Multi-Layered TiO2 Films towards Enhancement of Escherichia coli Inactivation
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Film Preparation and Characterization
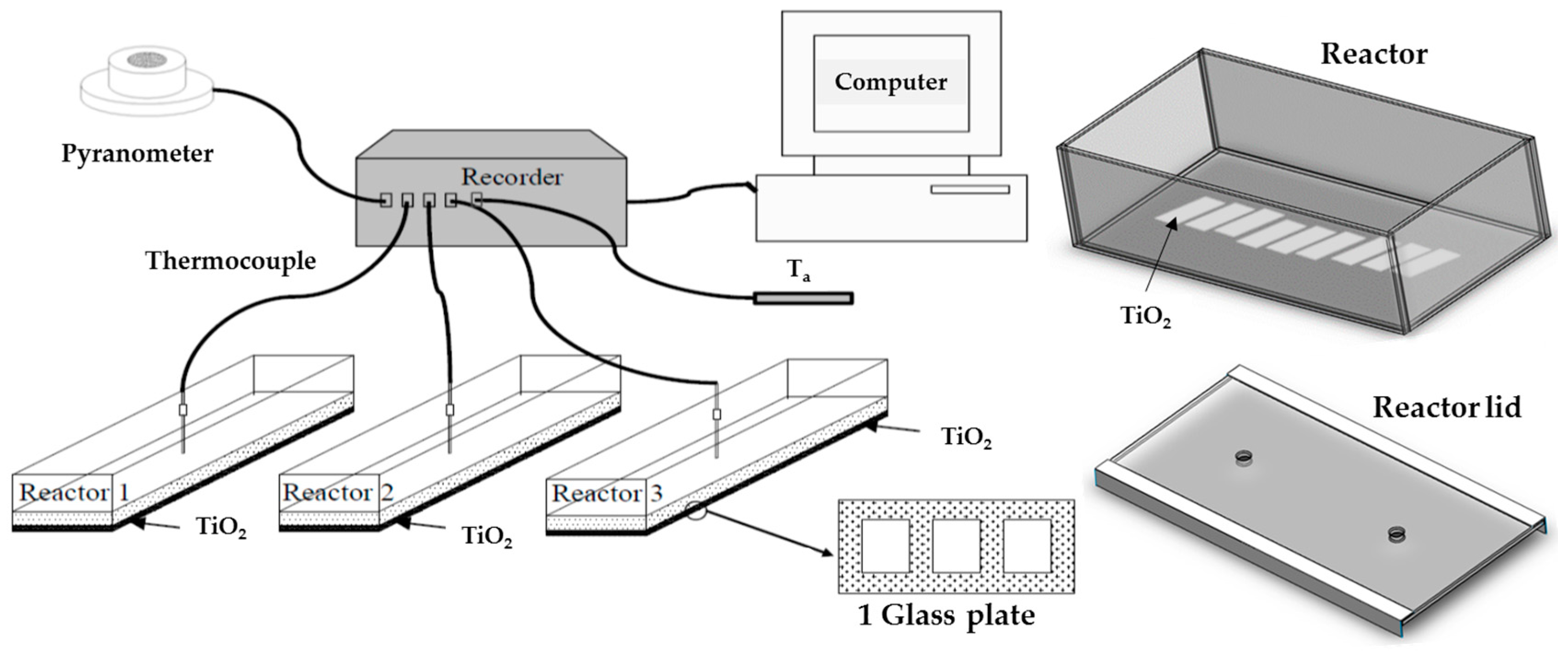
3.2. Inactivation Reactor
3.3. Unit Testing
3.4. Sample Collection
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mcloughlin, O.A.; Fernandez-Ibanez, P.; Gernjak, W.; Rodriguez, S.M.; Gill, L.W. Photocatalytic disinfection of water using low cost compound parabolic collector. Sol. Energy 2004, 77, 625–633. [Google Scholar] [CrossRef]
- Gomes, A.I.; Santos, J.C.; Vilar, V.J.P.; Boaventura, R.A.R. Inactivation of bacteria E. coli and photodegradation of humic acids using natural sunlight. Appl. Catal. B 2009, 88, 283–291. [Google Scholar] [CrossRef]
- Lonnen, J.; Kilvington, S.; Kehoe, S.C.; Al-Touati, F.; Mcguigan, K.G. Solar and photocatalytic disinfection of protozoan, fungal and bacterial microbes in drinking water. Water Res. 2005, 39, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.R.; Gomes, F.C.O.; Fonseca, M.P.P.; Parreira, J.S.; Santos, V.P. Efficiency of PET reactors in solar water disinfection for use in southeastern Brazil. Sol. Energy 2013, 87, 158–167. [Google Scholar] [CrossRef]
- Lopez, M.I.P.; Fernandez-Ibanez, P.; Ubomba-Jaswa, E.; Navntoft, C.; Garcia-Fernandez, I.; Dunlop, P.S.M.; Schmid, M.; Byrne, J.A.; Mcguigan, K.G. Elimination of water pathogens with solar radiation using an automated sequential batch CPC reactor. J. Hazard. Mater. 2011, 196, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Conroy, R.M.; Elmore-Meegan, M.; Joyce, T.; McGuigan, K.G.; Barnes, J. Solar disinfection of drinking water and diarrhoea in Maasai children: A controlled field trial. Lancet 1996, 348, 1695–1697. [Google Scholar] [CrossRef]
