Effect of Mn and Fe on the Formation of Fe- and Mn-Rich Intermetallics in Al–5Mg–Mn Alloys Solidified Under Near-Rapid Cooling
Abstract
:1. Introduction
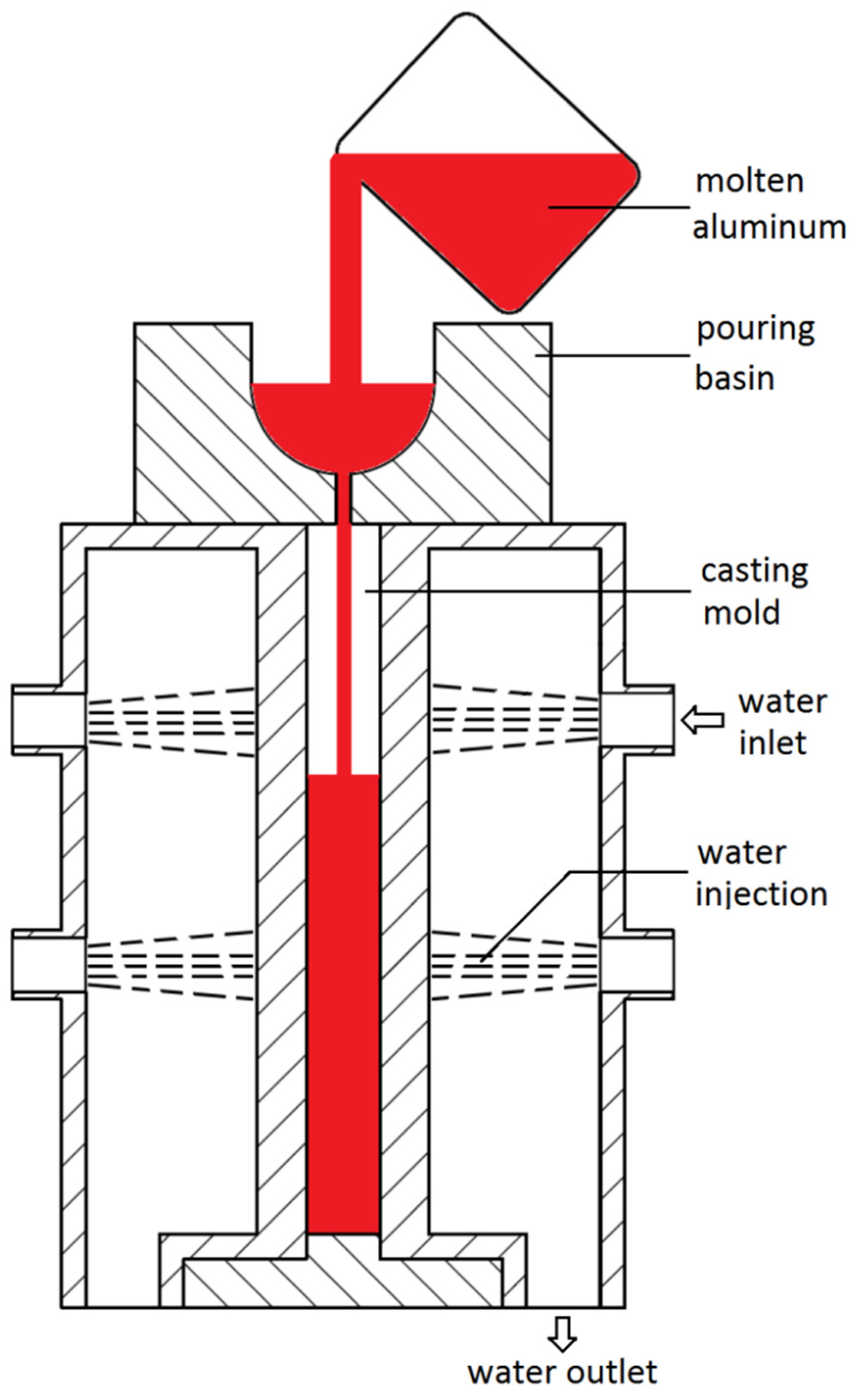
2. Experimental Procedures
| Group | Alloy Sample | Nominal Content of Mn | Measured Composition | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mg | Mn | Fe | Si | Cr | Ti | Al | |||
| I | I-1 | 0.4 | 4.75 | 0.39 | 0.085 | 0.034 | 0.006 | 0.018 | Bal. |
| I-2 | 0.8 | 5.19 | 0.80 | 0.092 | 0.035 | 0.006 | 0.016 | Bal. | |
| I-3 | 1.2 | 5.08 | 1.29 | 0.085 | 0.033 | 0.006 | 0.016 | Bal. | |
| I-4 | 1.6 | 4.83 | 1.63 | 0.089 | 0.036 | 0.006 | 0.017 | Bal. | |
| I-5 | 2.0 | 5.13 | 2.10 | 0.098 | 0.034 | 0.006 | 0.017 | Bal. | |
| II | II-1 | 0.4 | 4.81 | 0.39 | 0.455 | 0.233 | 0.006 | 0.017 | Bal. |
| II-2 | 0.8 | 4.87 | 0.73 | 0.485 | 0.241 | 0.006 | 0.023 | Bal. | |
| II-3 | 1.2 | 4.82 | 1.23 | 0.473 | 0.219 | 0.006 | 0.018 | Bal. | |
| II-4 | 1.6 | 5.12 | 1.51 | 0.502 | 0.238 | 0.006 | 0.022 | Bal. | |
| II-5 | 2.0 | 4.83 | 2.00 | 0.556 | 0.230 | 0.006 | 0.024 | Bal. | |
3. Results
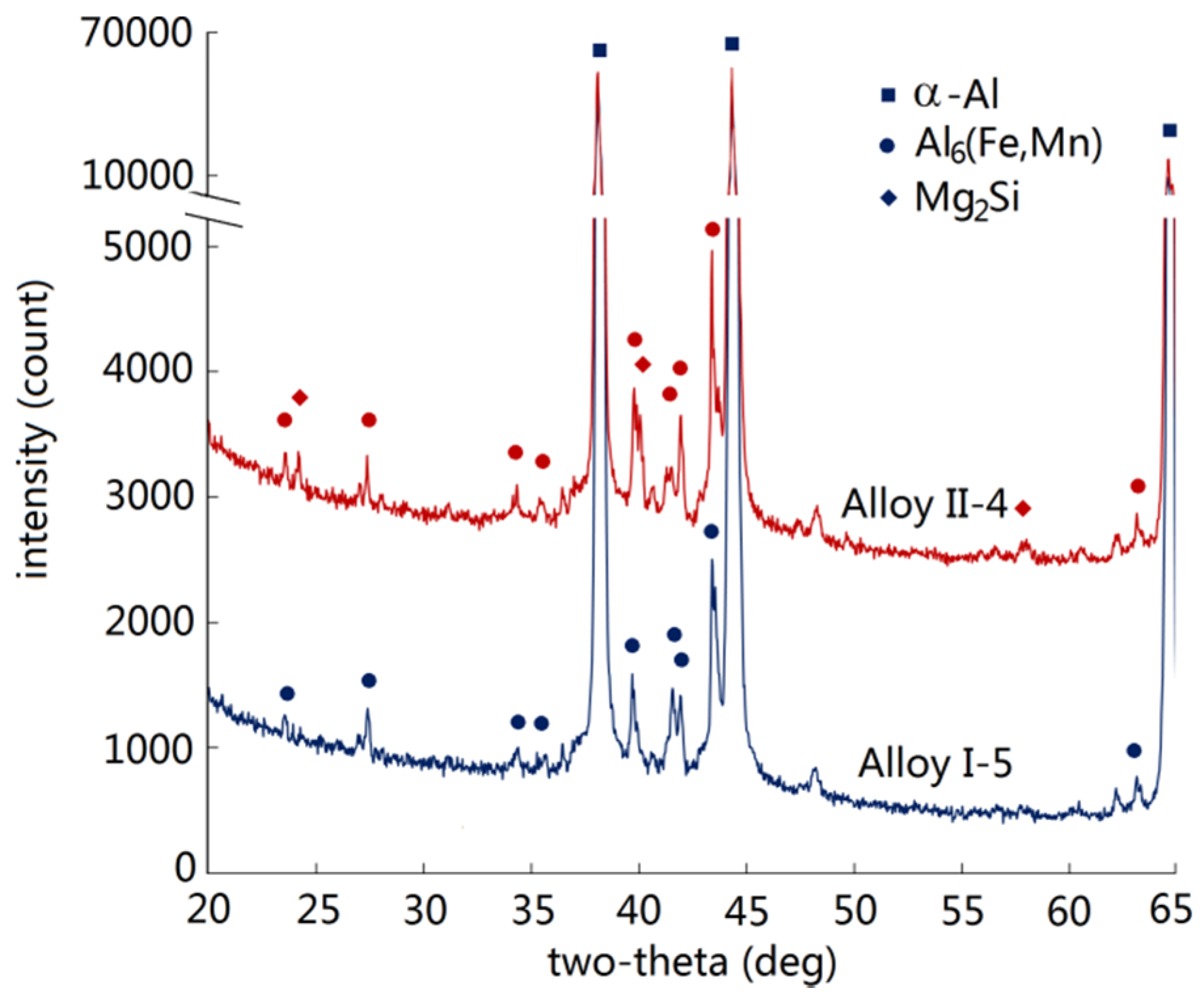
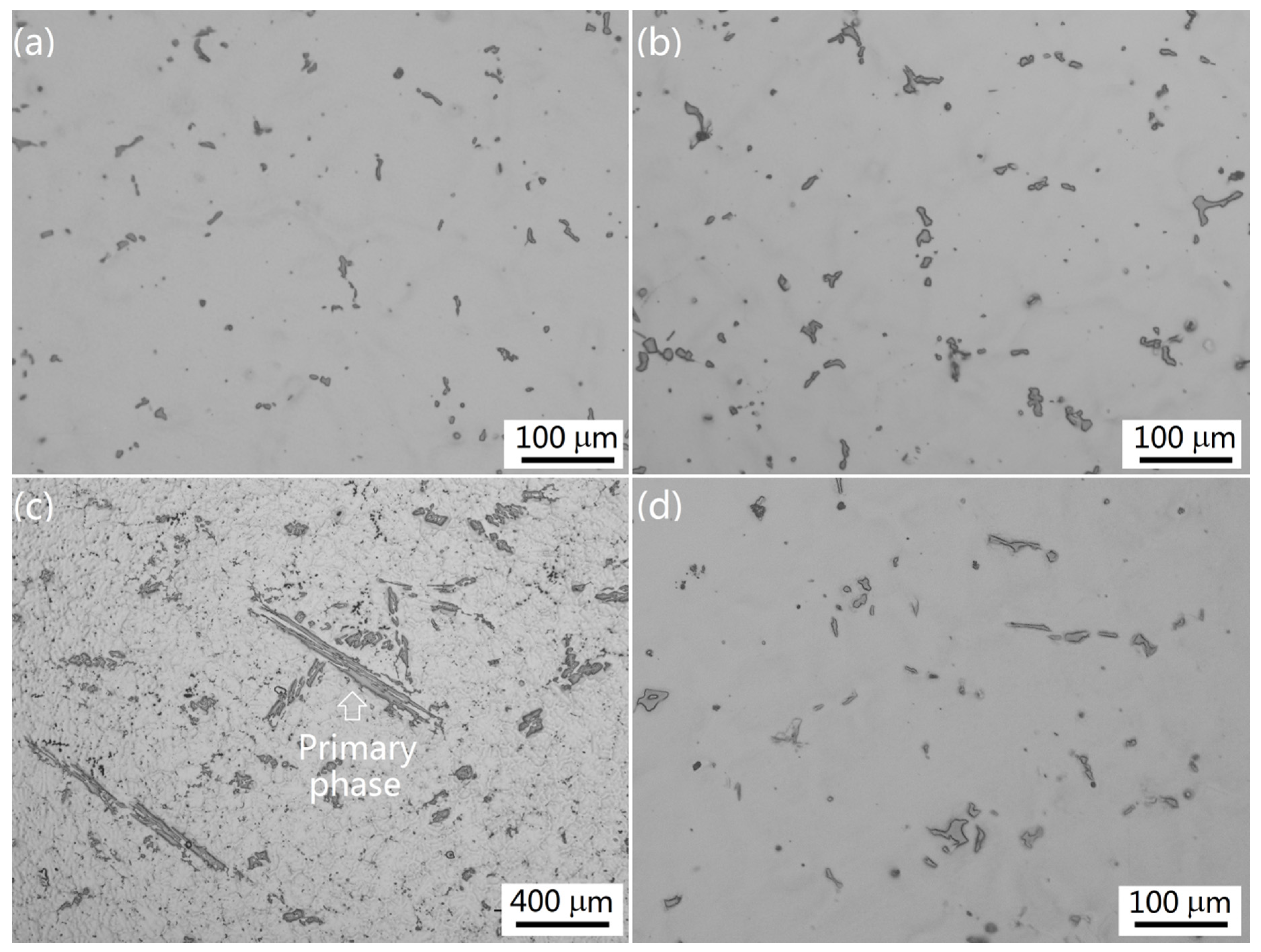
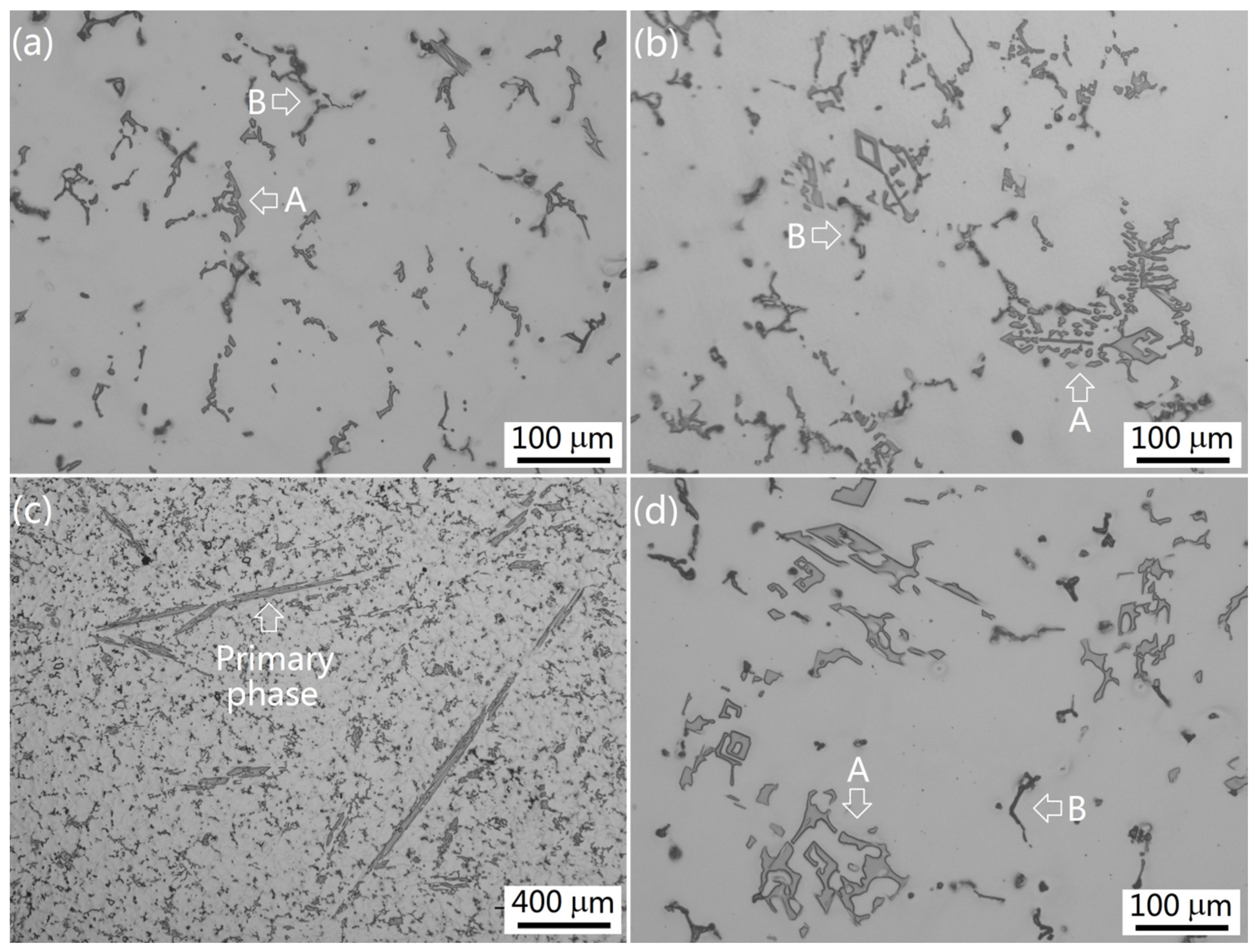
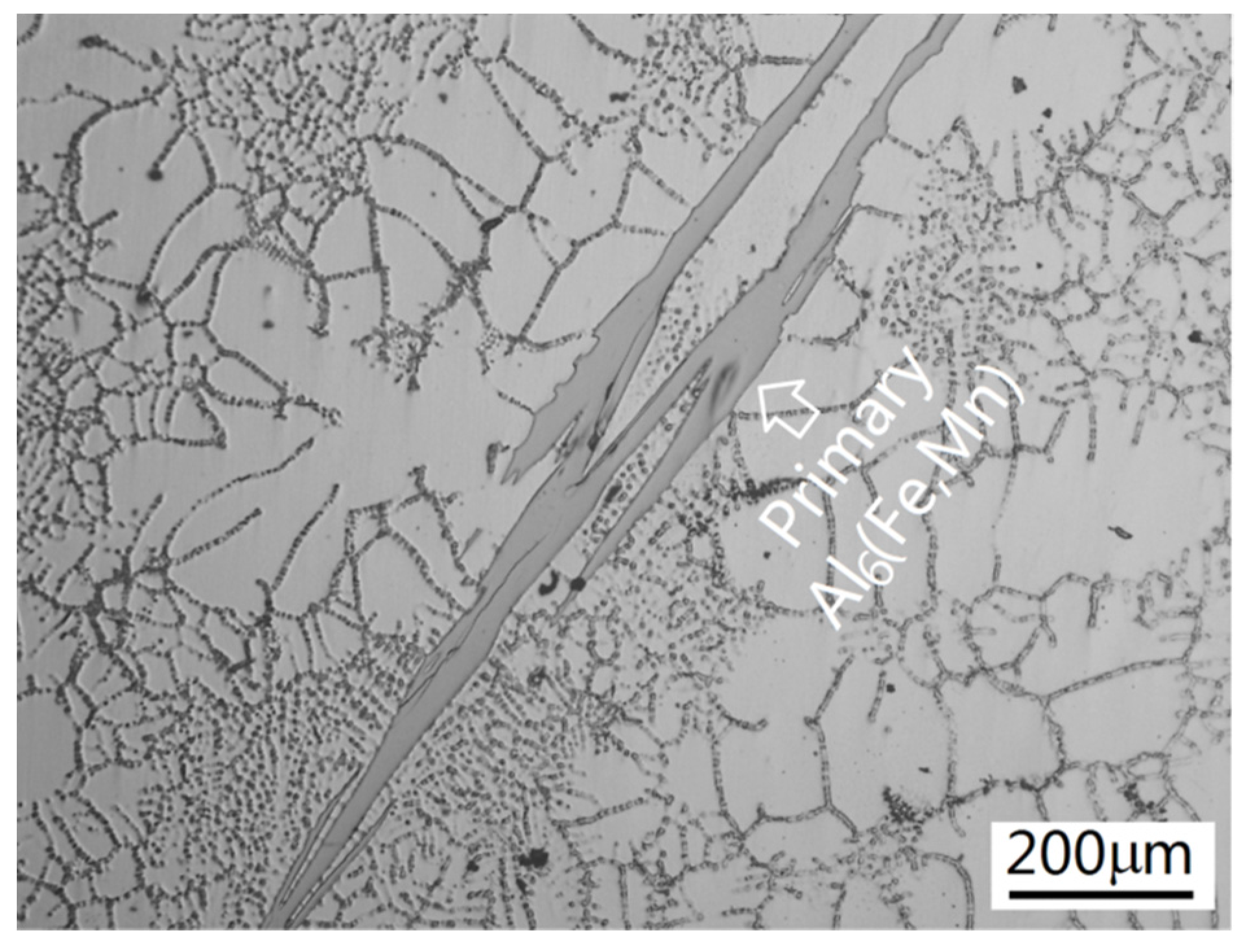
3.1. Intermetallics Formed under Near-Rapid Cooling
| Element | Sample I-4 | Sample I-5 | ||
|---|---|---|---|---|
| Composition Range | Composition Average | Composition Range | Composition Average | |
