Applications of Graphene-Modified Electrodes in Microbial Fuel Cells
Abstract
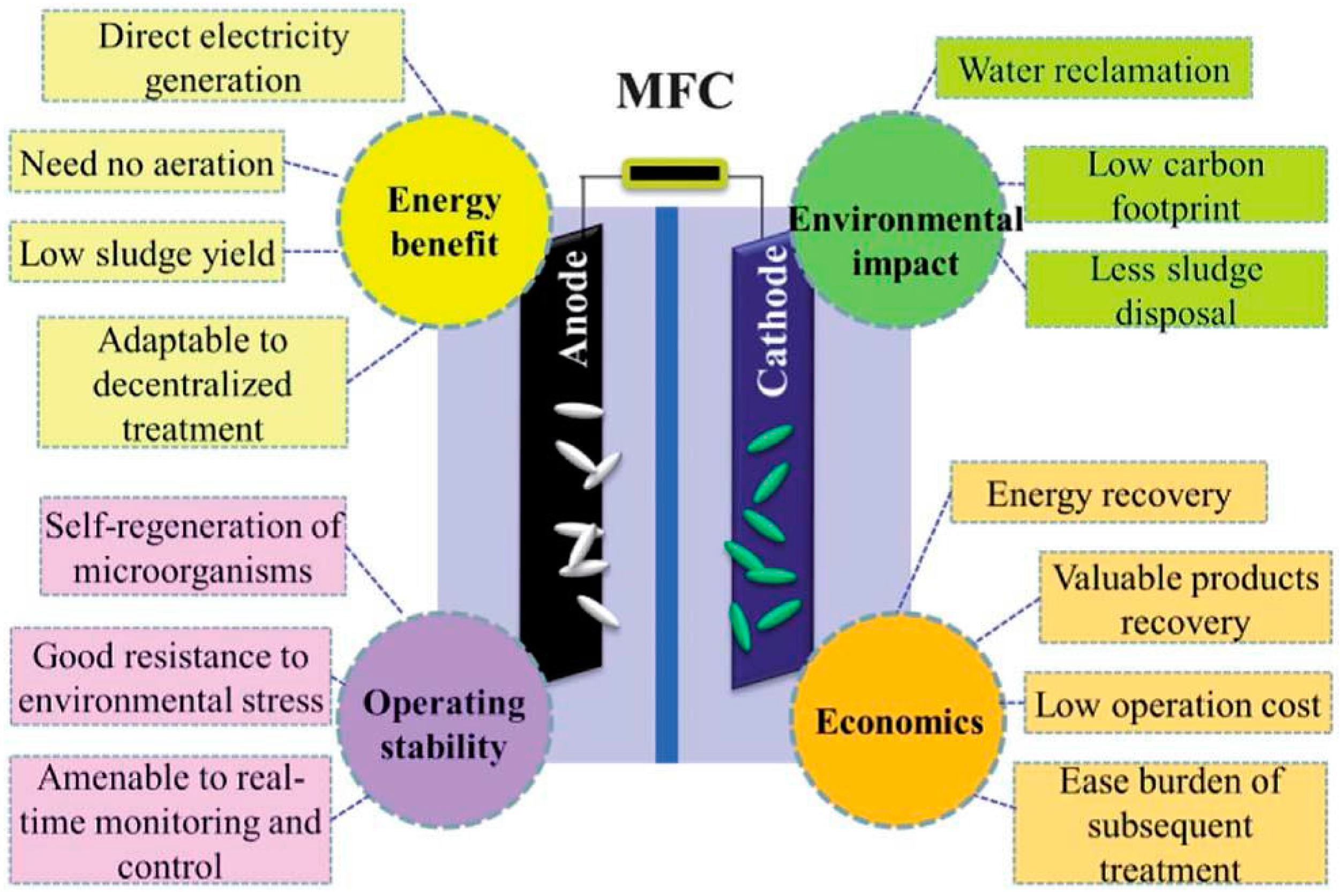
:1. Introduction
2. Synthesis Methods of Graphene-Modified Electrodes
2.1. Oxidization and Reduction Methods
2.2. Self-Assembly Methods