- McGuigan, K.G.; Joyce, T.N.; Conroy, R.M. Solar disinfection: Use of sunlight to decontaminate drinking water in developing countries. J. Med. Microbiol. 1999, 48, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Al Momani, F.A.; Shawaqfeh, A.T.; Shawaqfeh, M.A.S. Solar wastewater treatment plant for aqueous solution of pesticide. Sol. Energy 2007, 81, 1213–1218. [Google Scholar] [CrossRef]
- Jung, J.; Kim, J.O.; Choi, J.Y. Effect of various oxidants in a photocataysis/filtration system for the treatment of contaminants. Res. Chem. Intermed. 2009, 35, 243–248. [Google Scholar] [CrossRef]
- Paleologou, A.; Marakas, H.; Xekoukoulotakis, N.P.; Moya, A.; Vergara, Y.; Kalogerakis, N.; Gikas, P.; Mantzavinos, D. Disinfection of water and wastewater by TiO2 Photocatalysis, sonolysis and UV-C irradiation. Catal. Today 2007, 129, 136–142. [Google Scholar] [CrossRef]
- Jain, A.; Vaya, D.; Sharma, V.K.; Ameta, S.C. Photo-fenton degradation of phenol red catalyzed by inorganic additives: A technique for wastewater treatment. Kinet. Catal. 2011, 52, 40–47. [Google Scholar] [CrossRef]
- McCullagh, C.; Robertson, J.M.C.; Bahnemann, D.W.; Robertson, P.K.J. The application of TiO2 photocatalysis for disinfection of water contaminated with pathogenic micro-organisms: A review. Res. Chem. Intermed. 2007, 33, 359–375. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Kozlova, E.A.; Besove, A.S.; Kozlov, D.V.; Kiselev, S.A.; Safatov, A.S. Photocatalysis: Light energy conversion for the oxidation, disinfection, and decomposition of water. Kinet. Catal. 2010, 51, 801–808. [Google Scholar] [CrossRef]