| Al | 76.32–85.19 | 80.46 | 83.41–86.00 | 84.82 |
| Mn | 9.36–17.91 | 14.96 | 13.37–15.74 | 14.38 |
| Fe | 1.88–3.35 | 2.9 | 0.63–0.91 | 0.80 |
| Mg | 0–3.57 | 1.71 | 0 | 0 |
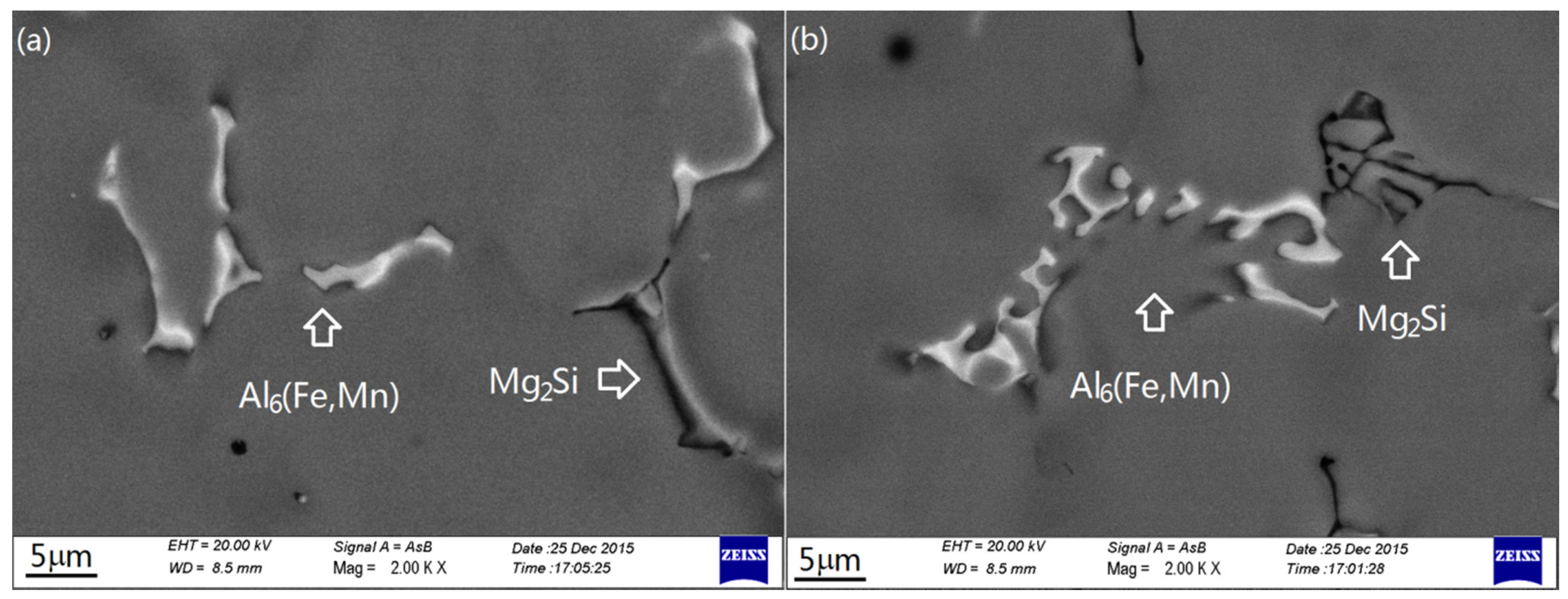
| Element | Alloy II-1 | Alloy II-4 | ||
|---|---|---|---|---|
| Composition Range | Composition Average | Composition Range | Composition Average | |
| Al | 85.62–86.03 | 85.83 | 81.08–82.25 | 81.83 |
| Mn | 2.17–2.34 | 2.27 | 12.08–13.53 | 12.82 |
| Fe | 10.58–11.35 | 10.95 | 4.98–5.67 | 5.35 |
| Mg | 0.74–1.09 | 0.81 | 0 | 0 |
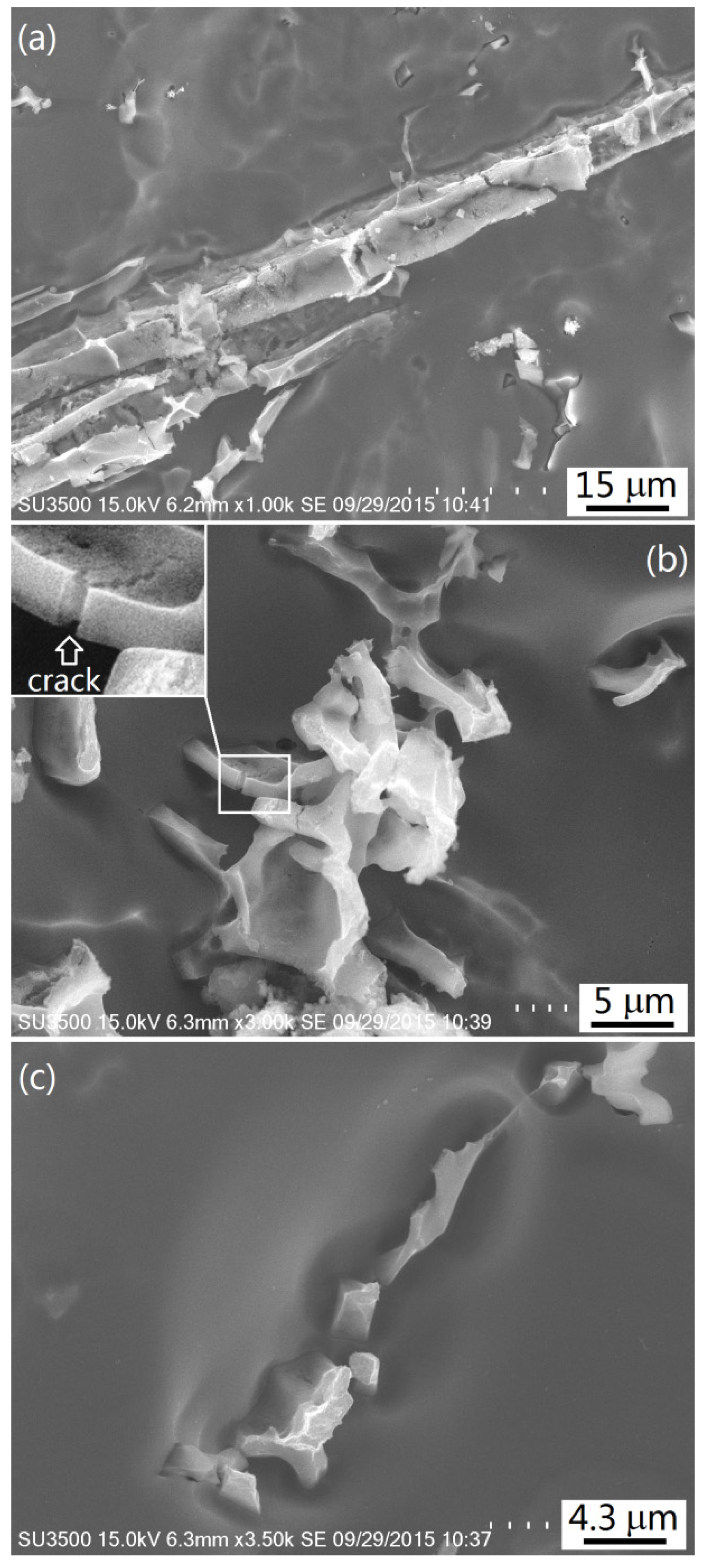
3.2. Intermetallics Formed under Slow Cooling

3.3. The Solidification Process
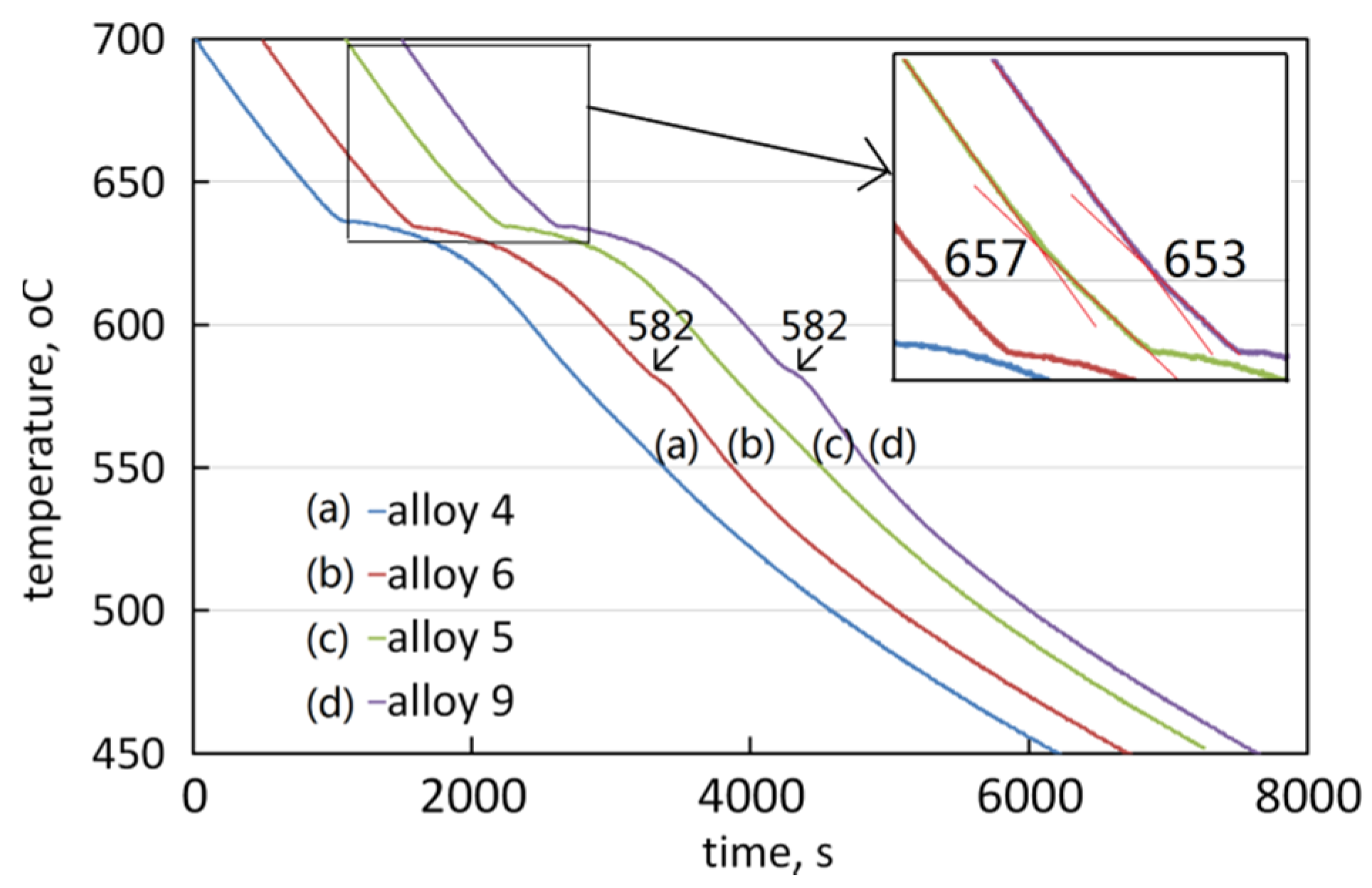
4. Discussion
4.1. The Influence of Mn and Fe
4.2. The Influence of Cooling Rate
5. Conclusions
- 1)
- In Al–5Mg–Mn alloy with low Fe content, intermetallic Al6(Fe,Mn) was small in size and amount. With the increase of Mn, intermetallic Al6(Fe,Mn) increased, but the increment was limited. Once the content of Mn passed beyond eutectic, intermetallic Al6(Fe,Mn) became the primary phase with extremely coarse platelet-like morphology and increased significantly in amount;
- 2)
- In high-Fe-containing Al–5Mg–Mn alloys, Fe promoted the formation of intermetallic Al6(Fe,Mn). Even in the alloy with low Mn content, it was in a large amount. With the increase of Mn content, intermetallic Al6(Fe,Mn) increased in both size and amount. Increasing the content of Fe caused intermetallic Al6(Fe,Mn) to become the primary phase at a lower Mn content;
