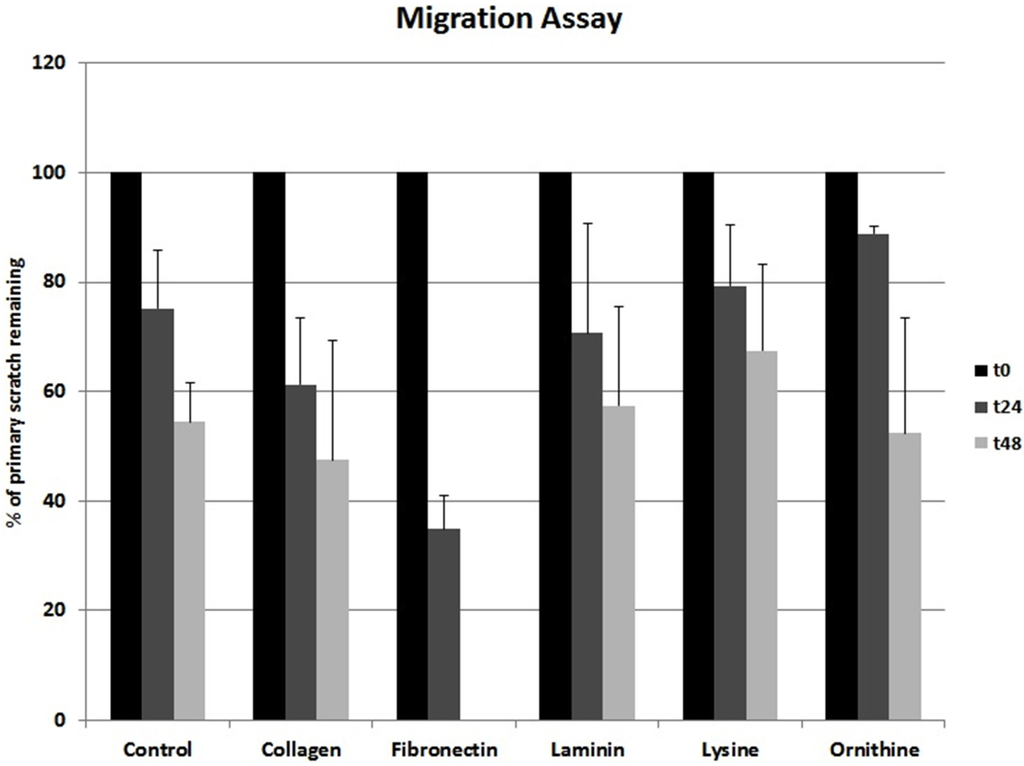
Figure 2.
Different ECMs and corresponding SC migration. Ordinate indicates remaining cell-free area as percentage values upon the day of creating the scratch.