- Elfeky, S.A.; Al-Sherbini, A.S.A. Photocatalytic decomposition of trypan blue over nanocomposite thin films. Kinet. Catal. 2011, 52, 391–396. [Google Scholar] [CrossRef]
- Nesic, J.; Rtimi, S.; Laub, D.; Roglic, G.M.; Pulgarin, C.; Kiwi, J. New evidence for TiO2 uniform surfaces leading to complete bacterial reduction in the dark: Critical issues. Coll. Surf. B Biointerfaces 2014, 123, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Benabbou, A.K.; Derriche, Z.; Felix, C.; Lejeune, P.; Guillard, C. Photocatalytic inactivation of Escherichia coli-effect of concentration of TiO2 and microorganism, nature, and intensity of UV irradiation. Appl. Catal. B 2007, 76, 257–263. [Google Scholar] [CrossRef]
- Malato, S.; Fernandez-Ibanez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Dexontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Robertson, J.M.C.; Robertson, P.K.J.; Lawton, L.A. A comparison of the effectiveness of TiO2 photocatalysis and UVA photolysis for the destruction of three pathogenic micro-organisms. J. Photochem. Photobiol. A Chem. 2005, 175, 51–56. [Google Scholar] [CrossRef]
- Sichel, C.; Cara, M.D.; Tello, J.; Blanco, J.; Fernandez-Ibanez, P. Solar photocatalytic disinfection of agricultural pathogenic fungi: Fusarium species. Appl. Catal. B Environ. 2007, 74, 152–160. [Google Scholar] [CrossRef]