2.3. Chemical Vapor Deposition
2.4. Template Methods
2.5. Other Synthesis Methods
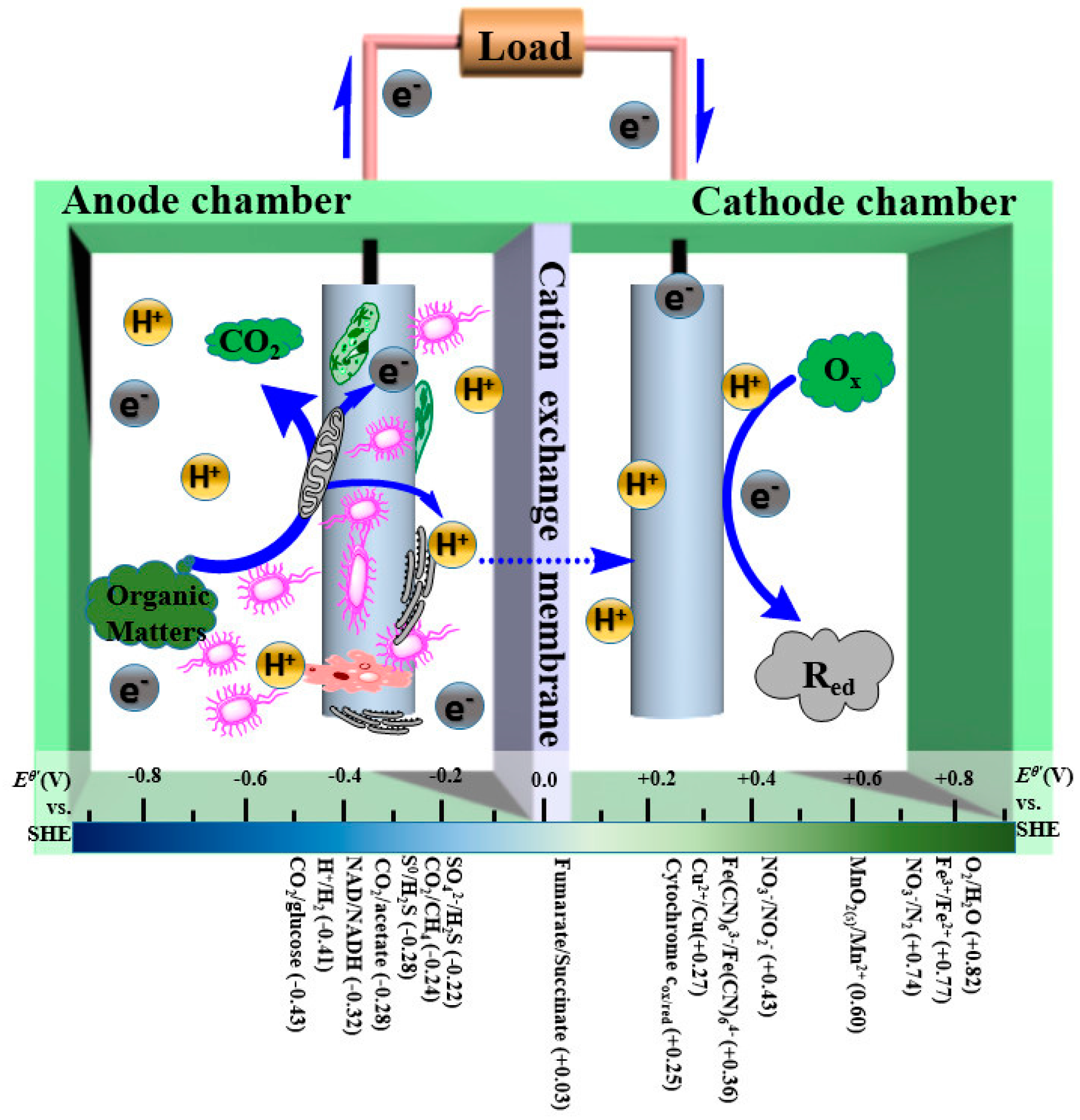
3. Development of Graphene-Modified Electrodes in Microbial Fuel Cells
3.1. Design and Development of Graphene-Modified Anode Materials in MFCs
