Design of Boiler Welding for Improvement of Lifetime and Cost Control
Abstract
:1. Introduction
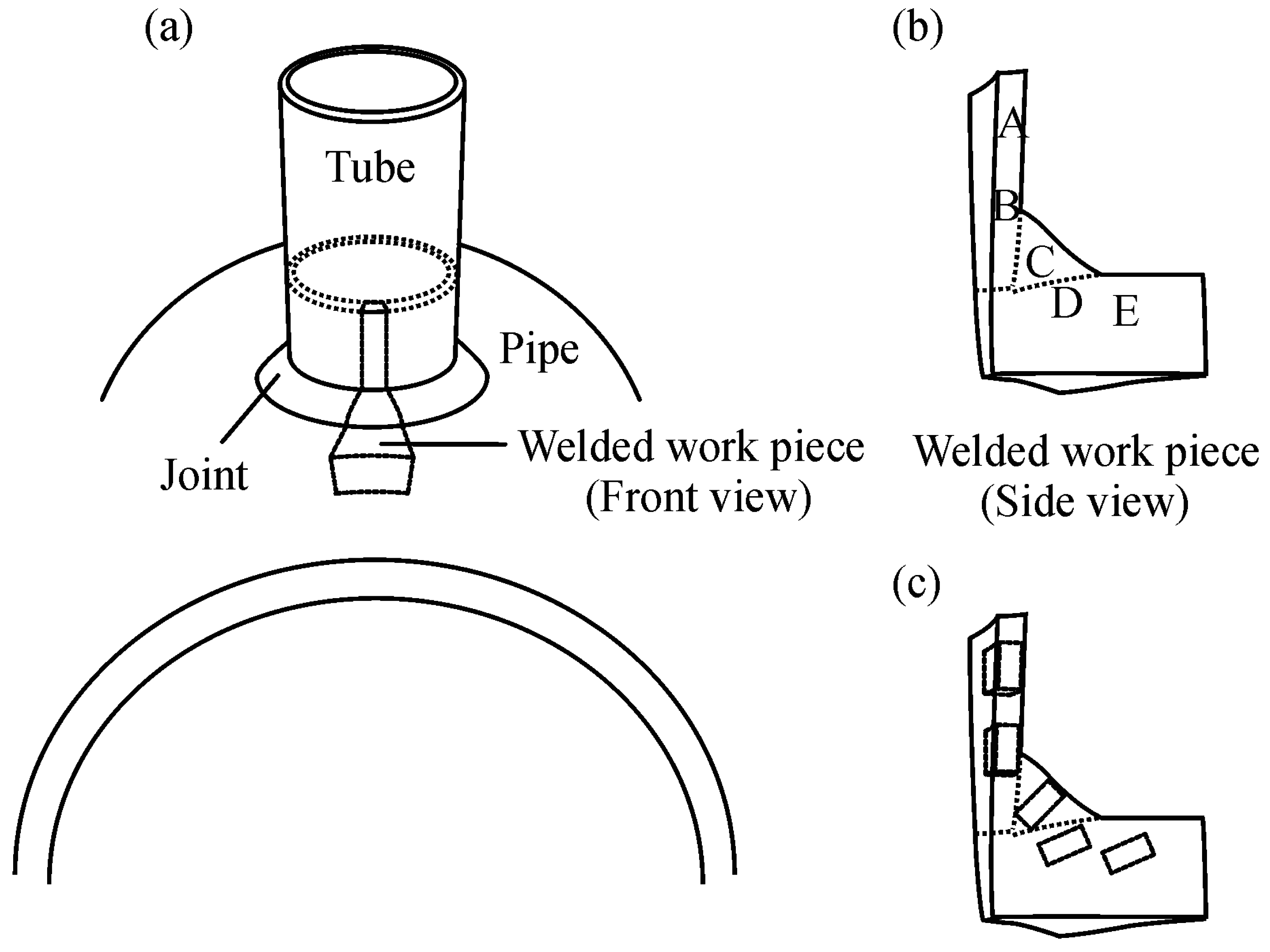
2. Materials and Methods
3. Results and Discussion
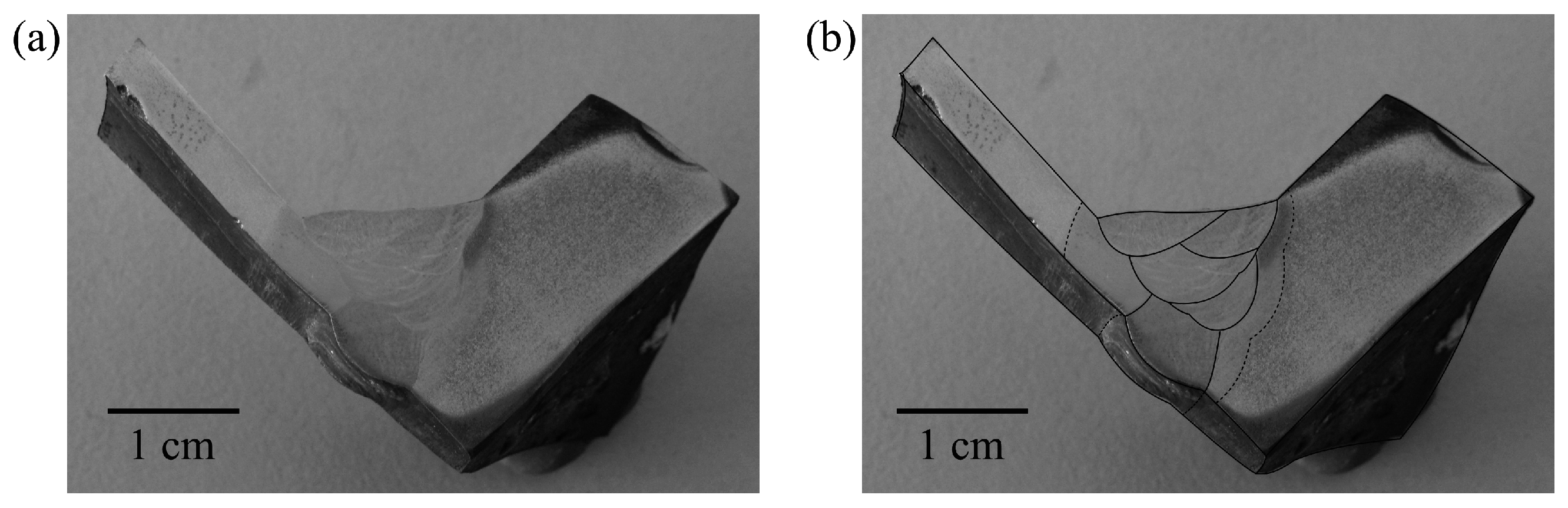
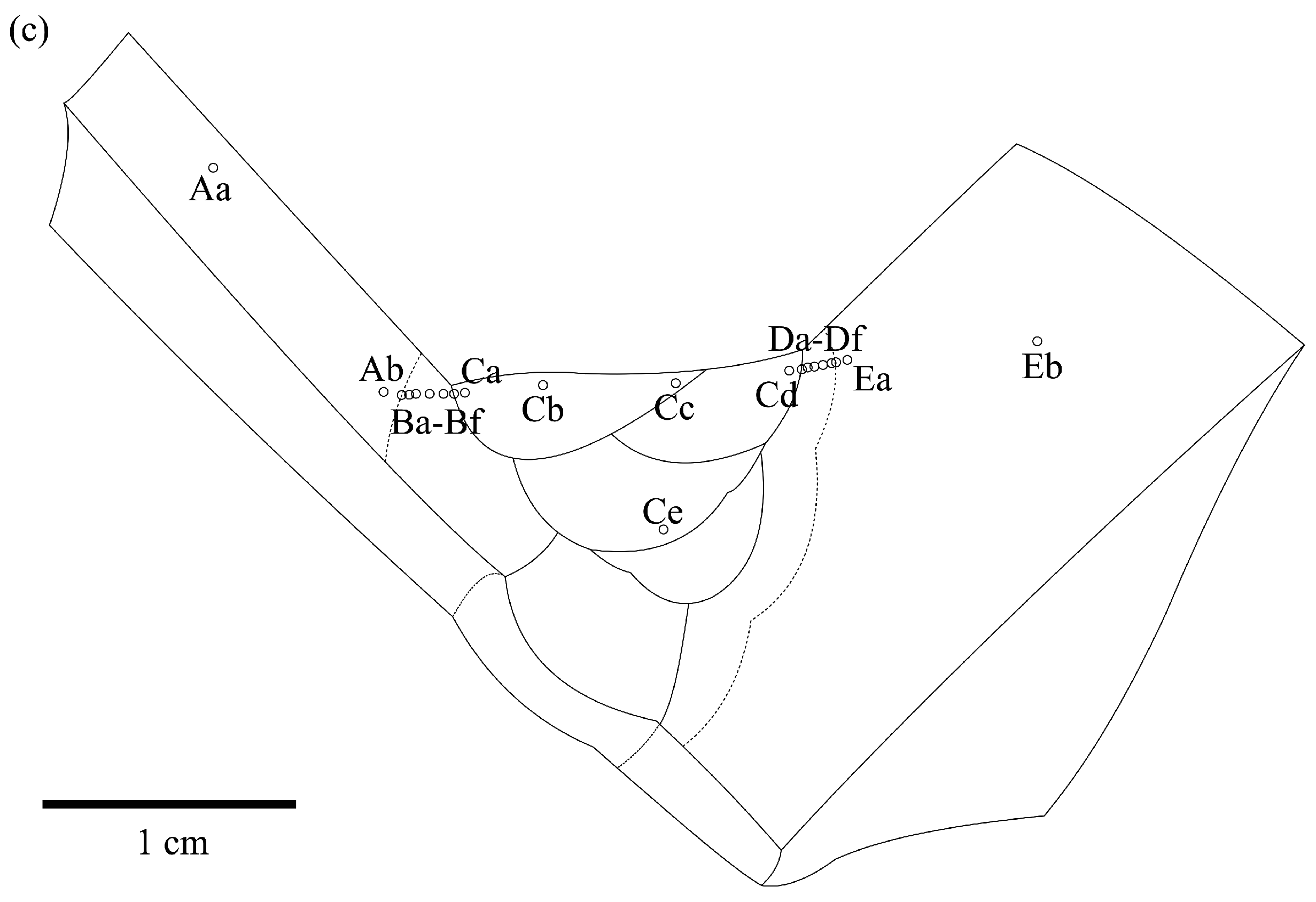
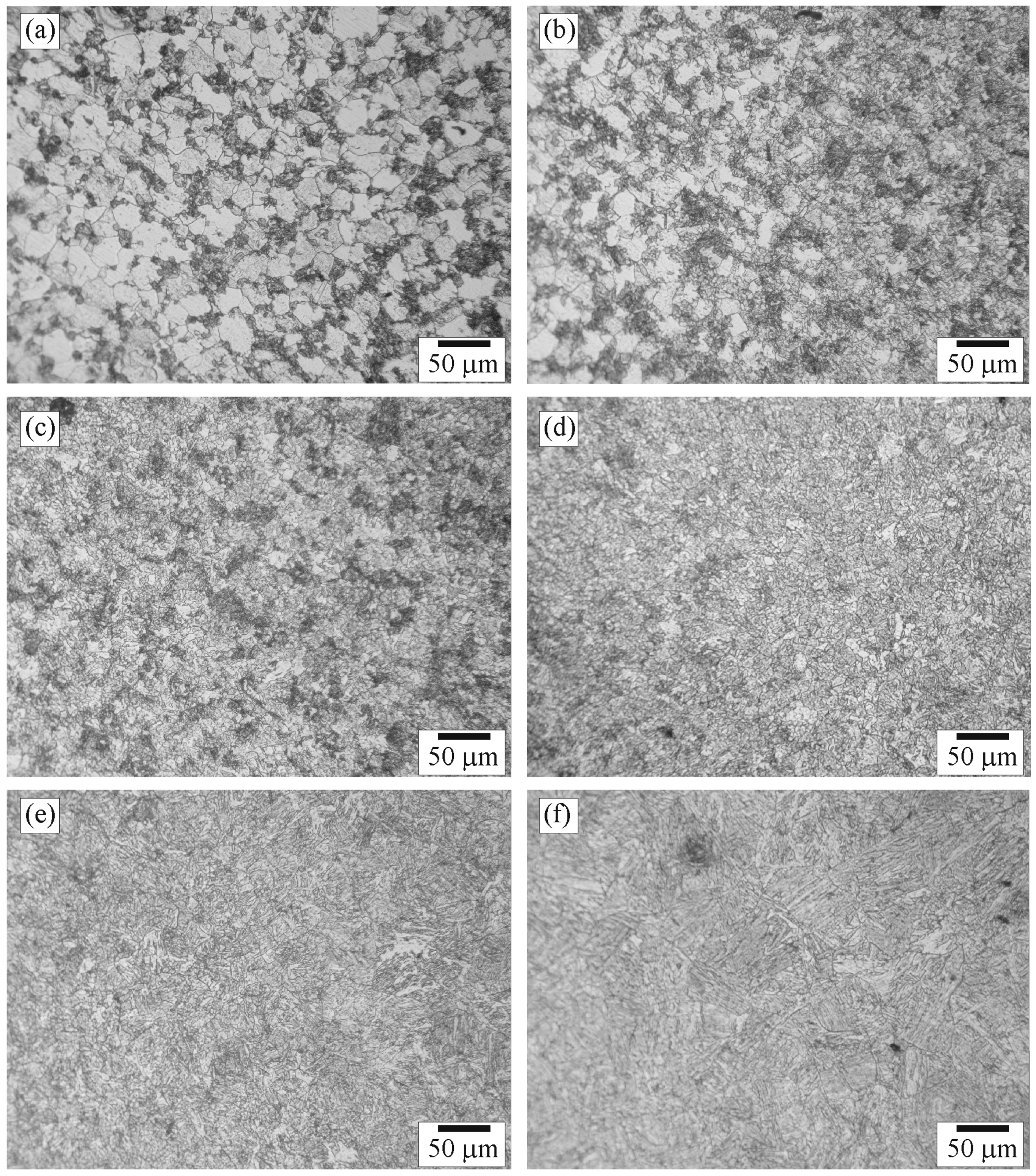
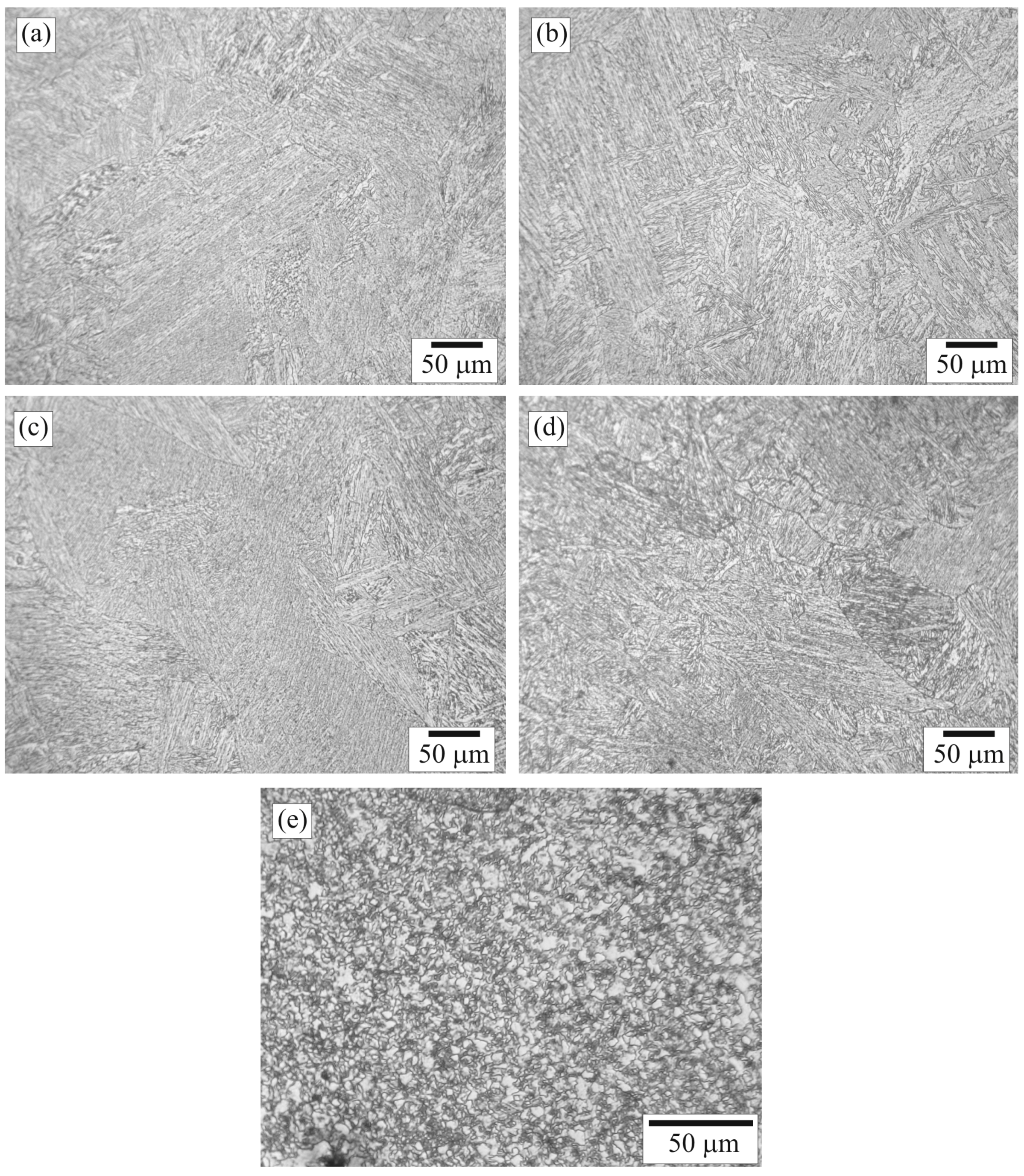
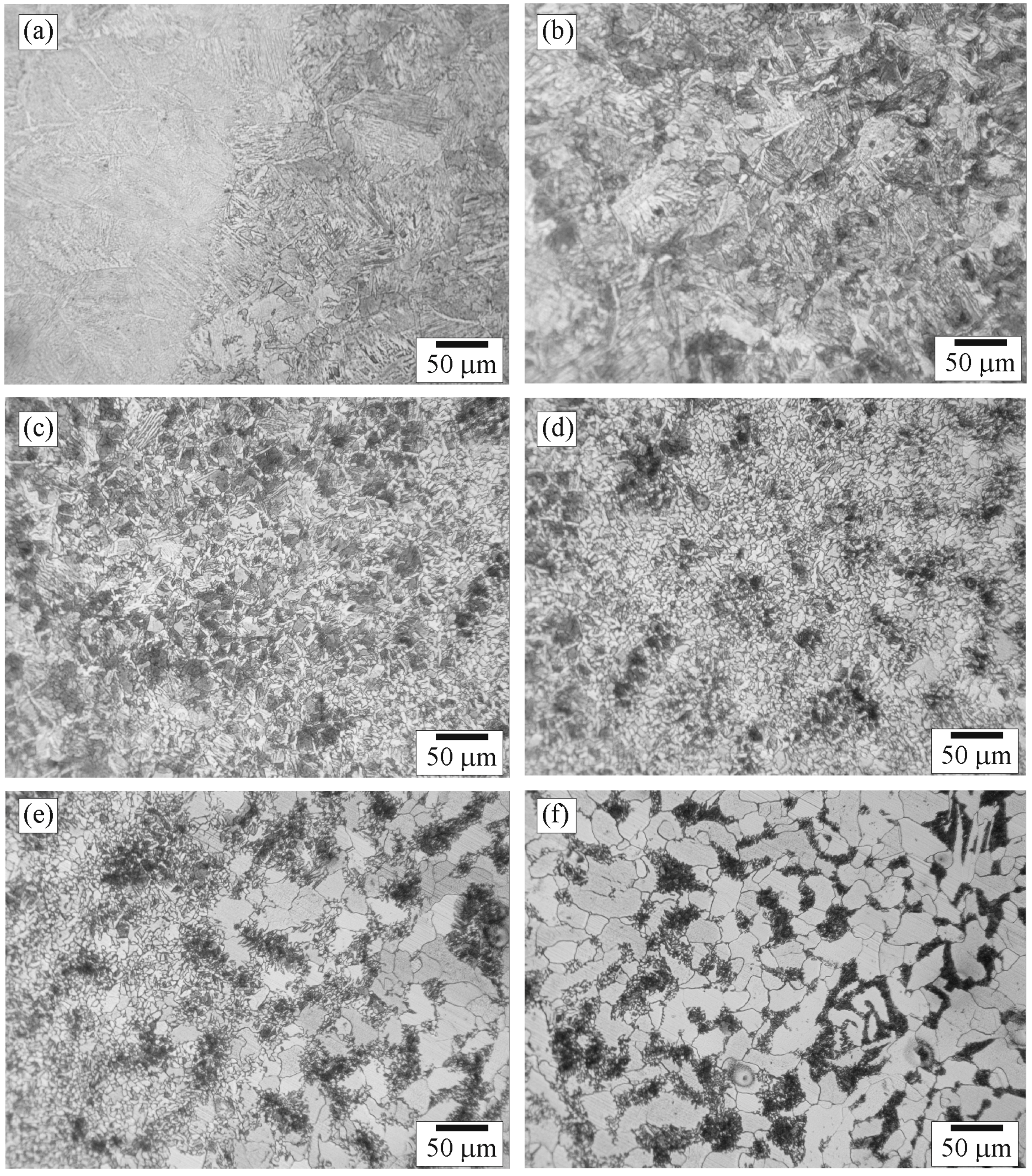
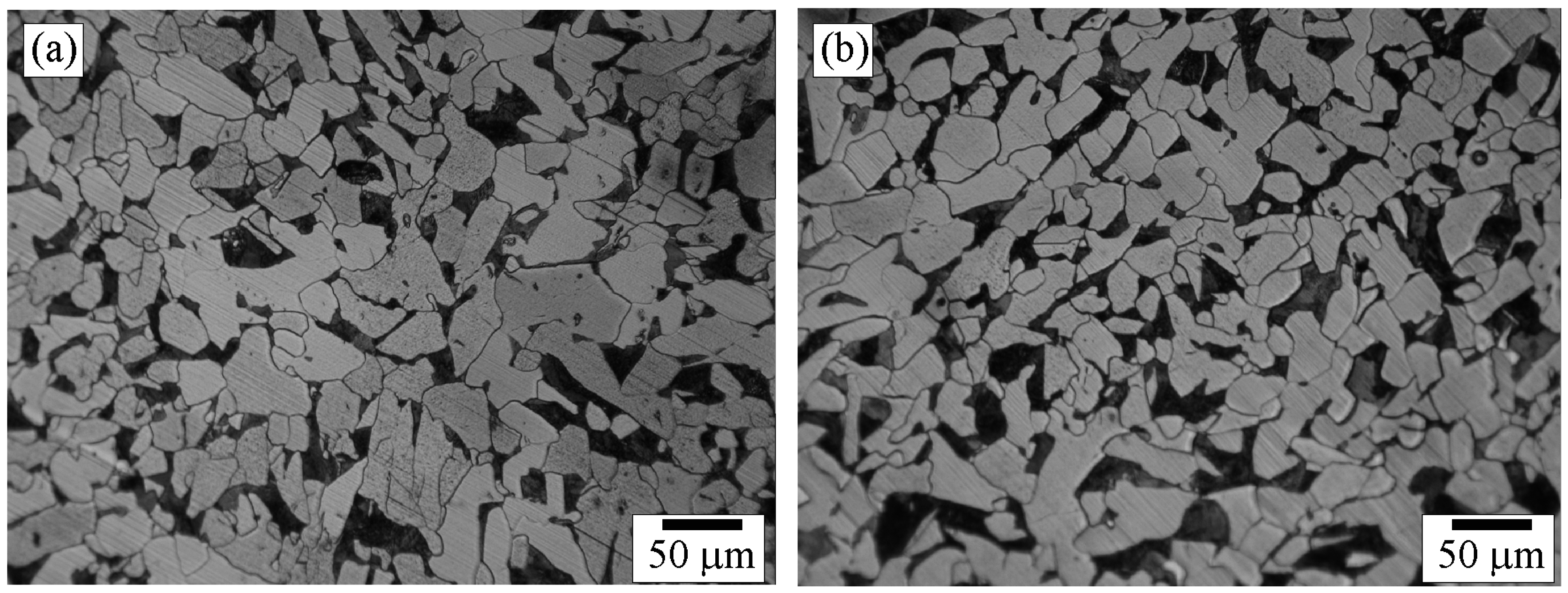
3.1. Composition and Structure of the Weld
3.2. Hardness of the Weld
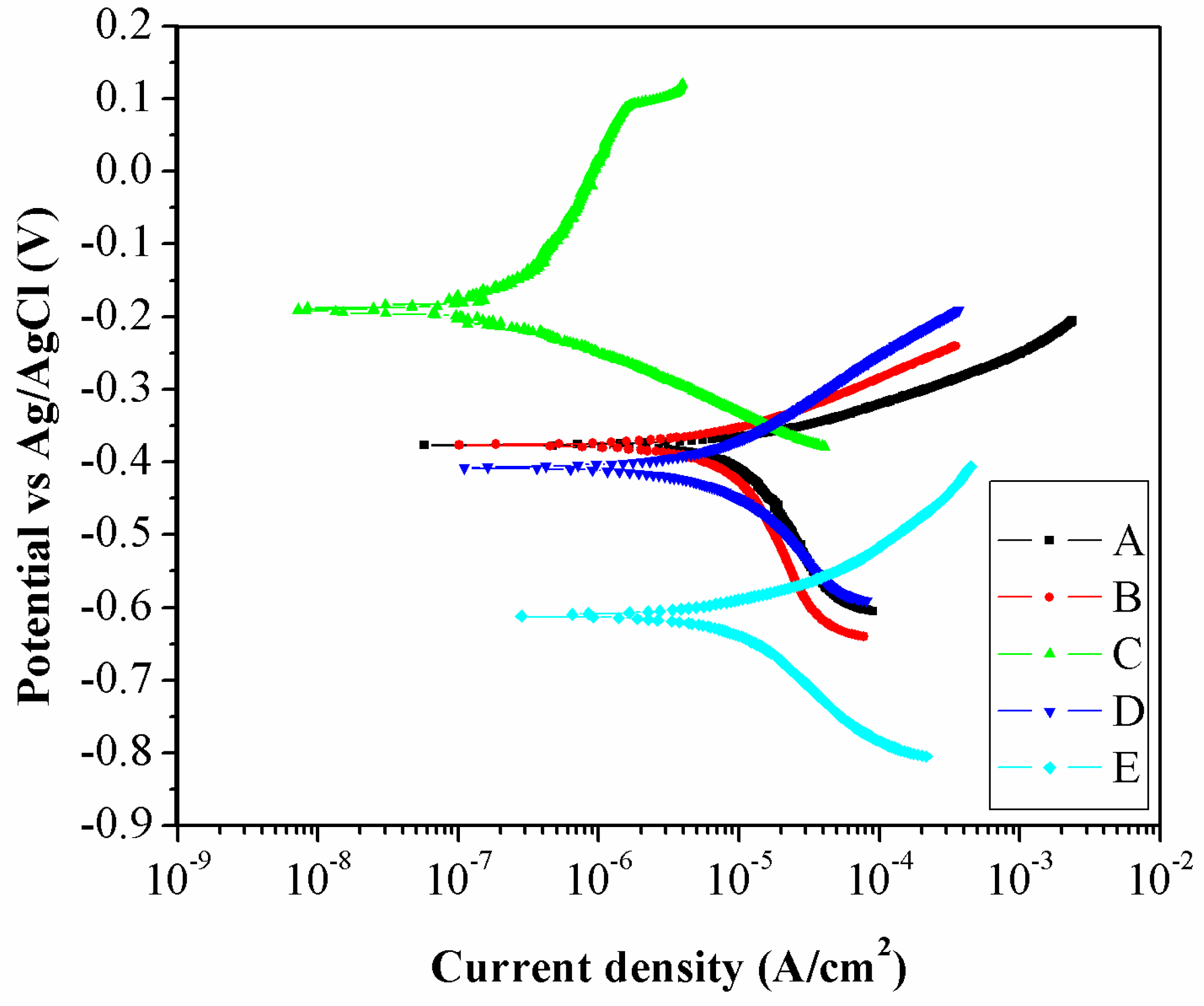
3.3. Corrosion Behavior
3.4. X-ray Photoelectron Spectroscopy
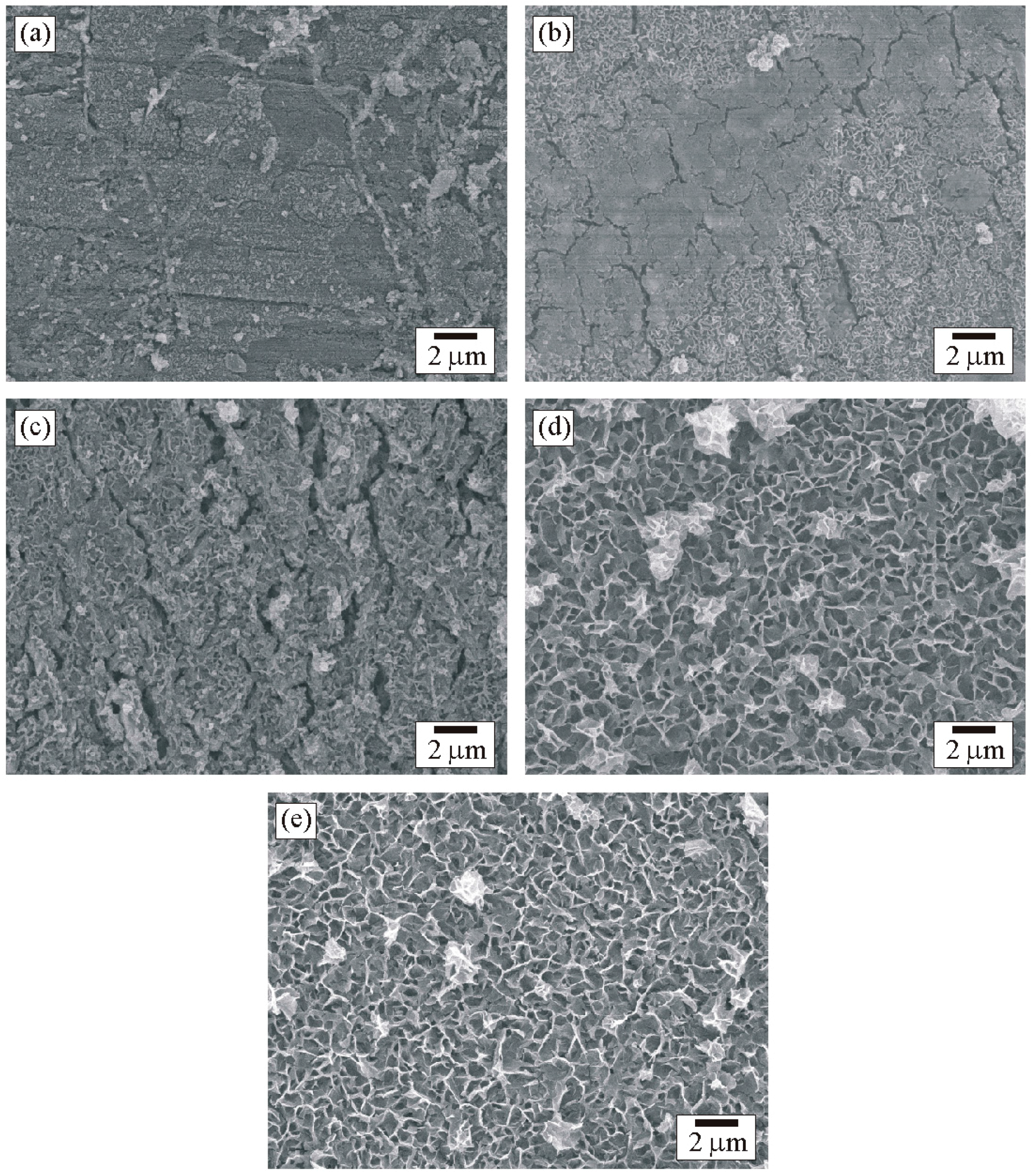
3.5. Scanning Electron Microscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peng, T.; Chi, Z.; Zhi-gang, Y.; Hiroyuki, T. Evolution and coarsening of carbides in 2.25Cr-lMo steel weld metal during high temperature tempering. J. Iron Steel. Res. Int. 2010, 17, 74–78. [Google Scholar]
- Mitchell, D.R.G.; Ball, C.J. A quantitative X-ray diffraction and analytical electron microscopy study of service-exposed 2.25Cr-1Mo steels. Mater. Charact. 2001, 47, 17–26. [Google Scholar] [CrossRef]