- 3)
- Cooling rate played a critical role in the refinement of intermetallics. The Al6(Fe,Mn) phase could be refined to a significant extent by casting under near-rapid cooling (around 20 °C·s−1). In the alloys with high Mn and/or high Fe contents solidified under near-rapid cooling, intermetallic Al6(Fe,Mn) demonstrated fine Chinese script structures, which could be achieved using the continuous strip casting process.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Backerud, L.; Krol, E.; Tamminen, J. Solidification Characteristics of Aluminum Alloys; Universitetsforlaget AS: Osle, Norway, 1986; p. 113. [Google Scholar]
- Seifeddine, S.; Johansson, S.; Svensson, I.L. The influence of cooling rate and manganese content on the β-Al5FeSi phase formation and mechanical properties of Al–Si-based alloys. Mater. Sci. Eng. A 2008, 490, 385–390. [Google Scholar] [CrossRef]
- Sreeja Kumari, S.S.; Pillai, R.M.; Rajan, T.P.D.; Pai, B.C. Effects of individual and combined additions of Be, Mn, Ca and Sr on the solidification behaviour, structure and mechanical properties of Al–7Si–0.3Mg–0.8Fe alloy. Mater. Sci. Eng. A 2007, 460, 561–573. [Google Scholar] [CrossRef]
- Liu, K.; Cao, X.; Chen, X.G. Tensile Properties of Al–Cu 206 Cast Alloys with Various Iron Contents. Metall. Mater. Trans. A 2014, 45, 2498–2507. [Google Scholar] [CrossRef]