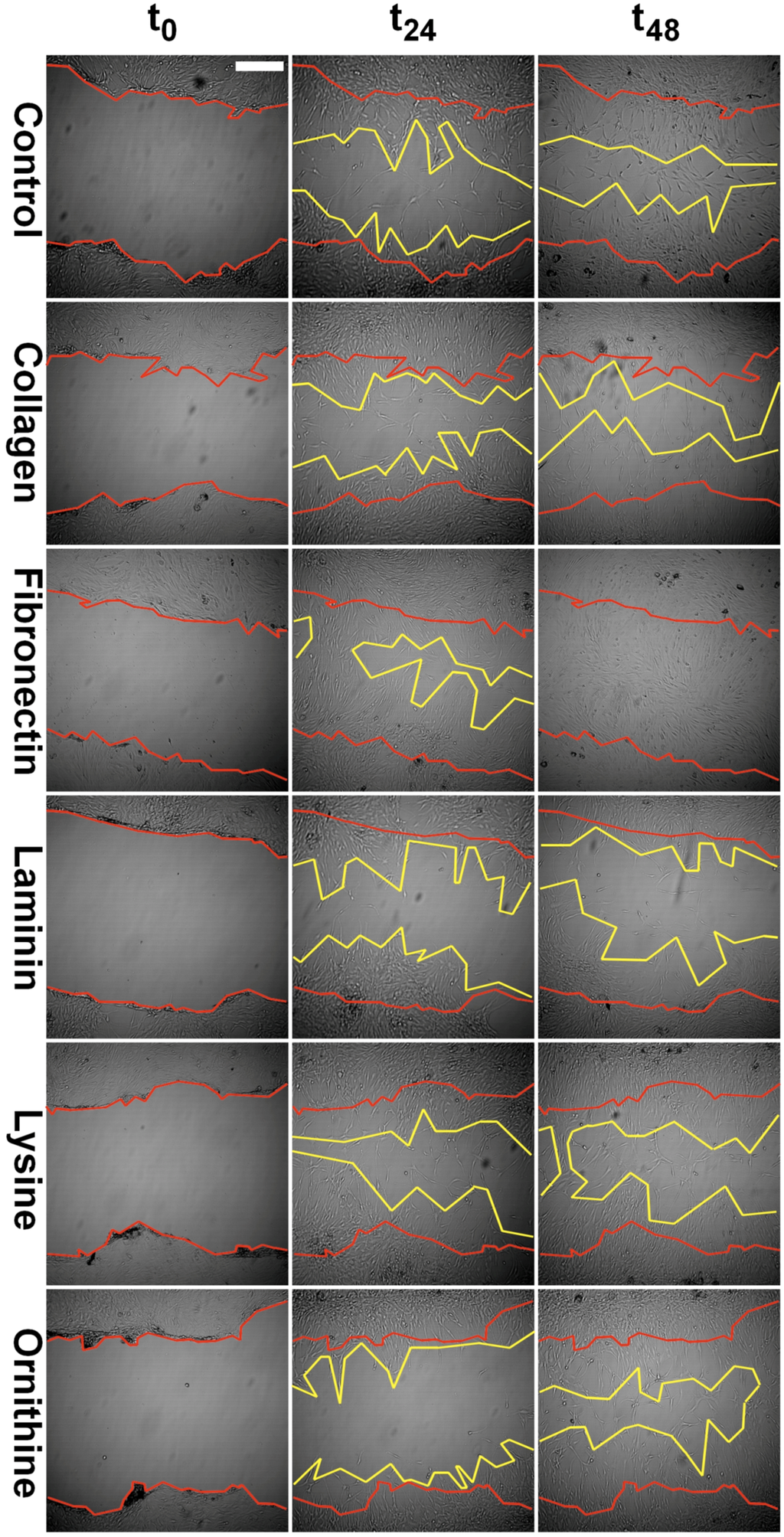
Figure 3.
Representative images of scratches in the confluent SC monolayer at day zero (t0), as well as at 24 h (t24) and 48 h (t48) intervals. Scratch margins are marked in red and yellow color for t0 and t48, respectively. Scale bar indicates 100 µm.
3. Discussion
As opposed to the central nervous system, peripheral nerves have a high potential to regenerate and thereby allow functional recovery [16]. Research on peripheral nerve regeneration revealed SCs as the regenerative source of recovery in the PNS [17]. In response to injury, SCs dedifferentiate into a state which has been termed the activated SC phenotype [11]. This activation includes a loss of myelinating proteins and, at the same time, allows secretion of neurotrophic and neurotropic factors that have been proven to be essential for regenerative processes in the PNS [18].
