A Study on the Susceptibility to SCC of 7050 Aluminum Alloy by DCB Specimens
Abstract
:1. Introduction
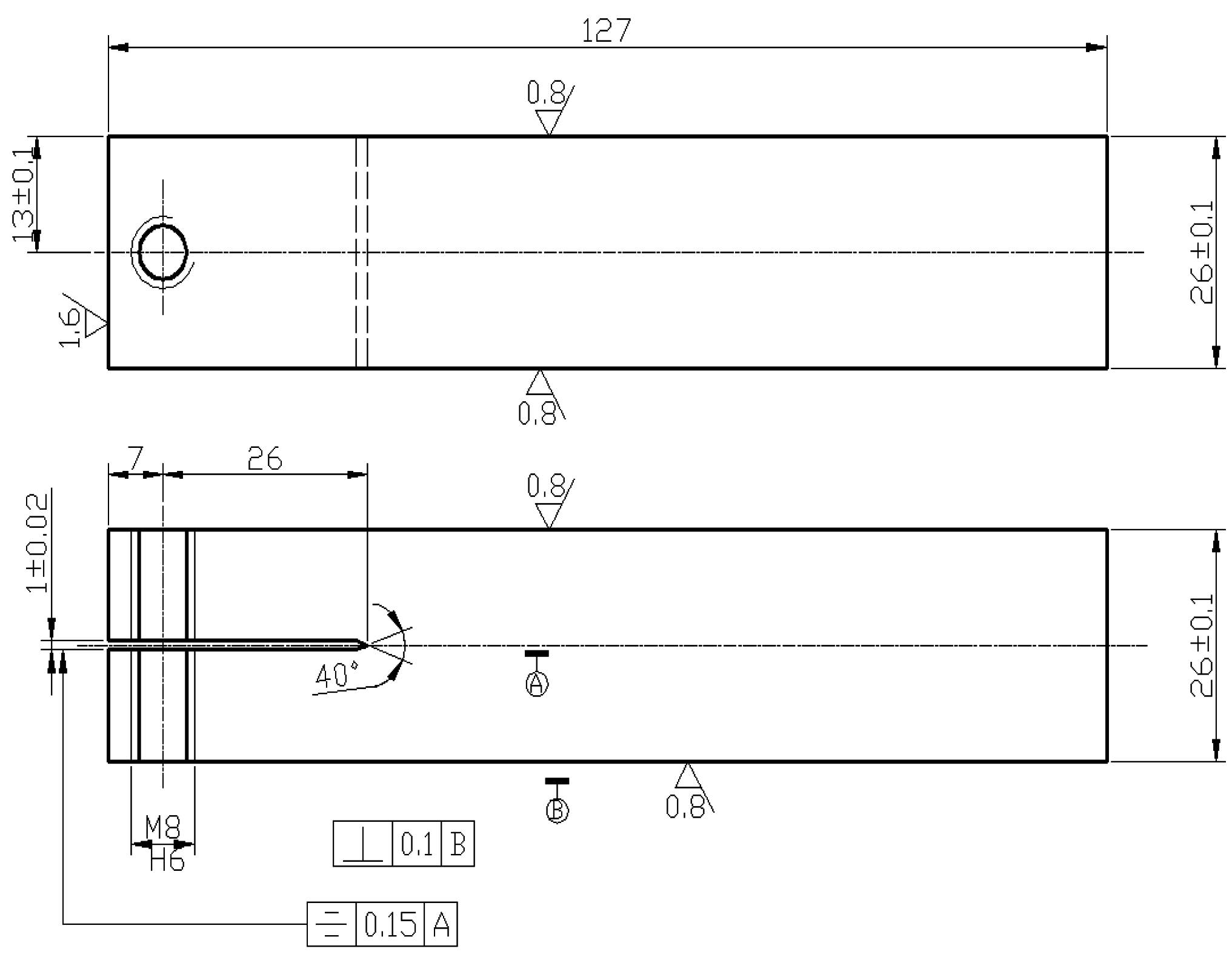
2. Experiments
3. Results and Discussion