- Li, J.; Birbilis, N.; Buchheit, R.G. Electrochemical Assessment of Interfacial Characteristics of Intermetallic Phases Present in Aluminium Alloy 2024-T3. Corros. Sci. 2015, 101, 155–164. [Google Scholar] [CrossRef]
- Cavanaugh, M.K.; Li, J.-C.; Buchheit, R.G. Electrochemical characterization of intermetallic phases common to aluminum alloys as a function of solution temperature. J. Electrochem. Soc. 2014, 161, C535–C543. [Google Scholar] [CrossRef]
- Davis, J.R. Alloying: Understanding the Basics; ASM International: Novelty, OH, USA, 2001; p. 382. [Google Scholar]
- Backerud, L.; Krol, E.; Tamminen, J. Solidification Characteristics of Aluminum Alloys; Universitetsforlaget AS: Osle, Norway, 1986; p. 119. [Google Scholar]
- Bauccio, M.L. ASM Metals Reference Book, 3rd ed.; ASM International: Novelty, OH, USA, 1993; pp. 405–415. [Google Scholar]
- Liu, Y.L.; Liu, M.; Luo, L.; Wang, J.Z.; Liu, C.Z. The Solidification Behavior of AA2618 Aluminum Alloy and the Influence of Cooling Rate. Materials 2014, 7, 7875–7890. [Google Scholar] [CrossRef]
- Sanders, R.E., Jr. Continuous Casting for Aluminum Sheet: A Product Perspective. JOM 2012, 64, 291–301. [Google Scholar] [CrossRef]
- Goswami, R.; Spanos, G.; Pao, P.S.; Holtz, R.L. Microstructural evolution and stress corrosion cracking behavior of Al-5083. Metall. Mater. Trans. A 2011, 42, 348–355. [Google Scholar] [CrossRef]