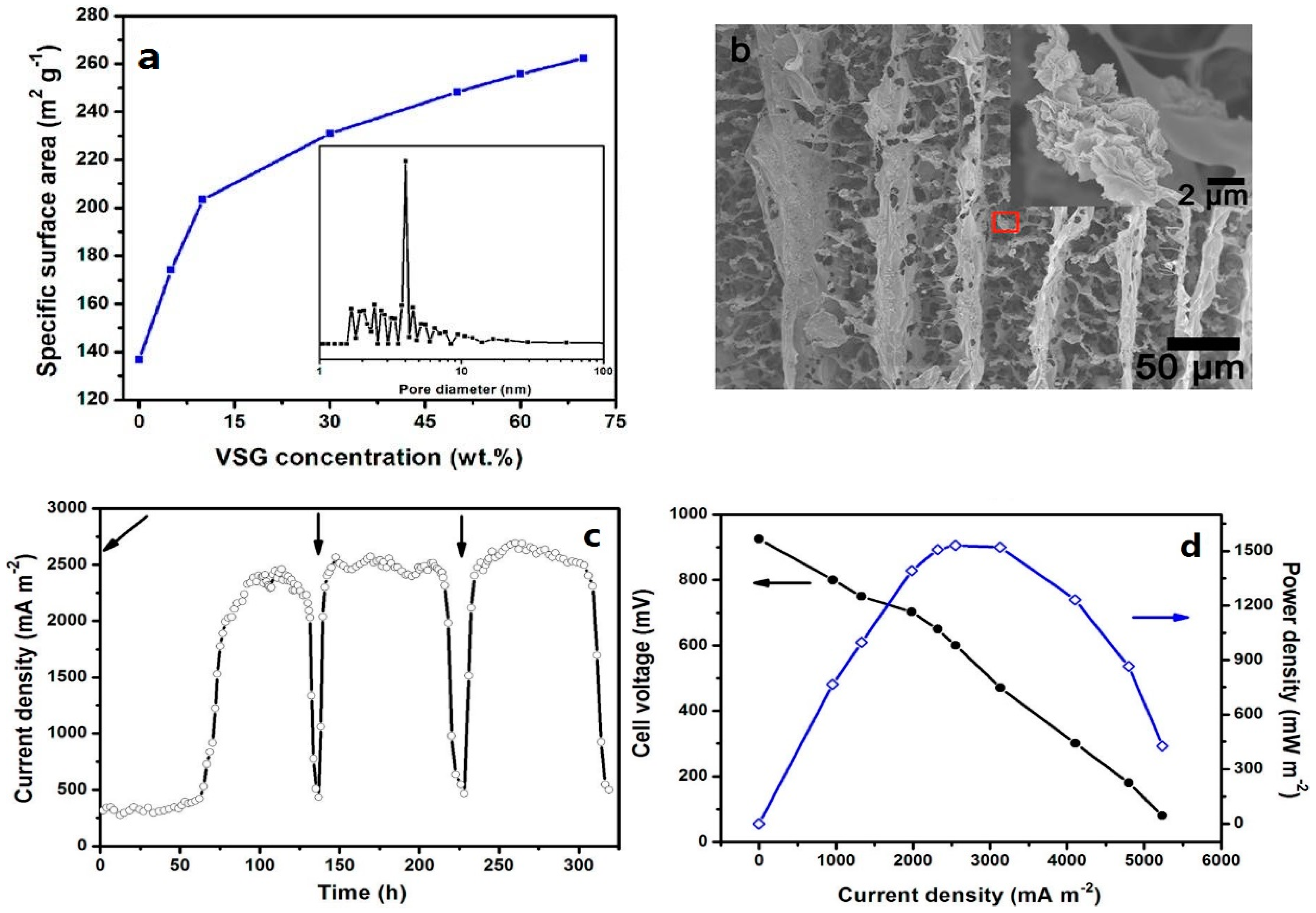
3.1.1. Specific Surface Area
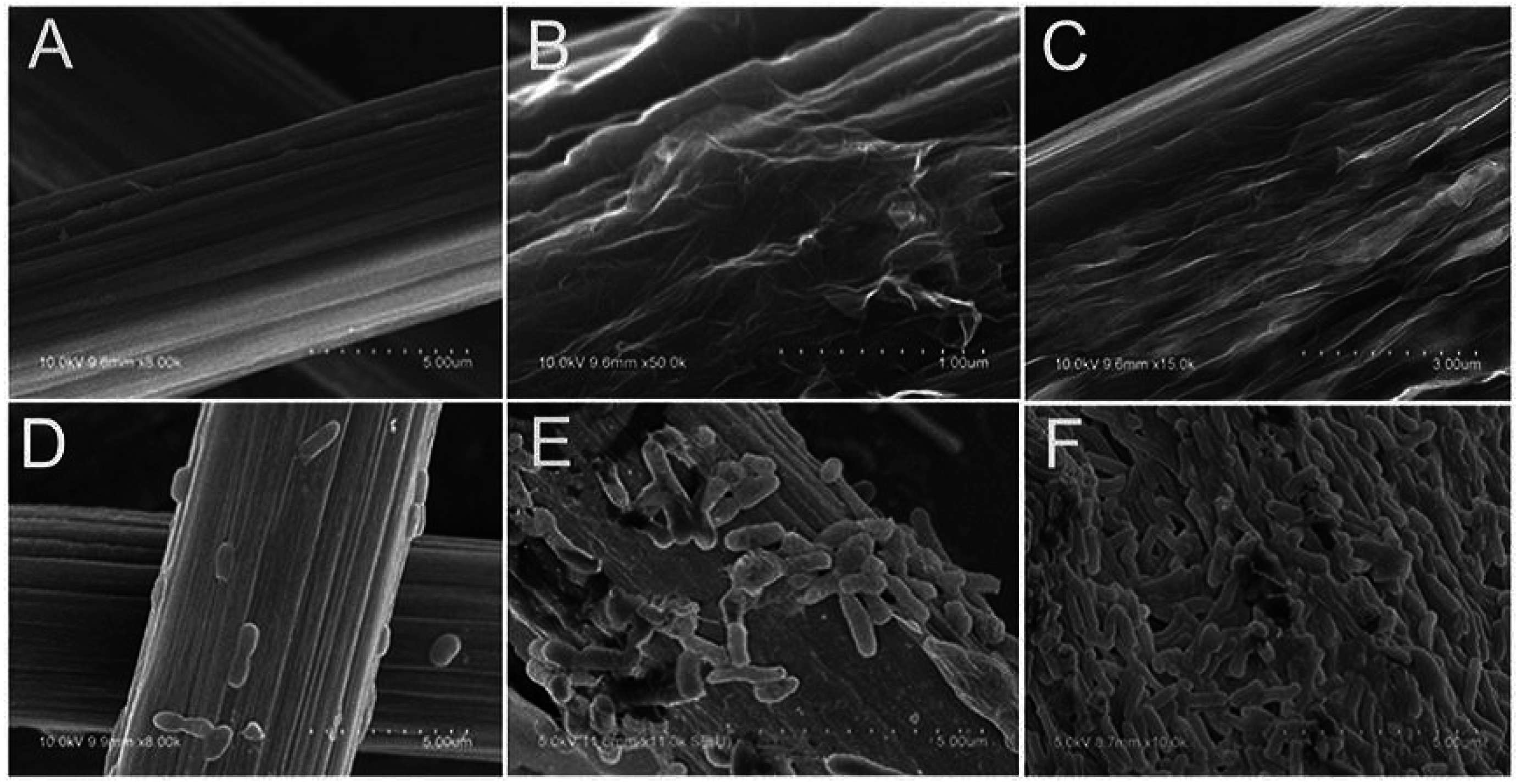
3.1.2. Biocompatibility
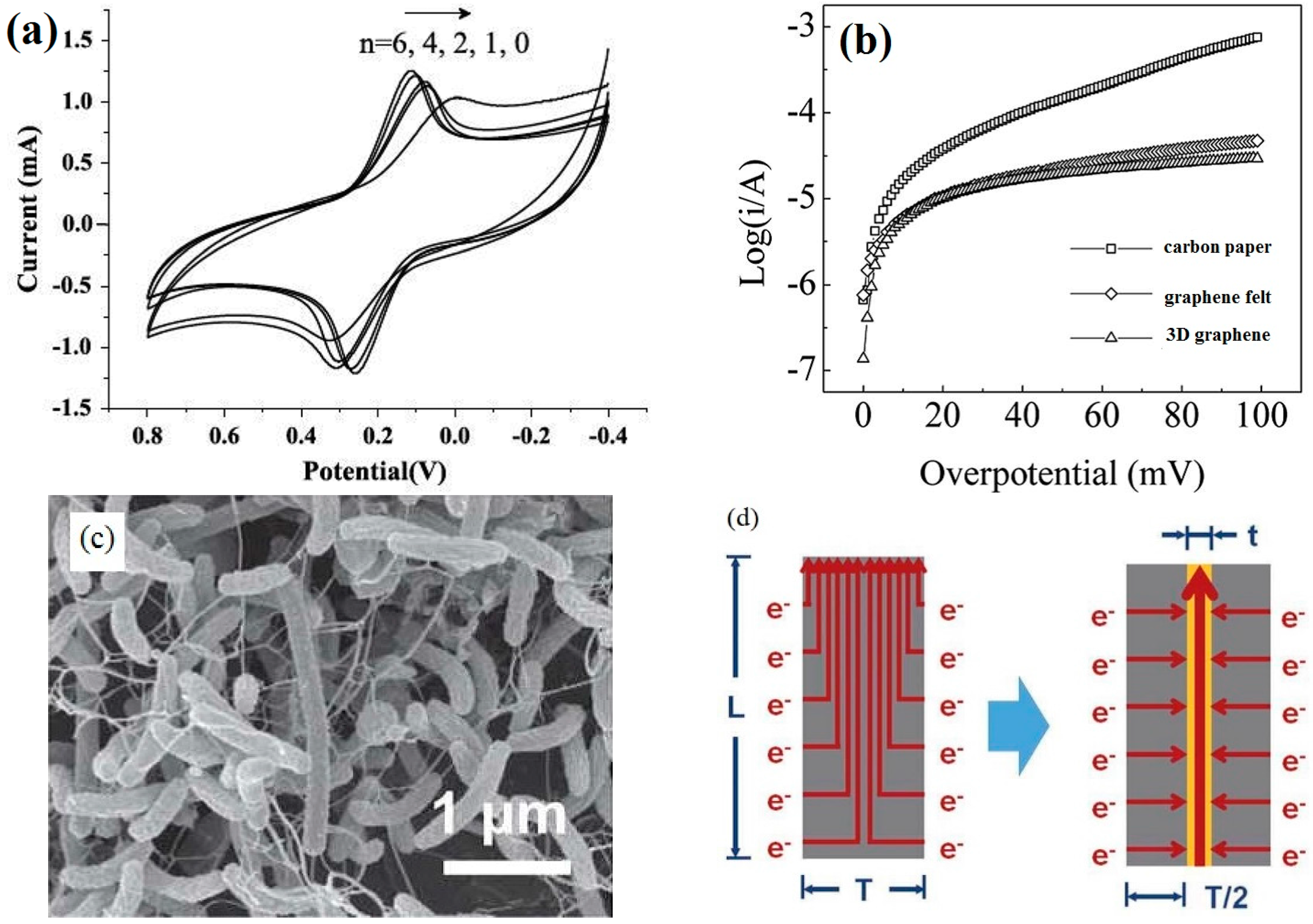
3.1.3. EET Efficiency
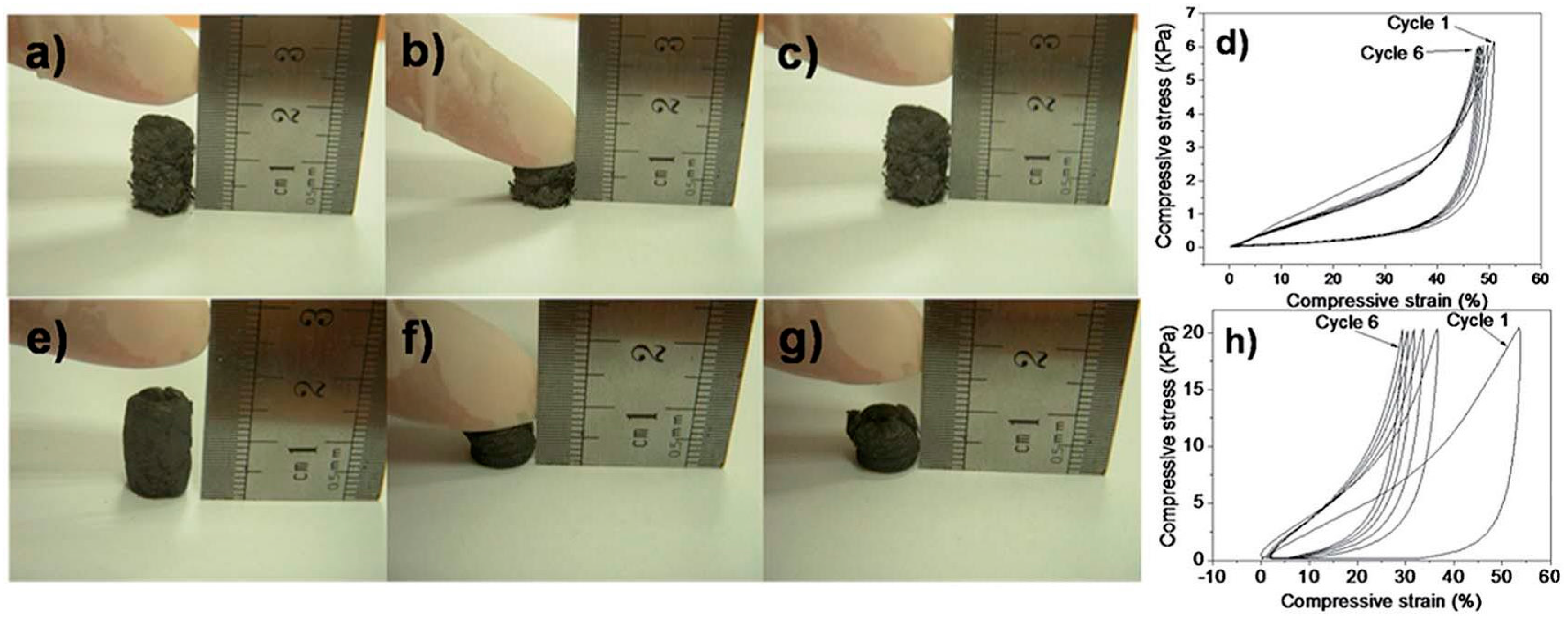
3.1.4. Mechanical Properties