- Deng, D.; Murakawa, H. Finite element analysis of temperature field, microstructure and residual stress in multi-pass butt-welded 2.25Cr-1Mo steel pipes. Comput. Mater. Sci. 2008, 43, 681–695. [Google Scholar] [CrossRef]
- Tsai, M.C.; Yang, J.R. Microstructural degeneration of simulated heat-affected zone in 2.25Cr-1Mo steel during high-temperature exposure. Mater. Sci. Eng. A 2003, 340, 15–32. [Google Scholar] [CrossRef]
- Mitchell, D.R.G. Some applications of analytical TEM to the characterisation of high temperature equipment. Micron 2001, 32, 831–840. [Google Scholar] [CrossRef]
- Wang, C.Y.; Fu, R.D.; Zhou, W.H.; Zhang, W.H.; Zheng, Y.Z. Effect of reheating processes on grain boundary heritance for 2.25Cr-1Mo-0.25V steel. Mater. Sci. Eng. A 2006, 438–440, 1135–1138. [Google Scholar] [CrossRef]
- Andrén, H.O.; Cai, G.; Svensson, L.E. Microstructure of heat resistant chromium steel weld metals. Appl. Surf. Sci. 1995, 87–88, 200–206. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Sawada, K.; Kushima, H.; Tabuchi, M.; Kimura, K. Change of precipitate free zone during long-term creep in 2.25Cr-1Mo steel. Mater. Sci. Eng. A 2014, 591, 130–135. [Google Scholar] [CrossRef]