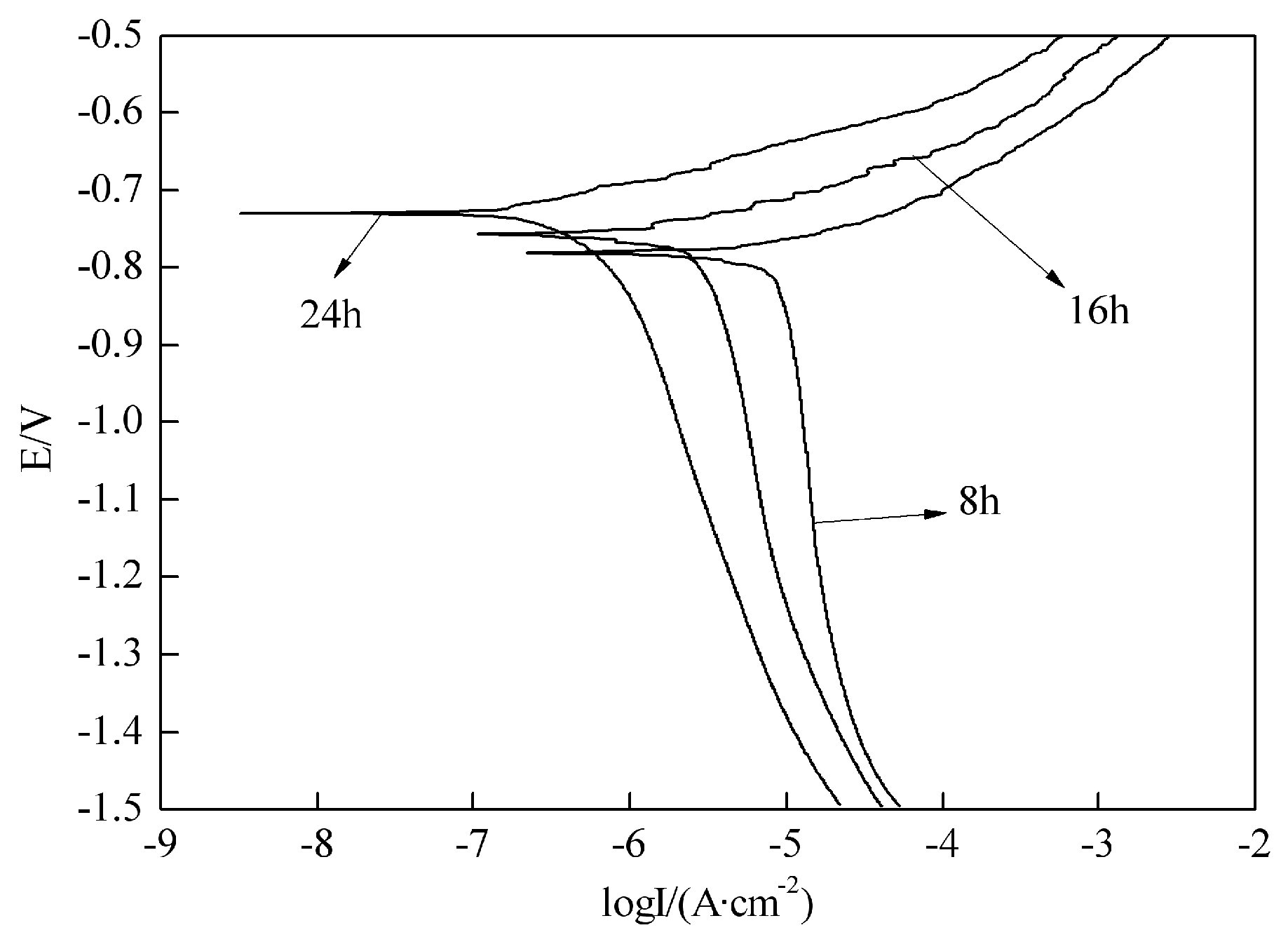
3.1. Polarization Curves of Different Aged Aluminum Alloy
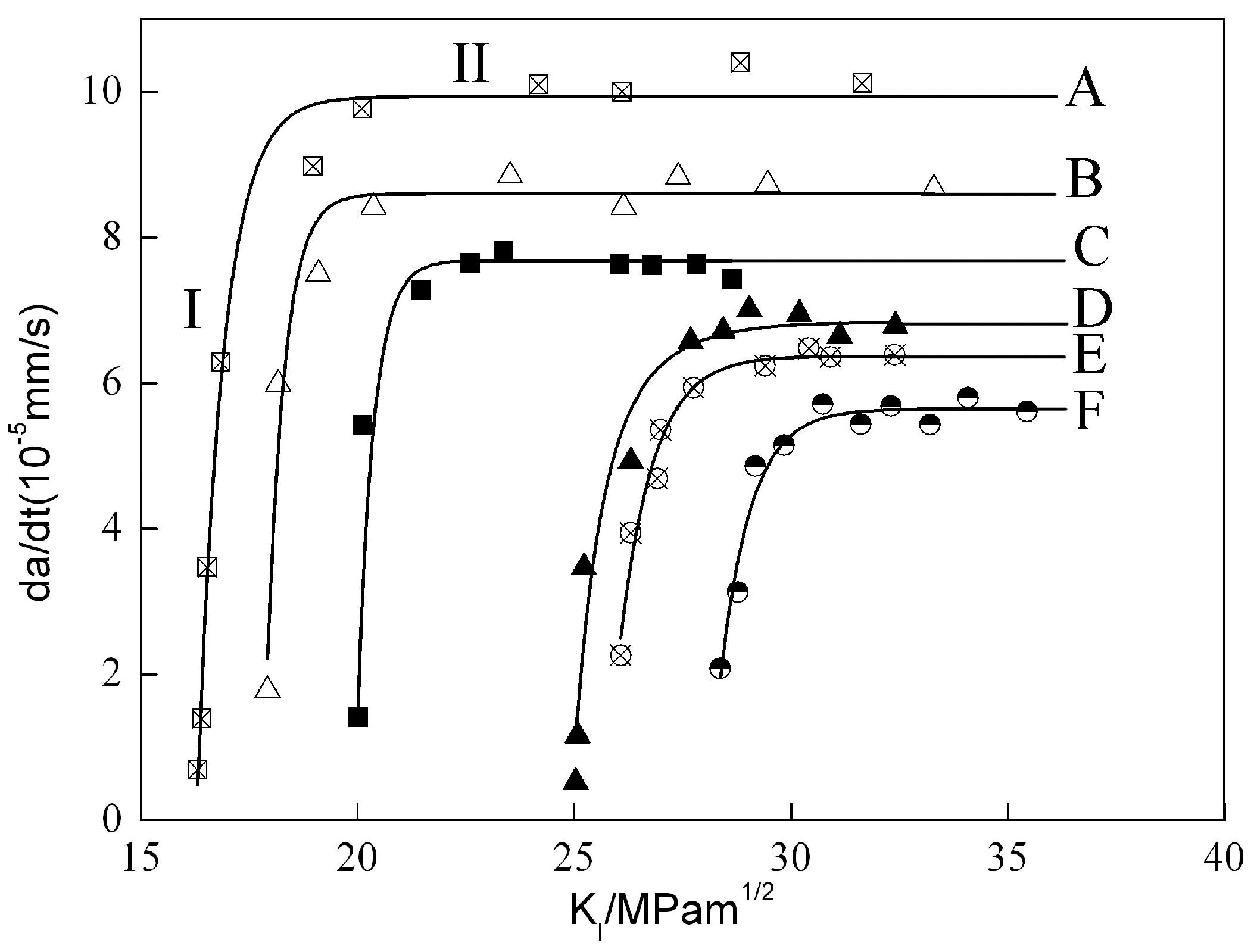
3.2. Effect of Aging and Cathodic