- Dunlop, P.S.M.; Ciavola, M.; Rizzo, L.; Byrne, J.A. Inactivation and injury assessment of Eschericia coli during solar and photocatalytic disinfection in LDPE bags. Chemosphere 2011, 85, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Rincon, A.G.; Pulgarin, C. Photocatalytical inactivation of E. coli: Effect of (continuous-intermittent) light intensity and of (suspended-fixed) TiO2 concentration. Appl. Catal. B Environ. 2003, 44, 263–284. [Google Scholar] [CrossRef]
- Grieken, R.V.; Marugan, J.; Pablos, C.; Furones, L.; Lopez, A. Comparison between the photocatalytic inactivation of Gram-positive E. faecalis and Gram-negative E. coli faecal contamination indicator microorganism. Appl. Catal. B Environ. 2010, 100, 212–220. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Nikazara, M.; Gholivand, K.; Mahanpoor, K. Using TiO2 supported on clinoptilolite as a catalyst for photocatalytic degradation of azo dye disperse yellow 23 in water. Kinet. Catal. 2007, 48, 214–220. [Google Scholar] [CrossRef]
- Fernandez, P.; Blanco, J.; Sichel, C.; Malato, S. Water disinfection by solar photocatalysis using compound parabolic collectors. Catal. Today 2005, 101, 345–352. [Google Scholar] [CrossRef]
- McLoughlin, O.A.; Kehoe, S.C.; McGuigan, K.G.; Duffy, E.F.; Touati, F.A.; Gernja, W.; Alberola, I.O.; Rodriguez, S.M.; Gill, L.W. Solar disinfection of contaminated water: A comparison of three small-scale reactors. Sol. Energy 2004, 77, 657–664. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Desai, V.S.; Kowshik, M. Antimicrobial activity of titanium dioxide nanoparticles synthesized by sol-gel technique. Res. J. Microbiol. 2009, 4, 97–103. [Google Scholar]