The most severe injuries in peripheral nerves are complete nerve disruptions with remaining nerve gaps. In the past two decades, synthetic nerve guidance conduits became an alternative for bridging nerve discontinuities to circumvent the disadvantages of autologous nerve grafts [19,20,21]. Conduits ideally not only guide the axonal outgrowth, but allow optimal conditions for SC-induced regenerative processes. Basement proteins such as collagen I, fibronectin and laminin are known to influence cell viability and migration [22,23,24]. Indeed, there is increasing evidence that collagen type I, fibronectin, laminin, lysine and ornithine are among the ECMs that contribute to the microenvironment of SC-induced nerve recovery during Wallerian degeneration [10,13,14,15,25]. When SC viability was assessed for the different surface materials in this in vitro experiment, the highest cell viability was detected for collagen I (Figure 1). At this time, commercially available biodegradable nerve conduits are mostly composed of collagen type I [10,15]. Therefore, collagen I might not only be favorable due to its biocompatibility [10], but also for the viability of migrating SC in the context of regenerating nerves. Interestingly, autologous muscle conduits have been investigated for their ability to regenerate peripheral nerve injuries and showed promising results [10,26]. The high amount of collagen I in musculoskeletal tissues together with the SC-proliferating property might be one explanation for this in vivo observation.
Interestingly, investigations in human SCs in a comparable study showed results that vary from our findings [27]. Vleggeert-Lankamp et al. observed the highest proliferative effect for fibronectin and laminin within the first three days after seeding [27]. In contrast, collagen I did not show a comparable stimulatory effect in their study. Possibly, these differences can be attributed to variations in the experimental setup. The study of Vleggeert-Lankamp et al. was conducted with human SCs, instead of rat SCs, and coated surfaces were glass cover slips instead of polystyrene. However, four to six days after seeding, the proliferation rates of human SCs on coated glass were in closer accordance with the results from our viability assay. At this particular time point, the highest viability was detected for collagen I and fibronectin, whereas viability on laminin was inferior in comparison to uncoated glass controls. Perhaps these differences in human and rat SCs’ behavior are one explanation for the higher regenerative potential observed in rodents.
Migration of SCs has been demonstrated as one critical factor not only in peripheral nerve development but also in nerve regeneration [12]. Further, axonal elongation is known to depend on surrounding ECM [13,28]. Based on these findings, we investigated the influence of the ECM proteins collagen I, fibronectin, and laminin on SC migration. Amino acids such as poly-l-lysine and poly-l-ornithine are known to be relevant for in vitro SC attachment as well as SC proliferation during the early phase of Wallerian degeneration [14]. Accordingly, the potential of these polymers to facilitate SC migration and viability was as well evaluated [14,25]. Whereas collagen I showed a slightly stimulating effect on SC migration, fibronectin strongly supported migration. After 48 h, the gaps in all fibronectin-coated wells were completely closed (Figure 2 and Figure 3). At this time point, on all other surfaces evaluated, the remaining cell-free area was around 50%, with only collagen I being below 50% (Figure 2 and Figure 3). This data strongly suggests that a fibronectin-coated nerve guide conduit might have a stimulating effect on SC migration and possibly on consecutive axonal regeneration [12]. Interestingly, Widhe et al. reported that changing the widely used cell attachment RGD (i.e., arginine-glycine-asparagine) motive using genetic engineering to a constellation more similar to that of fibronectin improves attachment, spreading, stress fiber formation and focal adhesions [29], which are crucial factors for cell migration. Scott et al. demonstrated that inhibition of fibronectin assembly strongly reduces the contractile force generation of human embryonic fibroblasts [30]. Further, fibronectin stimulated migration in murine embryonic stem cells [31]. These observations support the stimulatory effect of fibronectin not only in SCs.
In our experiment, collagen I showed a minor migration-promoting effect and laminin, poly-l-lysine, and poly-l-ornithine did not stimulate migration compared to the uncoated control (Figure 2 and Figure 3). Notably, a study using a prostate adenocarcinoma cell line together with collagen I, fibronectin, laminin, poly-l-lysine, and poly-l-ornithine coating showed conflicting results [32]. In that experiment, a migration-stimulatory effect was only demonstrated for laminin whereas all other coatings were inferior to the polystyrene controls. Remarkably, the prostate adenocarcinoma cell line used in that study is known for its very weak cell-surface adhesion. Consequently, cell-specific factors influence the migration potential in correspondence to surface materials.