Polarization on Susceptibility to SCC
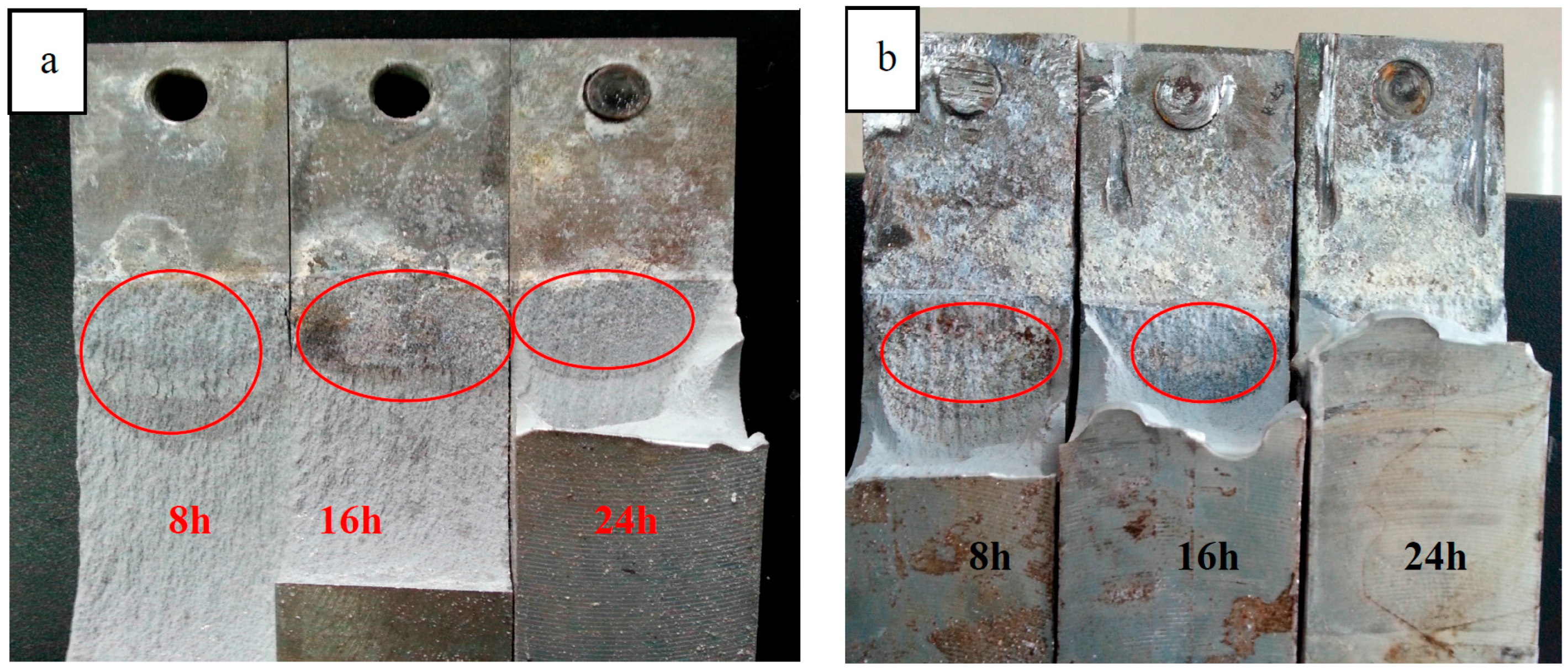
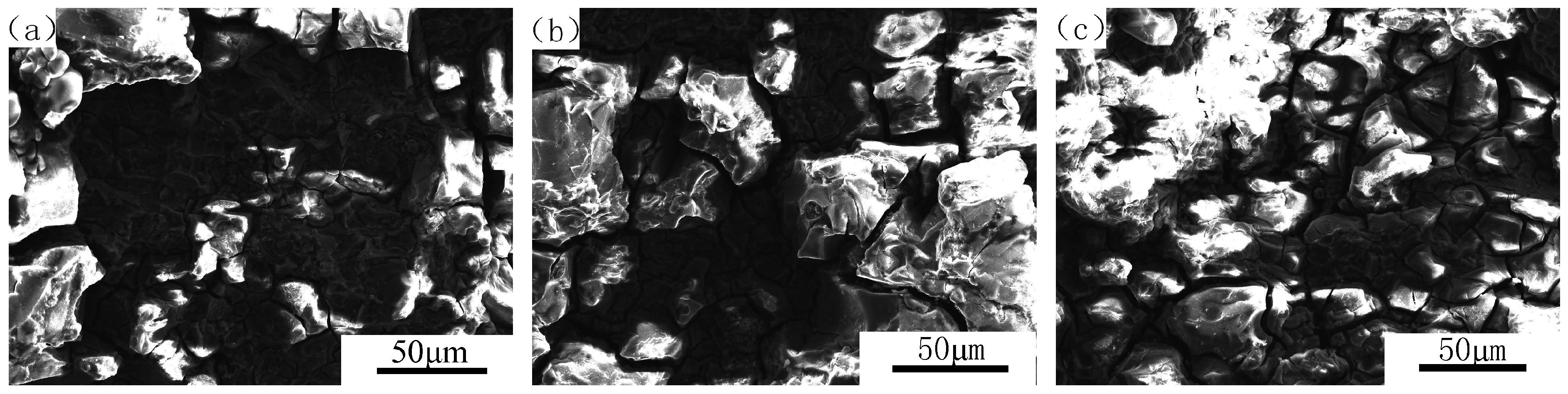
3.3. Effect of Aging States and Cathodic Polarization on SCC Fracture Morphology