- Chung, C.J.; Lin, H.I.; Chou, C.M.; Hsieh, P.Y.; Hsiao, C.H.; Shi, Z.Y.; He, J.L. Inactivation of Staphylococcus aureus and Escherichia coli under various light sources on photocatalytic titanium dioxide thin film. Surf. Coat. Technol. 2009, 203, 1081–1085. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Li, H.; Wang, S.; Wei, Y. Preparation of anatase of TiO2 nanoparticles with high thermal stability and specific surface area by alcohothermal method. Powder Technol. 2009, 194, 149–152. [Google Scholar] [CrossRef]
- Duffy, E.F.; Touati, F.A.; Kehoe, S.C.; McLoughlin, O.A.; Gill, L.W.; Gernjak, W.; Oller, I.; Maldonado, M.I.; Malato, S.; Cassidy, J.; et al. A novel TiO2-assisted solar photocatalytic batch-process disinfection reactor for the treatment of biological and chemical contaminants in domestic drinking water in developing countries. Sol. Energy 2004, 77, 649–655. [Google Scholar] [CrossRef]
- Rtimi, S.; Sanjines, R.; Andrzejczuk, M.; Pulagrin, C.; Kulik, A.; Kiwi, J. Innovative transparent non-scattering TiO2 bactericide thin films inducing increased E. coli wall fluidity. Surf. Coat. Technol. 2014, 254, 333–343. [Google Scholar] [CrossRef]
- Rtimi, S.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Kinetics and mechanism for transparent polyethylene-TiO2 films mediated self-cleaning leading to MB dye discoloration under sunlight irradiation. Appl. Catal. B Environ. 2015, 162, 236–244. [Google Scholar] [CrossRef]
- Ibrahim, A.; Lima, R.; Berndt, C.C.; Marple, B. Fatigue and mechanical properties of nanostructured and conventional titania (TiO2) thermal spray coatings. Surf. Coat. Technol. 2007, 201, 7589–7596. [Google Scholar] [CrossRef]
- Nararom, M.; Thepa, S.; Kongkiattikajorn, J.; Songprakorp, R. The effect of bacterial disinfection by solar illumination and photocatalytic disinfection. Chem. Intermed. 2015, 41, 6543–6558. [Google Scholar] [CrossRef]