- Goswami, R.; Holtz, R.L. Transmission electron microscopic investigations of grain boundary beta phase precipitation in Al 5083 aged at 373 K (100 °C). Metall. Mater. Trans. A 2013, 44, 1279–1289. [Google Scholar] [CrossRef]
- Harrell, T.J.; Topping, T.D.; Wang, H.M.; Hu, T.; Schoenung, J.M.; Lavernia, E.J. Microstructure and strengthening mechanisms in an ultrafine grained Al–Mg–Sc alloy produced by powder metallurgy. Metall. Mater. Trans. A 2014, 45, 6329–6343. [Google Scholar] [CrossRef]
- Verma, R.; Kim, S. Superplastic behavior of copper-modified 5083 aluminum alloy. J. Mater. Eng. Perform. 2007, 16, 185–191. [Google Scholar] [CrossRef]
- Smolej, A.; Skaza, B.; Dragojević, V. Superplastic behavior of Al–4.5Mg–0.46Mn–0.44Sc alloy sheet produced by a conventional rolling process. J. Mater. Eng. Perform. 2010, 19, 221–230. [Google Scholar] [CrossRef]
- Shin, D.H.; Hwang, D.-Y.; Oh, Y.-J.; Park, K.-T. High-strain-rate superplastic behavior of equal-channel angular-pressed 5083 Al-0.2 wt pct Sc. Metall. Mater. Trans. A 2004, 35, 825–837. [Google Scholar] [CrossRef]
- Singh, D.; Rao, P.N.; Jayaganthan, R. Effect of deformation temperature on mechanical properties of ultrafine grained Al–Mg alloys processed by rolling. Mater. Des. 2013, 50, 646–655. [Google Scholar] [CrossRef]
- Abdu, M.T.; Dheda, S.S.; Lavernia, E.J.; Topping, T.D.; Mohamed, F.A. Creep and microstructure in ultrafine-grained 5083 Al. J. Mater. Sci. 2013, 48, 3294–3303. [Google Scholar] [CrossRef]