- Deen, K.M.; Ahmad, R.; Khan, I.H.; Farahat, Z. Microstructural study and electrochemical behavior of low alloy steel weldment. Mater. Des. 2010, 31, 3051–3055. [Google Scholar] [CrossRef]
- Natarajan, S.; Kumaresh Babu, S.P. Corrosion and its inhibition in SA213-T22 TIG weldments used in power plants under neutral and alkaline environments. Mater. Sci. Eng. A 2006, 432, 47–51. [Google Scholar] [CrossRef]
- Amin, M.A.; Rehim, S.S.A.E.; Abdel-Fatah, H.T.M. Electrochemical frequency modulation and inductively coupled plasma atomic emission spectroscopy methods for monitoring corrosion rates and inhibition of low alloy steel corrosion in HCl solutions and a test for validity of the Tafel extrapolation method. Corros. Sci. 2009, 51, 882–894. [Google Scholar] [CrossRef]
- Issler, S.; Klenk, A.; Shibli, A.A.; Williams, J.A. Weld repair of ferritic welded materials for high temperature application. Int. Mater. Rev. 2004, 49, 299–324. [Google Scholar] [CrossRef]
- Bordbar, S.; Alizadeh, M.; Hashemi, S.H. Effects of microstructure alteration on corrosion behavior of welded joint in API X70 pipeline steel. Mater. Des. 2013, 45, 597–604. [Google Scholar] [CrossRef]