- Yasin, A.; Guo, F.; Demopoulos, G.P. Aqueous, screen-printable paste for fabrication of mesoporous composite anatase-rutile TiO2 nanoparticle thin films for (Photo)electrochemical devices. ACS Sustain. Chem. Eng. 2016, 4, 2173–2181. [Google Scholar] [CrossRef]
- Dhungel, S.K.; Park, J.G. Optimization of paste formulation for TiO2 nanoparticles with wide range of size distribution for its application in dye sensitized solar cells. Renew. Energy 2010, 35, 2776–2780. [Google Scholar] [CrossRef]
- Lee, K.M.; Suryanarayanan, V.; Ho, K.C. The influence of surface morphology of TiO2 coating on the performance of dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2006, 90, 2398–2404. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials; Wiley: New York, NY, USA, 1954. [Google Scholar]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Saito, T.; Iwase, T.; Horie, J.; Morioka, T. Mode of photocatalytic bactericidal action of powdered semiconductor TiO2 on mutans streptococci. J. Photochem. Photobiol. B 1992, 14, 369–379. [Google Scholar] [CrossRef]
- Watts, R.J.; Kong, S.; Orr, M.P.; Miller, G.C.; Henry, B.E. Photocatalytic inactivation of coliform bacteria and viruses in secondary wastewater effluent. Water Res. 1995, 29, 95–100. [Google Scholar] [CrossRef]
- Bock, C.; Dittmar, H.; Gemeinhardt, H.; Bauer, E.; Greulich, K.O. Comet assay detects cold repair of UV-A damages in human B-lymphoblast cell line. Mutat. Res. 1998, 408, 111–120. [Google Scholar] [CrossRef]