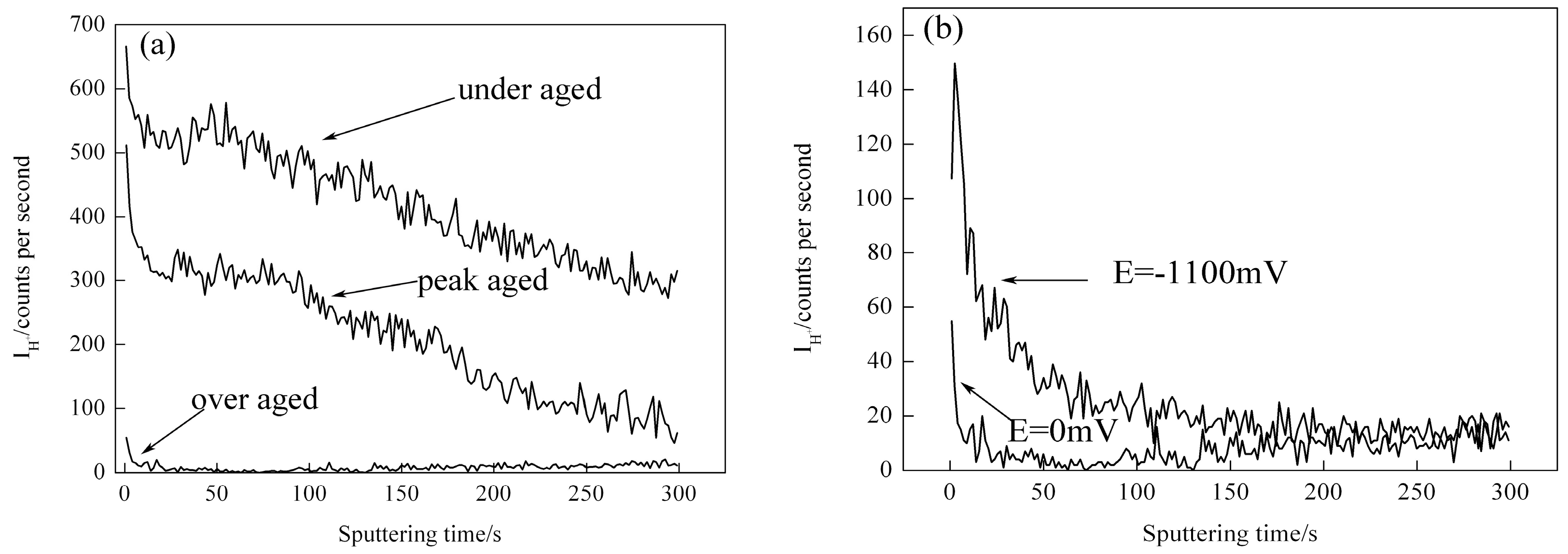
3.4. Effect of Aging and Cathodic Polarization on Hydrogen Distribution near Crack Tips
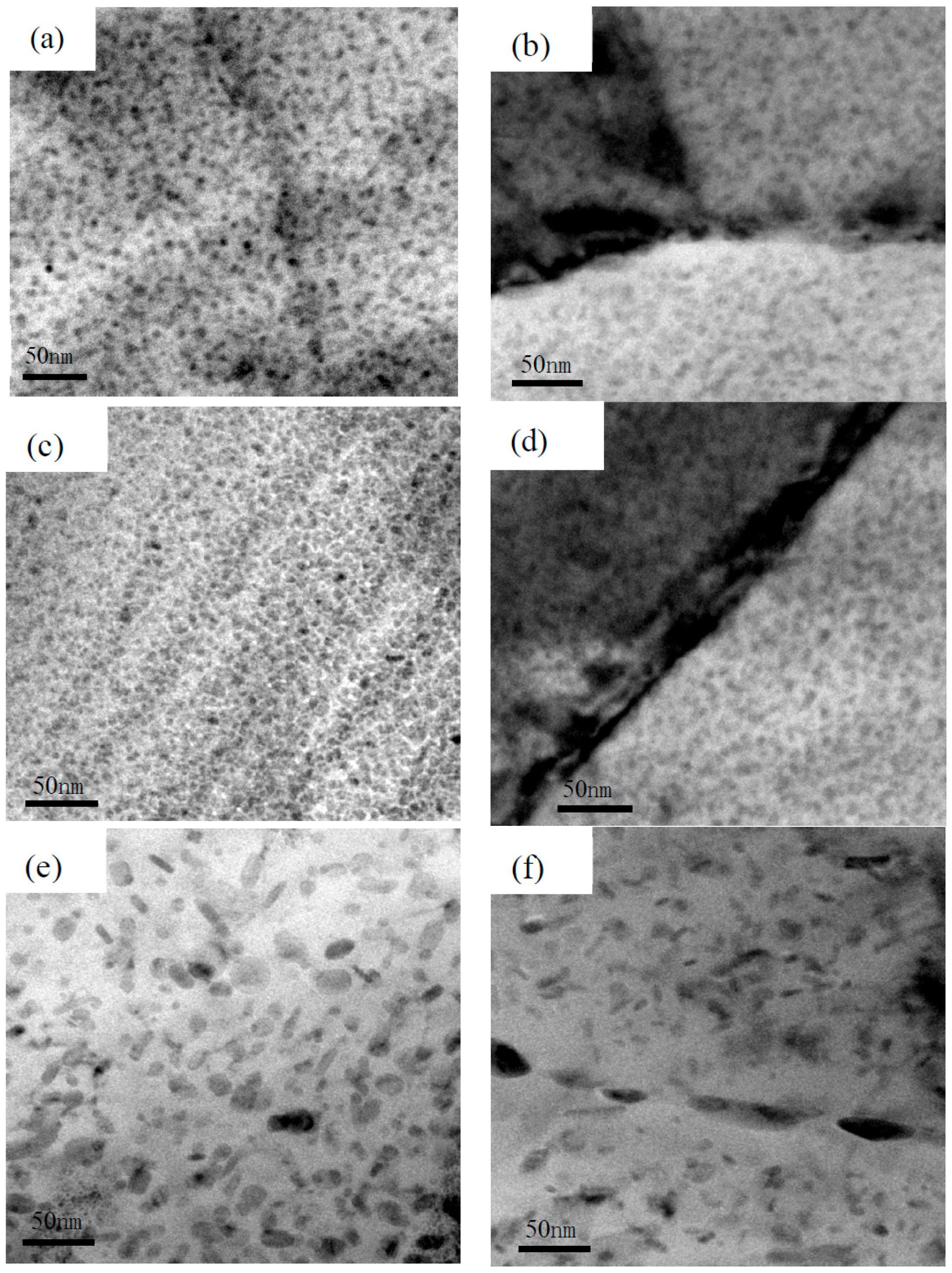
3.5. TEM Observations
4. Conclusions
- (1)
- The susceptibility to stress corrosion cracking of 7050 aluminum alloy decreased while the critical stress intensity factor KISCC increased with increasing the aging time. When applied a cathodic polarization potential of −1100 mV, the KISCC decreased significantly and aluminum alloy was much easier to be corroded.