- Chaudhuri, S. Some aspects of metallurgical assessment of boiler tubes—Basic principles and case studies. Mater. Sci. Eng. A 2006, 432, 90–99. [Google Scholar] [CrossRef]
- Wang, L.W.; Liu, Z.Y.; Cui, Z.Y.; Du, C.W.; Wang, X.H.; Li, X.G. In situ corrosion characterization of simulated weld heat affected zone on API X80 pipeline steel. Corros. Sci. 2014, 85, 401–410. [Google Scholar] [CrossRef]
- Subcommittee G01.11 on Electrochemical Measurements in Corrosion Testing. G102-89 (Reapproved 2010) Standard practice for calculation of corrosion rates and related information from electrochemical measurements. In ASTM Volume 03.02 Corrosion of Metals; Wear and Erosion; ASTM Committee G01 on Corrosion of Metals; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Jones, D.A. Electrochemical kinetics of corrosion. In Principles and Prevention of Corrosion, 2nd ed.; Johnstone, D., Ed.; Macmillan Pub. Co.: New York, NY, USA, 1992; pp. 74–114. [Google Scholar]
- Choi, Y.S.; Shim, J.J.; Kim, J.G. Corrosion behavior of low alloy steels containing Cr, Co and W in synthetic potable water. Mater. Sci. Eng. A 2004, 385, 148–156. [Google Scholar] [CrossRef]