- McGuigan, K.G.; Joyce, T.M.; Conroy, R.M.; Gillespie, J.B.; Elmore-Meegan, M. Solar disinfection of drinking water contained in transparent plastic bottles: Characterizing the bacterial inactivation process. J. Appl. Microbiol. 1998, 84, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photolysis of chloroform and other organic molecules in aqueous TiO2 suspensions. Environ. Sci. Technol. 1991, 25, 494–500. [Google Scholar] [CrossRef]
- Gilbert, P.; Evans, D.J.; Evans, E.; Duguld, I.G.; Brown, M.R.W. Surface characteristics and adhesion of E. coli and S. epidermis. J. Appl. Bacteriol. 1991, 71, 72–77. [Google Scholar]
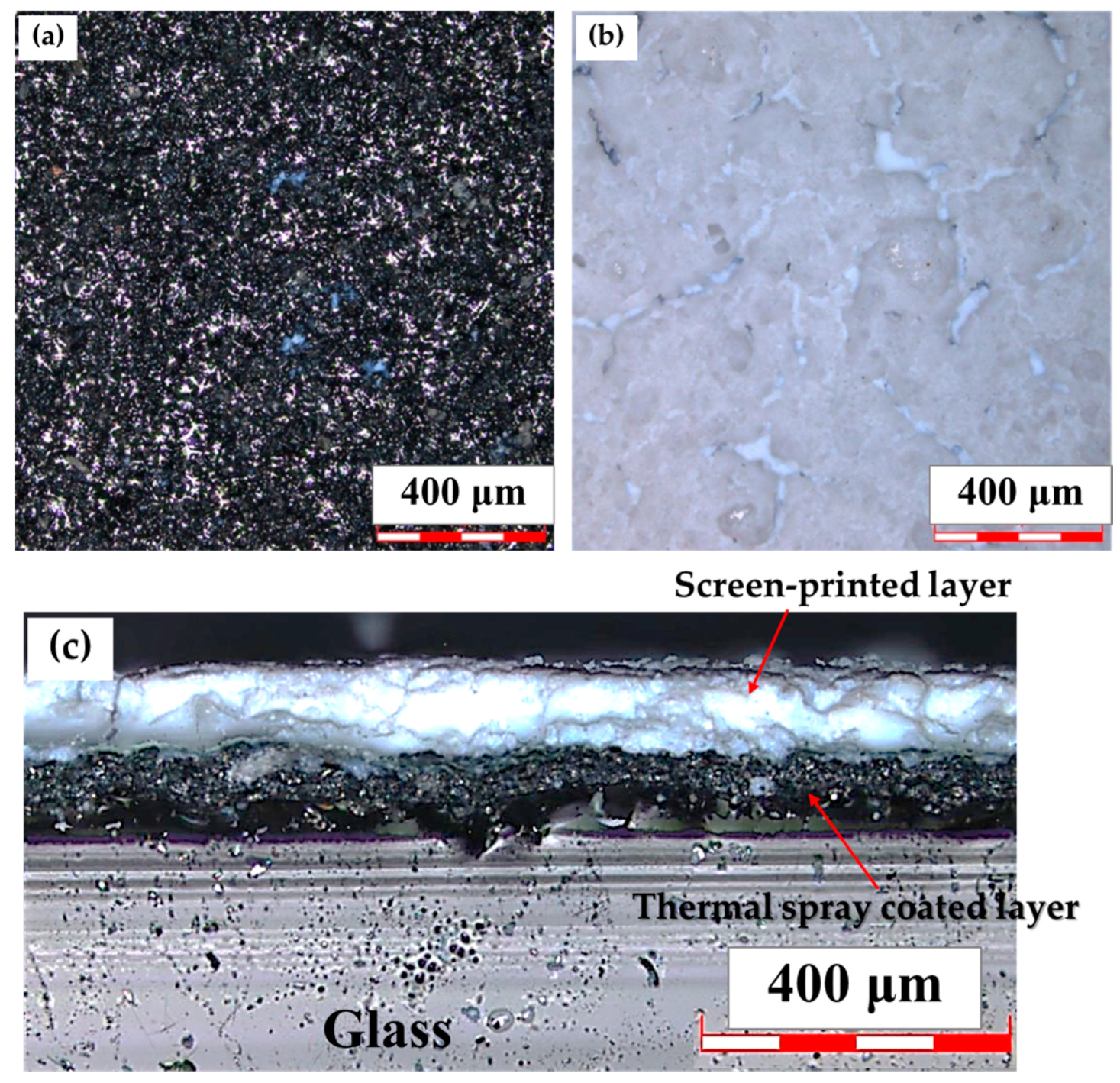

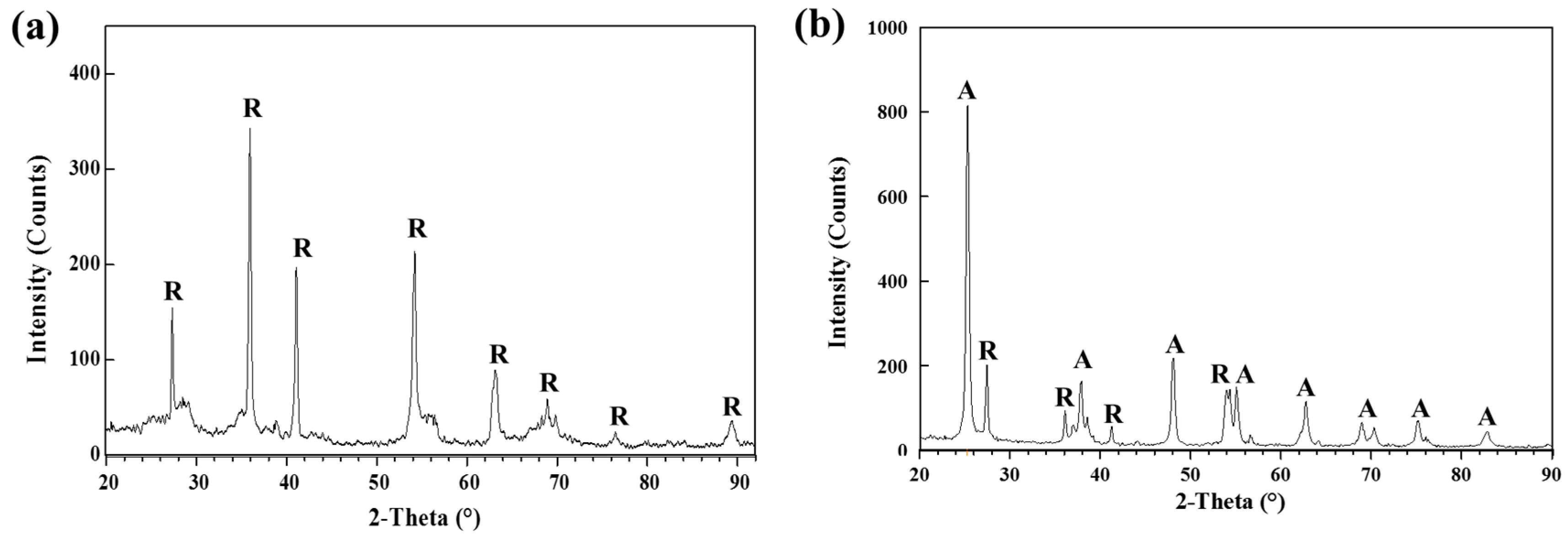

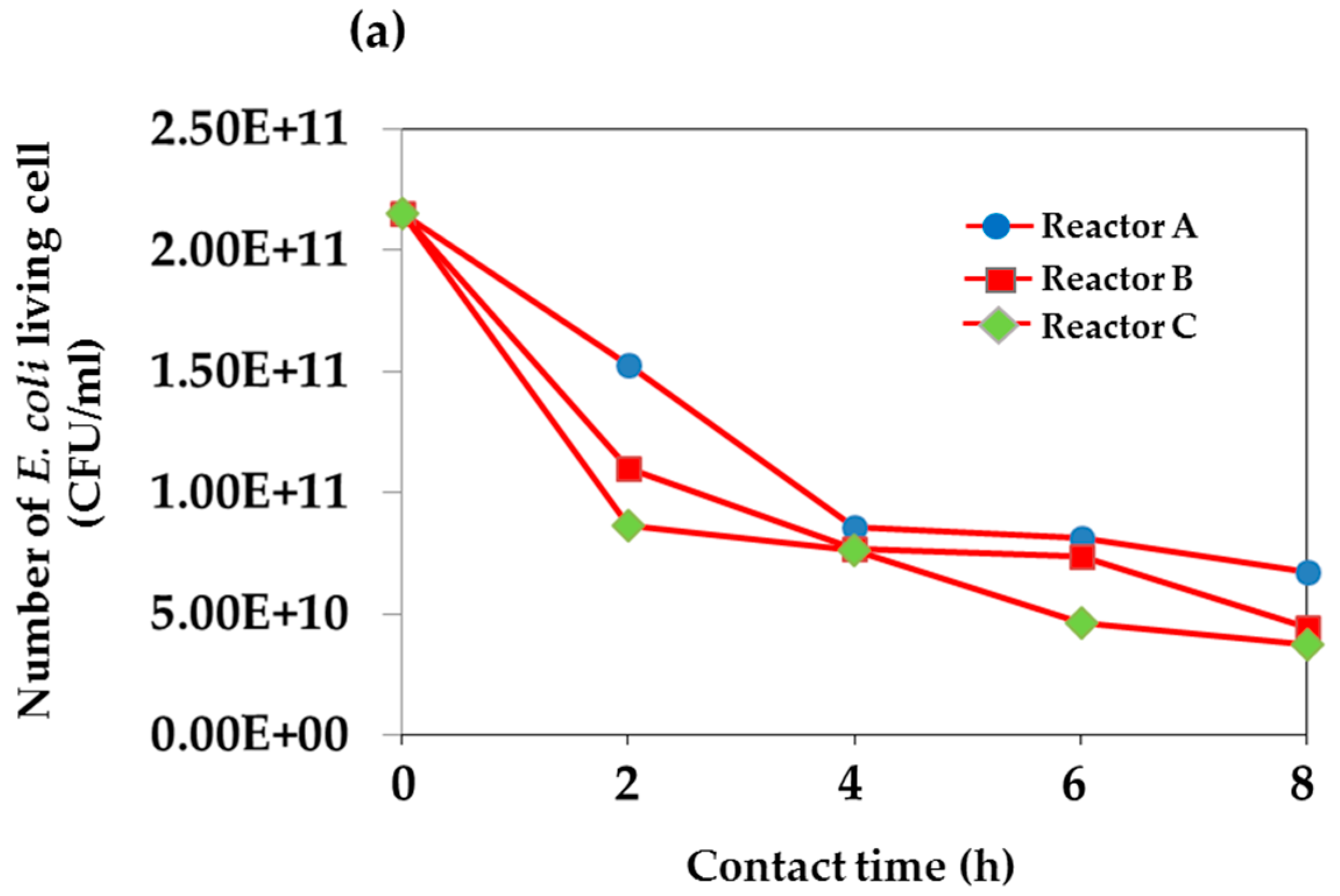
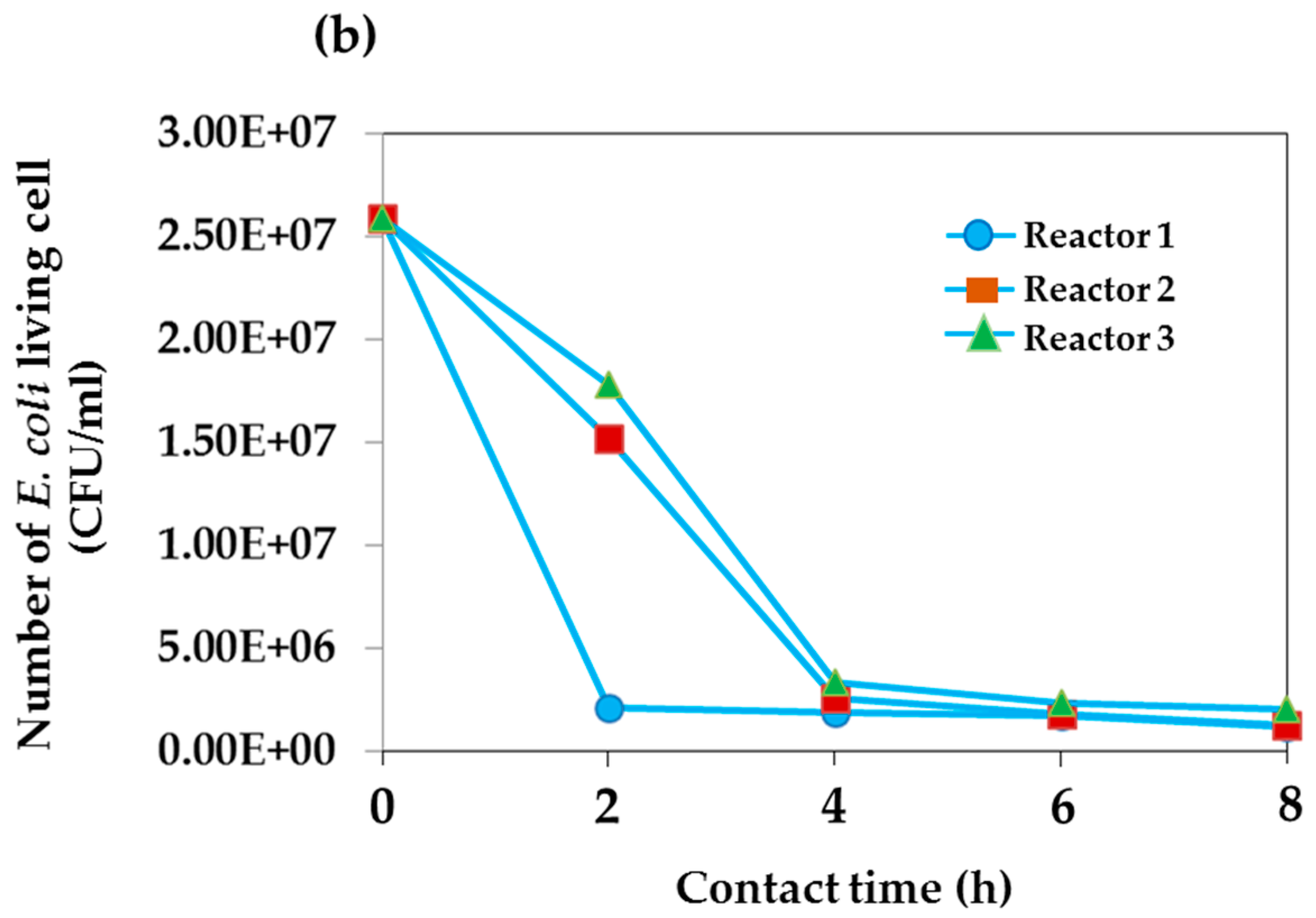
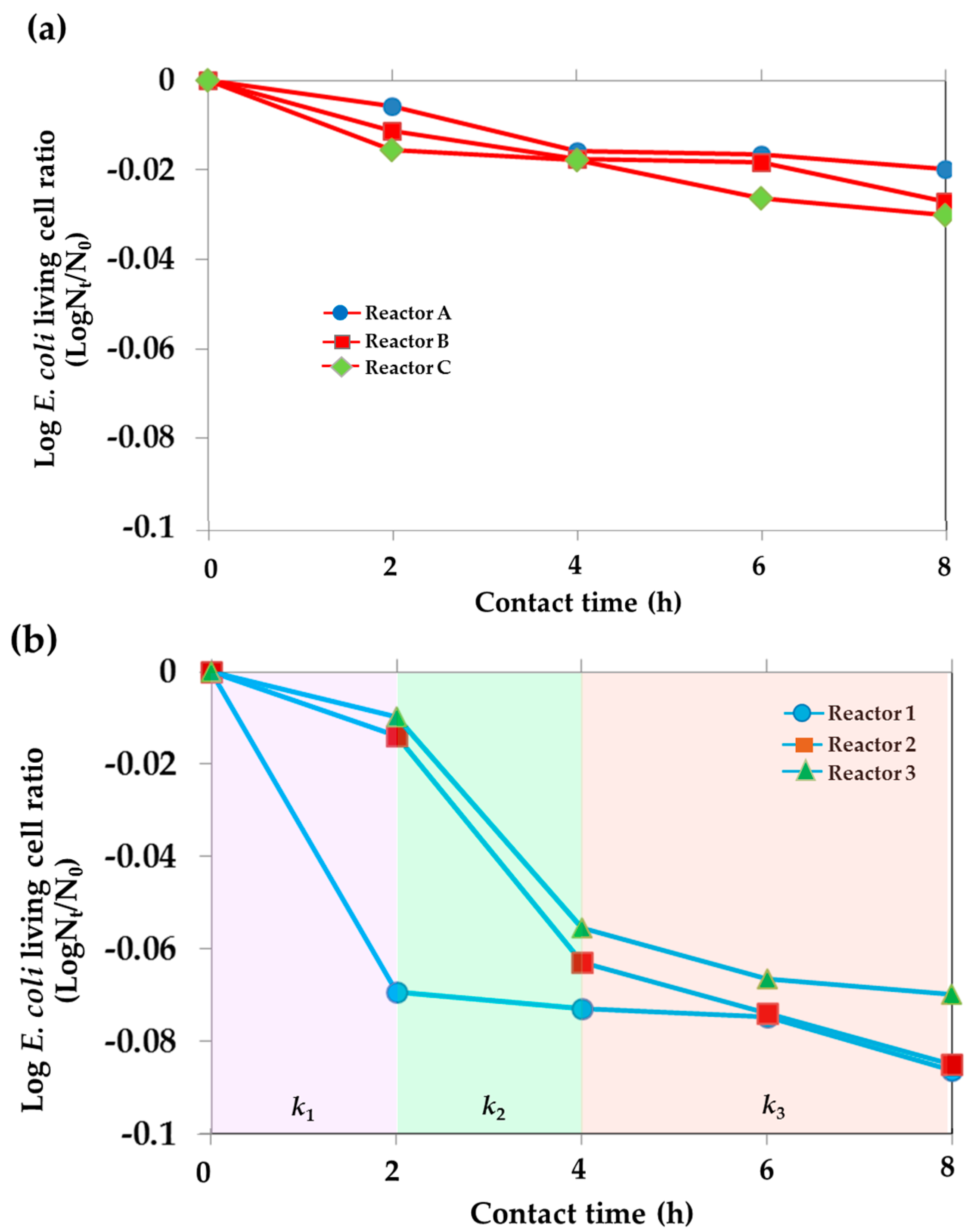
| Time | Total Number of Living E. coil (CFU/mL) | |||||
|---|---|---|---|---|---|---|
| Reactor A 1 (Water + E. coli + Sun) | Reactor B 1,† (Water + E. coli + TiO2 + Sun) | Reactor C 1,† (Water + E. coli + TiO2 + UV) | Reactor 1 † | Reactor 2 † | Reactor 3 † | |
| 8:30 | 2.15 × 1011 | 2.15 × 1011 | 2.15 × 1011 | 2.59 × 107 | 2.59 × 107 | 2.59 × 107 |
| 10:30 | 1.53 × 1011 | 1.10 × 1011 | 8.65 × 1010 | 2.10 × 106 | 1.52 × 107 | 1.78 × 107 |
| 12:30 | 8.55 × 1010 | 7.70 × 1010 | 7.60 × 1010 | 1.85 × 106 | 2.60 × 106 | 3.35 × 106 |
| 14:30 | 8.15 × 1010 | 7.40 × 1010 | 4.65 × 1010 | 1.75 × 106 | 1.80 × 106 | 2.30 × 106 |
| 16:30 | 6.75 × 1010 | 4.45 × 1010 | 3.75 × 1010 | 1.20 × 106 | 1.25 × 106 | 2.05 × 106 |
| Contact Time (h) | Percentage of Number of Living E. coli (%) | |||||
|---|---|---|---|---|---|---|