According to the results of this experiment, collagen I and fibronectin presented to be the most optimal surface materials for nerve guidance conduits. The composition of these two ECM proteins promotes SC survival and sufficient cell migration at the same time, and hence provides favorable conditions for peripheral nerve regeneration.
4. Materials and Methods
4.1. Cell Culture
Rat Schwann cells and Schwann cell medium were purchased from Sciencell Research Laboratories (Carlsbad, CA, USA). Cells were isolated from postnatal day 8 rat sciatic nerve and used in passage 5 for all experiments. Cells were incubated at 37 °C and 5% CO2.
4.2. Surface Coating
Well plates were coated prior to the experiments with ECM proteins or poly-amino acids, respectively. We used collagen I solution from bovine skin, fibronectin from bovine plasma, laminin from murine sarcoma basement membrane, poly-l-lysine solution, and poly-l-ornithine solution (all Sigma Aldrich, St. Louis, MO, USA) according to the manufacturers’ instructions. Briefly, coating molecules were prepared with PBS (Sigma Aldrich, Schnelldorf, Germany) in the desired concentration (see below) and 150 µL/cm2 was given to the wells. Wells coated with poly-l-lysine and poly-l-ornithine solution were incubated for five minutes, whereas wells coated with collagen I, fibronectin, and laminin were incubated for several h. After incubation, the coating solution was discarded, and the wells were washed with ddH2O, and air-dried. Table 1 shows the coating concentration. Uncoated wells were used as control.

Table 1.
Molecules and corresponding coating conditions.
4.3. Viability Assay
Cell viability was evaluated using resazurin sodium salt (Sigma Aldrich, Schnelldorf, Germany) solution. Metabolically active cells reduce the oxidized form of this blue dye to the highly fluorescent resorufin (red). To assess cell vitality on the differently coated surfaces, cells were seeded into pre-coated well plates at a density of 2500 cells/cm2 and allowed to proliferate for two or five days, respectively. Prior to measurement, cells were incubated with a 10% resazurin in medium solution (final concentration of 70 µM) for 2 h. Subsequently, fluorescence intensity at 600 nm wavelength was measured using the VarioskanFlash and the SkanIt Software 2.4.5 RE (Scanlab AG, Puchheim, Germany). Measurement was done with five replicates for each coating. After subtraction of blanks the values were normalized to the control. A Levene’s test was conducted to assess the equality of variances (d2: p = 0.079; d5: p = 0.095) between the groups. One-way ANOVA analysis revealed significant differences between the groups (d2: F(5,24) = 8.693, p = 0.000082; d5: F(5,24) = 3.166, p = 0.025). A Bonferroni post-hoc analysis was used to identify the specific differences between the individual groups. Statistical significance was considered as p-values < 0.05 (Figure 1).
4.4. Migration Assay
A scratch assay was used to evaluate SC migration on the differently coated surfaces. Cells were seeded into pre-coated well plates at a density of 5000 cells/cm2 and allowed to proliferate until confluency. Subsequently, a scratch was inserted by scraping the cell monolayer in a straight line with a pipet tip. The resulting cell debris was removed by washing the cells. Pictures were taken directly after the monolayer was scraped and after 24 and 48 h, respectively. Pictures were taken with the Wilovert S microscope (Helmut Hund GmbH, Wetzlar, Germany) and the ScopeTec DCM 800 camera, using the Scope Photo 3.0 software (Figure 3). SC migration was assessed by measuring the cell-free area after 24 and 48 h compared to the cell-free area directly after the scratch was inflicted (Figure 2).
5. Conclusions
SCs are known to be the indispensable source of functional recovery in the peripheral nervous system. Insufficient SC dispersal within nerve guidance conduits has been suggested to cause a local SC deficit in nerve defect injuries with corresponding inadequate axonal regeneration.