- Topping, T.D.; Ahn, B.; Li, Y.; Nutt, S.R.; Lavernia, E.J. Influence of process parameters on the mechanical behavior of an ultrafine-grained Al alloy. Metall. Mater. Trans. A 2012, 43, 505–519. [Google Scholar] [CrossRef]
- Roy, I.; Chauhan, M.; Mohamed, F.A.; Lavernia, E.J. Thermal stability in bulk cryomilled ultrafine-grained 5083 Al alloy. Metall. Mater. Trans. A 2006, 37, 721–730. [Google Scholar] [CrossRef]
- Witkin, D.; Han, B.Q.; Lavernia, E.J. Mechanical behavior of ultrafine-grained cryomilled Al 5083 at elevated temperature. J. Mater. Eng. Perform. 2005, 14, 519–527. [Google Scholar] [CrossRef]
- Lin, S.; Nie, Z.; Huang, H.; Li, B. Annealing behavior of a modified 5083 aluminum alloy. Mater. Des. 2010, 31, 1607–1612. [Google Scholar] [CrossRef]
- Yang, D.; Li, X.; He, D.; Huang, H. Effect of minor Er and Zr on microstructure and mechanical properties of Al–Mg–Mn alloy (5083) welded joints. Mater. Sci. Eng. A 2013, 561, 226–231. [Google Scholar]
- Malopheyev, S.; Kaibyshev, R. Strengthening mechanisms in a Zr-modified 5083 alloy deformed to high strains. Mater. Sci. Eng. A 2015, 620, 246–252. [Google Scholar] [CrossRef]
- Meng, C.; Zhang, D.; Cui, H.; Zhuang, L.; Zhang, J. Mechanical properties, intergranular corrosion behavior and microstructure of Zn modified Al–Mg alloys. J. Alloy. Compd. 2014, 617, 925–932. [Google Scholar] [CrossRef]
- Xia, S.L.; Ma, M.; Zhang, J.X.; Wang, W.X.; Liu, W.C. Effect of heating rate on the microstructure, texture and tensile properties of continuous cast AA 5083 aluminum alloy. Mater. Sci. Eng. A 2014, 609, 168–176. [Google Scholar] [CrossRef]