- (2)
- The hydrogen concentration near crack tips of fracture zone enhanced when applied a cathodic polarization potential of −1100 mV and the crack propagation moved deeper towards grain interior. In addition, the diffusion depth hydrogen into 7050 aluminum alloy matrix reduced with increasing the aging time and the hydrogen enrichment also gradually diminished.
- (3)
- The stress corrosion cracking behavior of 7050 aluminum alloy was correlated with precipitated phases, i.e., the better stress corrosion resistance is due to small particle size and discontinuous grain boundary precipitated phases as well as wide precipitate-free zone (PFZ).
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheng, C.B.; Yan, B.H.; Zhang, K. Electrochemical investigation of hydrogen permeation behavior of 7075 t6 al alloy and its implication on stress corrosion cracking. Int. J. Min. Metall. Mater. 2015, 22, 729–737. [Google Scholar] [CrossRef]
- Fridlyander, I.N. Russian aluminum alloys for aerospace and transport application. Mater. Sci. Forum 2000, 331, 921–926. [Google Scholar] [CrossRef]
- Imanura, T. Current status and trend of applicable material technology for aerospace structure. J. Jpn. Light Met. 1999, 49, 302–309. [Google Scholar] [CrossRef]
- Li, Q.; Xi, M.; Wu, C. The role of hydrogen in stress-corrosion cracking of austenitic stainless steel in hot MgCl2 solution. Metall. Mater. Trans. A 1995, 26, 1777–1784. [Google Scholar]
- Amoush, E.; Saleh, A. Investigation of corrosion behaviour of hydrogenated 7075-T6 aluminum alloy. J. Alloys Compd. 2007, 443, 171–177. [Google Scholar] [CrossRef]
- Liao, H.L.; Lin, J.C.; Lee, S.L. Effect of pre-immersion on the SCC of heat-treated AA7050 in an alkaline 3.5%NaCl. Corros. Sci. 2009, 51, 209–216. [Google Scholar] [CrossRef]
- Qi, W.J.; Song, R.G.; Qi, X.; Li, H.; Wang, C.; Jin, J.R. Hydrogen embrettlement susceptibility and hydrogen induced additive stress of 7050 aluminum alloy under various aging states. J. Mater. Eng. Perform. 2015, 24, 3343–3355. [Google Scholar] [CrossRef]
- Sun, X.Y.; Zhang, B.; Lin, H.Q.; Zhou, Y.; Sun, L.; Wang, J.Q.; Han, E.H.; Ke, W. Correlations between stress corrosion cracking susceptibility and grain boundary microstructures for an Al-Zn-Mg alloy. Corros. Sci. 2013, 77, 103–112. [Google Scholar] [CrossRef]
- Shahriari, A.; Shahrabi, T.; Oskuie, A.A. Effect of cathodic potential, bicarbonate, and chloride ions on SCC of X70 pipeline steel. J. Mater. Eng. Perform. 2013, 22, 1421–1429. [Google Scholar] [CrossRef]
- Liu, M.; Schmutz, P.; Zanna, S.; Seyeux, A.; Ardelean, H.; Song, G.; Atrens, A. Electrochemical reactivity, surface composition and corrosion mechanisms of the complex metallic alloy Al3Mg2. Corros. Sci. 2010, 52, 562–578. [Google Scholar] [CrossRef]