Various ECMs have been suggested to regulate SC behavior. Among the ECMs investigated in this experiment collagen type I demonstrated favorable conditions for SC viability, whereas fibronectin predominantly induced SC migration. These stimulatory effects of ECMs might improve the microenvironment of nerve defects and thus should be considered as one potential target to eliminate the local SC deficit associated with nerve gap injuries.
Author Contributions
Silvan Klein contributed to the conception of the experiment, interpreted data and wrote the manuscript together with Oliver Felthaus. Lukas Prantl provided field expertise and feedback on the study conception. Jody Vykoukal interpreted data and contributed important intellectual content. Markus Loibl contributed with help to conceive the study design and interpreted data. Oliver Felthaus contributed with data acquisition, analysis, interpretation and wrote the manuscript together with Silvan Klein.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Novak, C.B.; Anastakis, D.J.; Beaton, D.E.; Katz, J. Patient-reported outcome after peripheral nerve injury. J. Hand Surg. 2009, 34, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Boyd, J.B.; Olsavsky, A.; Olsavski, A.; Gelfand, M.; Putnam, B. Outcomes of complex gunshot wounds to the hand and wrist: A 10-year level I urban trauma center experience. Ann. Plast. Surg. 2012, 68, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Hori, S.; Sasaki, S.; Maruo, S. Effects of tension at the site of coaptation on recovery of sciatic nerve function after neurorrhaphy: Evaluation by walking-track measurement, electrophysiology, histomorphometry, and electron probe X-ray microanalysis. Microsurgery 1999, 19, 200–207. [Google Scholar] [CrossRef]
- Hentz, V.R.; Rosen, J.M.; Xiao, S.J.; McGill, K.C.; Abraham, G. The nerve gap dilemma: A comparison of nerves repaired end to end under tension with nerve grafts in a primate model. J. Hand Surg. 1993, 18, 417–425. [Google Scholar] [CrossRef]
- Dienstknecht, T.; Klein, S.; Vykoukal, J.; Gehmert, S.; Koller, M.; Gosau, M.; Prantl, L. Type I collagen nerve conduits for median nerve repairs in the forearm. J. Hand Surg. 2013, 38, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Taras, J.S.; Jacoby, S.M.; Lincoski, C.J. Reconstruction of digital nerves with collagen conduits. J. Hand Surg. 2011, 36, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.M.; Pham, H.N.; Abraham, G.; Harold, L.; Hentz, V.R. Artificial nerve graft compared to autograft in a rat model. J. Rehabil. Res. Dev. 1989, 26, 1–14. [Google Scholar] [PubMed]
- Lundborg, G.; Rosén, B.; Dahlin, L.; Danielsen, N.; Holmberg, J. Tubular versus conventional repair of median and ulnar nerves in the human forearm: Early results from a prospective, randomized, clinical study. J. Hand Surg. 1997, 22, 99–106. [Google Scholar] [CrossRef]
- Keeley, R.; Atagi, T.; Sabelman, E.; Padilla, J.; Kadlcik, S.; Keeley, A.; Nguyen, K.; Rosen, J. Peripheral nerve regeneration across 14-mm gaps: A comparison of autograft and entubulation repair methods in the rat. J. Reconstr. Microsurg. 1993, 9, 349–358, discussion 359–360. [Google Scholar] [CrossRef] [PubMed]
- Stang, F.; Keilhoff, G.; Fansa, H. Biocompatibility of different nerve tubes. Materials 2009, 2, 1480–1507. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. Negative regulation of myelination: Relevance for development, injury, and demyelinating disease. Glia 2008, 56, 1552–1565. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.N.; Nadim, W.; Turmaine, M. Schwann cell migration through freeze-killed peripheral nerve grafts without accompanying axons. Acta Neuropathol. Berl. 1991, 82, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, Y.; Chen, J.; Peng, J. The role of peripheral nerve ECM components in the tissue engineering nerve construction. Rev. Neurosci. 