- Tsao, C.-Y.A.; Grant, N.J. Effects of in-situ alloying and microalloying on the microstructure and recrystallization behavior of spray-deposited SAE 1008 steel sheet. Mater. Sci. Eng. A 1995, 201, 261–268. [Google Scholar] [CrossRef]
- Yang, Z.-G.; Fang, H.-S. An overview on bainite formation in steels. Curr. Opin. Solid State Mater. Sci. 2005, 9, 277–286. [Google Scholar] [CrossRef]
- Babu, S.S. The mechanism of acicular ferrite in weld deposits. Curr. Opin. Solid State Mater. Sci. 2004, 8, 267–278. [Google Scholar] [CrossRef]
- Grong, O.; Matlock, D.K. Microstructural development in mild and low-alloy steel weld metals. Int. Mater. Rev. 1986, 31, 27–48. [Google Scholar] [CrossRef]
- Feng, R.; Li, S.; Li, Z.; Tian, L. Variations of microstructure and properties of 690 MPa grade low carbon bainitic steel after tempering. Mater. Sci. Eng. A 2012, 558, 205–210. [Google Scholar] [CrossRef]
- Mohammadi, F.; Eliyan, F.F.; Alfantazi, A. Corrosion of simulated weld HAZ of API X-80 pipeline steel. Corros. Sci. 2012, 63, 323–333. [Google Scholar] [CrossRef]
- DuPont, J.N.; Siefert, J.A.; Shingledecker, J.P. Microstructural evolution and mechanical properties of Grades 23 and 24 creep strength enhanced ferritic steels. Int. Mater. Rev. 2017, 62, 32–56. [Google Scholar] [CrossRef]