- García-Bernal, M.A.; Mishra, R.S.; Verma, R.; Hernández-Silva, D. Hot deformation behavior of friction-stir processed strip-cast 5083 aluminum alloys with different Mn contents. Mater. Sci. Eng. A 2012, 534, 186–192. [Google Scholar] [CrossRef]
- Kumar, S.; Babu, N.H.; Scamans, G.M.; Fan, Z.; O’Reilly, K.A.Q. Twin Roll Casting of Al–Mg Alloy with High Added Impurity Content. Metall. Mater. Trans. A 2014, 45, 2842–2854. [Google Scholar] [CrossRef]
- Liu, W.C.; Morris, J.G. Quantitative Analysis of Texture Evolution in Cold-Rolled, Continuous-Cast AA 5xxx-Series Aluminum Alloys. Metall. Mater. Trans. A 2004, 35, 265–277. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Morris, J.G. Comparison of the texture evolution of direct chill and continuous cast AA5052 hot bands during isothermal annealing. Metall. Mater. Trans. A 2005, 36, 2505–2515. [Google Scholar] [CrossRef]
- Liu, Y.L.; Hu, Z.Q.; Zhang, Y.; Shi, C.X. The solidification behavior of 8090 AI–Li alloy. Metall. Trans. B 1993, 24, 857–865. [Google Scholar] [CrossRef]
- Liu, K.; Cao, X.; Chen, X.-G. Formation and phase selection of iron-rich intermetallics in Al–4.6Cu–0.5Fe cast alloys. Metall. Mater. Trans. A 2013, 44, 682–695. [Google Scholar] [CrossRef]
- Mondolfo, L.F. Aluminium Alloys: Structure and Properties; Butterworths & co.: London, UK, 1976; p. 324. [Google Scholar]