2013, 24, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Hibasami, H.; Hineno, T.; Shi, D.; Morita, A.; Inada, H.; Fujisawa, K.; Nakashima, K.; Ogihara, Y. Role of ornithine decarboxylase in proliferation of Schwann cells during Wallerian degeneration and its enhancement by nerve expansion. Muscle Nerve 1995, 18, 1341–1343. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.C.; Lacour, S.P.; Raffoul, W.; di Summa, P.G. Extracellular matrix components in peripheral nerve repair: How to affect neural cellular response and nerve regeneration? Neural Regen. Res. 2014, 9, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, N.M. Peripheral nerve injury. J. Neurosurg. 2011, 114, 1516–1517, discussion 1517–1519. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.M. The effect of inhibiting Schwann cell mitosis on the re-innervation of acellular autografts in the peripheral nervous system of the mouse. Neuropathol. Appl. Neurobiol. 1986, 12, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Hollis, E.R.; Tuszynski, M.H. Neurotrophins: potential therapeutic tools for the treatment of spinal cord injury. Neurother. J. Am. Soc. Exp. Neurother. 2011, 8, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Nectow, A.R.; Marra, K.G.; Kaplan, D.L. Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng. Part B Rev. 2012, 18, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Belkas, J.S.; Shoichet, M.S.; Midha, R. Peripheral nerve regeneration through guidance tubes. Neurol. Res. 2004, 26, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Somaiah, C.; Kumar, A.; Mawrie, D.; Sharma, A.; Patil, S.D.; Bhattacharyya, J.; Swaminathan, R.; Jaganathan, B.G. Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS ONE 2015, 10, e0145068. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Reilly, G.C.; Engler, A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 2010, 43, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Vozzi, G.; Vozzi, F.; Salvadori, C.; Dini, F.; Carlucci, F.; Arispici, M.; Burchielli, S.; Di Scipio, F.; Geuna, S.; et al. Melt-extruded guides for peripheral nerve regeneration. Part I: poly(epsilon-caprolactone). Biomed. Microdevices 2009, 11, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Meek, M.F.; Varejão, A.S.P.; Geuna, S. Use of skeletal muscle tissue in peripheral nerve repair: Review of the literature. Tissue Eng. 2004, 10, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Vleggeert-Lankamp, C.L.A.-M.; Pêgo, A.P.; Lakke, E.A.J.F.; Deenen, M.; Marani, E.; Thomeer, R.T.W.M. Adhesion and proliferation of human Schwann cells on adhesive coatings. Biomaterials 2004, 25, 2741–2751. [Google Scholar] [CrossRef] [PubMed]
- Bovolenta, P.; Fernaud-Espinosa, I. Nervous system proteoglycans as modulators of neurite outgrowth. Prog. Neurobiol. 2000, 61, 113–132. [Google Scholar] [CrossRef]
- Widhe, M.; Shalaly, N.D.; Hedhammar, M. A fibronectin mimetic motif improves integrin mediated cell biding to recombinant spider silk matrices. Biomaterials 2016, 74, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.E.; Mair, D.B.; Narang, J.D.; Feleke, K.; Lemmon, C.A. Fibronectin fibrillogenesis facilitates mechano-dependent cell spreading, force generation, and nuclear size in human embryonic fibroblasts. Integr. Biol. Quant. Biosci. Nano Macro 2015, 7, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Ryu, J.M.; Yun, S.P.; Kim, M.O.; Han, H.J. Fibronectin stimulates migration through lipid raft dependent NHE-1 activation in mouse embryonic stem cells: Involvement of RhoA, Ca(2+)/CaM, and ERK. Biochim. Biophys. Acta 2012, 1820, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Liberio, M.S.; Sadowski, M.C.; Soekmadji, C.; Davis, R.A.; Nelson, C.C. Differential effects of tissue culture coating substrates on prostate cancer cell adherence, morphology and behavior. PLoS ONE 2014, 9, e112122. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).