- Ramalho, A.L.; Ferreira, J.A.M.; Branco, C.A.G.M. Fatigue behaviour of T welded joints rehabilitated by tungsten inert gas and plasma dressing. Mater. Des. 2011, 32, 4705–4713. [Google Scholar] [CrossRef]
- Yang, Y.; Joshi, G.R.; Akid, R. Electrochemical Investigation of the Corrosion of Different Microstructural Phases of X65 Pipeline Steel under Saturated Carbon Dioxide Conditions. Materials 2015, 8, 2635–2649. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Ningshen, S.; Sakairi, M.; Suzuki, K.; Ukai, S. The passive film characterization and anodic polarization behavior of 11% Cr ferritic/martensitic and 15% Cr oxide dispersion strengthened steels in different electrolytic solutions. Appl. Surf. Sci. 2013, 274, 345–355. [Google Scholar] [CrossRef]
- Aronniemi, M.; Sainio, J.; Lahtinen, J. Chemical state quantification of iron and chromium oxides using XPS: The effect of the background subtraction method. Surf. Sci. 2005, 578, 108–123. [Google Scholar] [CrossRef]
- Cao, X.Y.; Ding, X.F.; Lu, Y.H.; Zhu, P.; Shoji, T. Influences of Cr content and PWHT on microstructure and oxidation behavior of stainless steel weld overlay cladding materials in high temperature water. J. Nucl. Mater. 2015, 467, 32–41. [Google Scholar] [CrossRef]