| Reactor A 1 (Water + E. coli + Sun) | Reactor B 1,† (Water + E. coli + TiO2 + Sun) | Reactor C 1,† (Water + E. coli + TiO2 + UV) | Reactor 1 † | Reactor 2 † | Reactor 3 † | |
| 0 | 100 | 100 | 100 | 100 | 100 | 100 |
| 2 | 71.2 | 51.2 | 40.2 | 8.1 | 58.7 | 68.7 |
| 4 | 39.8 | 35.8 | 35.4 | 7.1 | 10.0 | 12.9 |
| 6 | 37.9 | 34.4 | 21.6 | 6.8 | 6.9 | 8.9 |
| 8 | 31.4 | 20.7 | 17.4 | 4.6 | 4.8 | 7.9 |
| Condition | Rate Constant (k)[Contact Time Duration] | ||
|---|---|---|---|
| Reactor A (Water + E. coli + Sun) | 0.0050[2–8 h] | ||
| Reactor B (Water + E. coli + TiO2 + Sun) | 0.0061[2–8 h] | ||
| Reactor C (Water + E. coli + TiO2 + UV) | 0.0071[2–8 h] | ||
| k1[0–2 h] | k2[2–4 h] | k3[4–8 h] | |
| Reactor 1 (TiO2 film area:water volume = 1:2.62) | 0.0691 | 0.0053[2–8 h] | |
| Reactor 2 (TiO2 film area:water volume = 1:5.24) | 0.0138 | 0.0490 | 0.0110 |
| Reactor 3 (TiO2 film area:water volume = 1:7.86) | 0.0096 | 0.0458 | 0.0072 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoriya, S.; Chumphu, A.; Pookmanee, P.; Laithong, W.; Thepa, S.; Songprakorp, R. Multi-Layered TiO2 Films towards Enhancement of Escherichia coli Inactivation. Materials 2016, 9, 808. https://doi.org/10.3390/ma9100808
Yoriya S, Chumphu A, Pookmanee P, Laithong W, Thepa S, Songprakorp R. Multi-Layered TiO2 Films towards Enhancement of Escherichia coli Inactivation. Materials. 2016; 9(10):808. https://doi.org/10.3390/ma9100808
Chicago/Turabian StyleYoriya, Sorachon, Angkana Chumphu, Pusit Pookmanee, Wreerat Laithong, Sirichai Thepa, and Roongrojana Songprakorp. 2016. "Multi-Layered TiO2 Films towards Enhancement of Escherichia coli Inactivation" Materials 9, no. 10: 808. https://doi.org/10.3390/ma9100808