- Oliveira, A.F.; Barros, M.C.; Cardoso, K.R.; Travessa, D.N. The effect of RRA on the strength and SCC resistance on AA7050 and AA7150 aluminium alloys. Mater. Sci. Eng. A 2004, 379, 321–326. [Google Scholar] [CrossRef]
- Qi, X.; Song, R.G.; Qi, W.J.; Jin, J.R.; Wang, C.; Li, H. Effects of polarisation on mechanical properties and stress corrosion cracking susceptibility of 7050 aluminum alloy. Corros. Eng. Sci. Technol. 2014, 49, 643–650. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Clark, D.A.; Johonson, W.S. Temperatures effects on fatigue performance of cold expanded holes in 7050-T7451 aluminum alloy. Int. J. Fatigue 2003, 25, 159–165. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, J.L. Effect of hydrogen on disorder of passive films and pitting susceptibility of type 310 stainless steel. J. Electrochem. Soc. 2001, 148, 29–35. [Google Scholar] [CrossRef]
- Marion, C.F.; Nicolas, N.; Severine, B.; Michel, T. Characterization by TEM and ToF-SIMS of the oxide layer formed during anaphoretic paint electrodeposition on Al-alloys. Appl. Surf. Sci. 2013, 277, 186–191. [Google Scholar]
- Li, H.; Mao, Q.Z.; Wang, Z.X.; Miao, F.F.; Fang, B.J.; Song, R.G.; Zheng, Z.Q. Simultaneously enhancing the tensile properties and intergranular corrosion resistance of Al-Mg-Si-Cu alloys by a thermo-mechanical treatment. Mater. Sci. Eng. A 2014, 617, 165–174. [Google Scholar] [CrossRef]
- Song, R.G.; Dietzel, W.; Zhang, B.J.; Liu, W.J.; Tseng, M.K.; Atrens, A. Stress corrosion and hydrogen embrittlement of an Al-Zn-Mg-Cu alloy. Acta Mater. 2004, 52, 4727–4743. [Google Scholar] [CrossRef]

| Element | Zn | Mg | Cu | Zr | Ti | Mn | Cr | Fe | Si | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| wt % | 6.42 | 2.25 | 2.02 | 0.13 | 0.03 | 0.10 | 0.04 | 0.11 | 0.07 | Balance |
| Aging State | Polarization Potential (mV) | Plateau Velocity/mm·s−1 | KISCC/MPa·m1/2 |
|---|---|---|---|
| Under aged (8 h) | / | 7.64 × 10−5 | 20.02 |
| Under aged (8 h) | −1100 | 9.97 × 10−5 | 16.32 |
| Peak aged (16 h) | / | 6.65 × 10−5 | 25.04 |
| Peak aged (16 h) | 1100 | 8.67 × 10−5 | 17.93 |
| Over aged (24 h) | / | 5.61 × 10−5 | 28.38 |
| Over aged (24 h) | 1100 | 6.36 × 10−5 | 26.01 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, X.; Jin, J.; Dai, C.; Qi, W.; He, W.; Song, R. A Study on the Susceptibility to SCC of 7050 Aluminum Alloy by DCB Specimens. Materials 2016, 9, 884. https://doi.org/10.3390/ma9110884
Qi X, Jin J, Dai C, Qi W, He W, Song R. A Study on the Susceptibility to SCC of 7050 Aluminum Alloy by DCB Specimens. Materials. 2016; 9(11):884. https://doi.org/10.3390/ma9110884
Chicago/Turabian StyleQi, Xing, Jirong Jin, Chunli Dai, Wenjuan Qi, Wangzhao He, and Renguo Song. 2016. "A Study on the Susceptibility to SCC of 7050 Aluminum Alloy by DCB Specimens" Materials 9, no. 11: 884. https://doi.org/10.3390/ma9110884