- Sun, J.; Sun, C.; Lin, X.; Cheng, X.; Liu, H. Effect of chromium on corrosion behavior of P110 steels in CO2-H2S environment with high pressure and high temperature. Materials 2016, 9, 200. [Google Scholar] [CrossRef]
- Costa, D.; Marcus, P. Electronic core levels of hydroxyls at the surface of chromia related to their XPS O 1s signature: A DFT+U study. Surf. Sci. 2010, 604, 932–938. [Google Scholar] [CrossRef]
- Melo-Máximo, L.; Salas, O.; Melo-Máximo, D.; Oseguera, J.; López-Hirata, V.M.; Torres, R.D.; Lepienski, C.M.; de Souza, R.M. Performance of Cr oxide coatings on 304 steel against metal dusting. Surf. Coat. Technol. 2013, 237, 39–50. [Google Scholar] [CrossRef]







| Materials | Composition (wt.%) | Dimension (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | P | S | Cr | Mo | Fe | OD 1 | ID 2 | |
| Tube | 0.10 | 0.27 | 0.45 | 0.12 | 0.04 | 2.12 | 0.96 | Bal. | 38.1 | 34.6 |
| Pipe | 0.15 | 0.49 | 0.61 | 0.00 | 0.00 | 0.07 | 0.22 | Bal. | 219.1 | 207.1 |
| Filler rod | 0.08 | 0.56 | 0.66 | 0.01 | 0.00 | 2.42 | 0.97 | Bal. | 2.4 | - |