- Kattner, U.R.; Burton, B.P. ASM Handbook Volume 3: Alloy Phase Diagrams; ASM International: Geauga County, OH, USA, 1992; pp. 2–44. [Google Scholar]
- Mondolfo, L.F. Aluminium Alloys: Structure and Properties; Butterworths & Co.: London, UK, 1976; p. 529. [Google Scholar]
- Liu, Y.L.; Luo, L.; Han, C.; Ou, L.; Wang, J.; Liu, C. Effect of Fe, Si and Cooling Rate on the Formation of Fe- and Mn-rich Intermetallics in Al–5Mg–0.8Mn Alloy. J. Mater. Sci. Technol. 2015. Available online: http://www.sciencedirect.com/science/article/pii/S1005030215001760 (accessed on 19 October 2015). [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Huang, G.; Sun, Y.; Zhang, L.; Huang, Z.; Wang, J.; Liu, C. Effect of Mn and Fe on the Formation of Fe- and Mn-Rich Intermetallics in Al–5Mg–Mn Alloys Solidified Under Near-Rapid Cooling. Materials 2016, 9, 88. https://doi.org/10.3390/ma9020088
Liu Y, Huang G, Sun Y, Zhang L, Huang Z, Wang J, Liu C. Effect of Mn and Fe on the Formation of Fe- and Mn-Rich Intermetallics in Al–5Mg–Mn Alloys Solidified Under Near-Rapid Cooling. Materials. 2016; 9(2):88. https://doi.org/10.3390/ma9020088
Chicago/Turabian StyleLiu, Yulin, Gaoren Huang, Yimeng Sun, Li Zhang, Zhenwei Huang, Jijie Wang, and Chunzhong Liu. 2016. "Effect of Mn and Fe on the Formation of Fe- and Mn-Rich Intermetallics in Al–5Mg–Mn Alloys Solidified Under Near-Rapid Cooling" Materials 9, no. 2: 88. https://doi.org/10.3390/ma9020088