| Zone | Composition (wt.%) | Cr:Fe | ||
|---|---|---|---|---|
| Fe | Cr | Mo | ||
| A | 97.01 | 1.77 | 1.22 | 0.0182 |
| B | 96.95 | 1.90 | 1.15 | 0.0196 |
| C | 96.11 | 2.43 | 1.46 | 0.0253 |
| D | 97.17 | 2.01 | 0.82 | 0.0207 |
| E | 99.99 | 0.01 | 0.00 | 0.0001 |
| Zone | βa (V/dec) | βc (V/dec) | Ecorr (V) | icorr (µA/cm²) | RC (mm/Year) |
|---|---|---|---|---|---|
| A | 0.0666 | −0.2406 | −0.389 | 9.35 | 0.1069 |
| B | 0.0806 | −0.2654 | −0.377 | 7.31 | 0.0835 |
| C | 0.2741 | −0.0828 | −0.192 | 0.22 | 0.0024 |
| D | 0.1182 | −0.2212 | −0.393 | 7.44 | 0.0851 |
| E | 0.1091 | −0.1861 | −0.627 | 11.66 | 0.1353 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thong-On, A.; Boonruang, C. Design of Boiler Welding for Improvement of Lifetime and Cost Control. Materials 2016, 9, 891. https://doi.org/10.3390/ma9110891
Thong-On A, Boonruang C. Design of Boiler Welding for Improvement of Lifetime and Cost Control. Materials. 2016; 9(11):891. https://doi.org/10.3390/ma9110891
Chicago/Turabian StyleThong-On, Atcharawadi, and Chatdanai Boonruang. 2016. "Design of Boiler Welding for Improvement of Lifetime and Cost Control" Materials 9, no. 11: 891. https://doi.org/10.3390/ma9110891