3.2. Design and Development of Graphene-Modified Cathode Materials in MFCs
3.2.1. Reduction Pathways
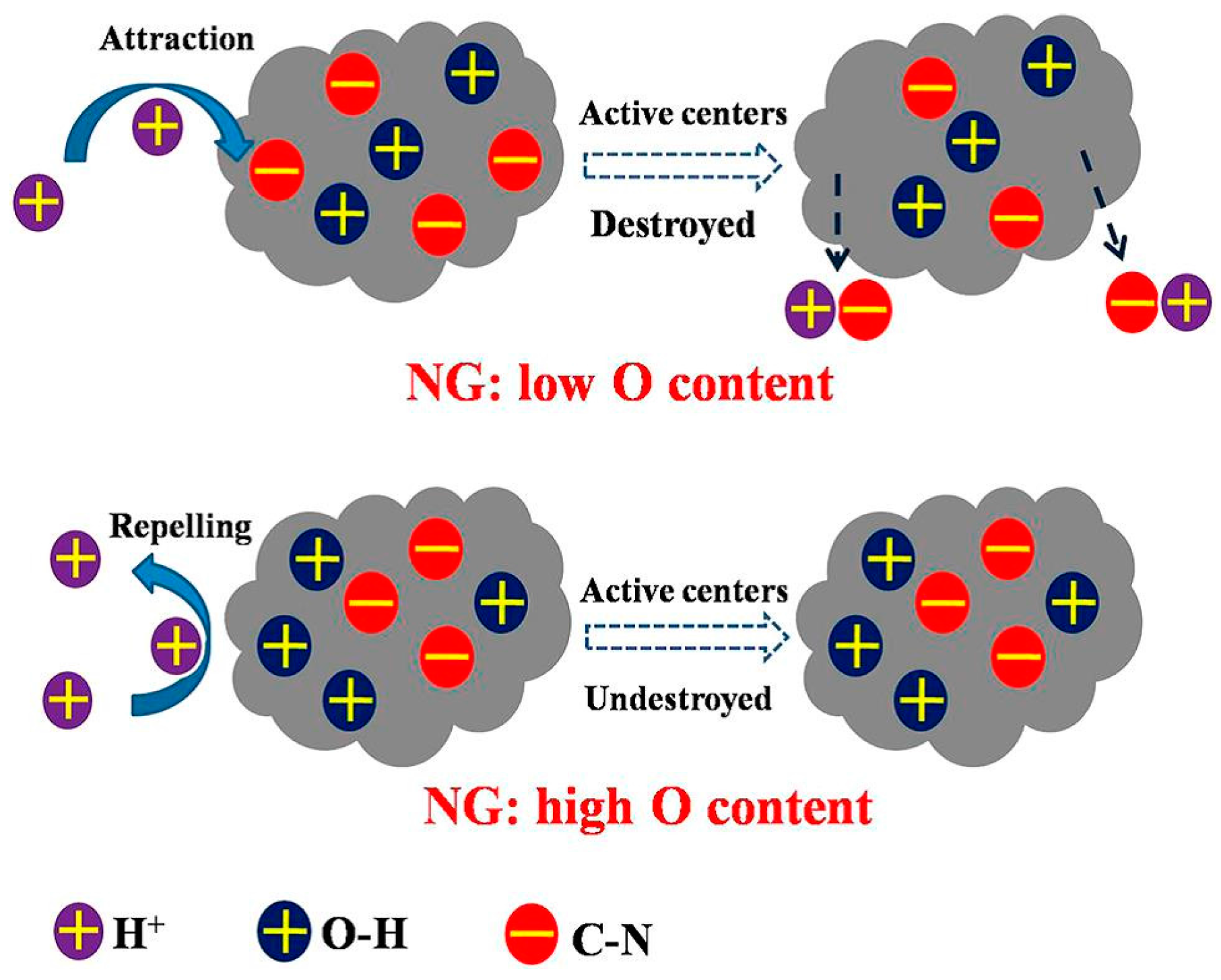
3.2.2. Active Sites
3.2.3. Electrical Conductivity
3.2.4. Electron Acceptor
4. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
References
- Chou, H.T.; Lee, H.J.; Lee, C.Y.; Tai, N.H.; Chang, H.Y. Highly durable anodes of microbial fuel cells using a reduced graphene oxide/carbon nanotube-coated scaffold. Bioresour. Technol. 2014, 169, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Kirubaharan, C.J.; Santhakumar, K.; Kumar, G.G.; Senthilkumar, N.; Jang, J.H. Nitrogen doped graphene sheets as metal free anode catalysts for the high performance microbial fuel cells. Int. J. Hydrogen Energy 2015, 40, 13061–13070. [Google Scholar] [CrossRef]
- Chen, W.F.; Huang, Y.X.; Li, D.B.; Yu, H.Q.; Yan, L.F. Preparation of a macroporous flexible three dimensional graphene sponge using an ice-template as the anode material for microbial fuel cells. RSC Adv. 2014, 4, 21619–21624. [Google Scholar] [CrossRef]
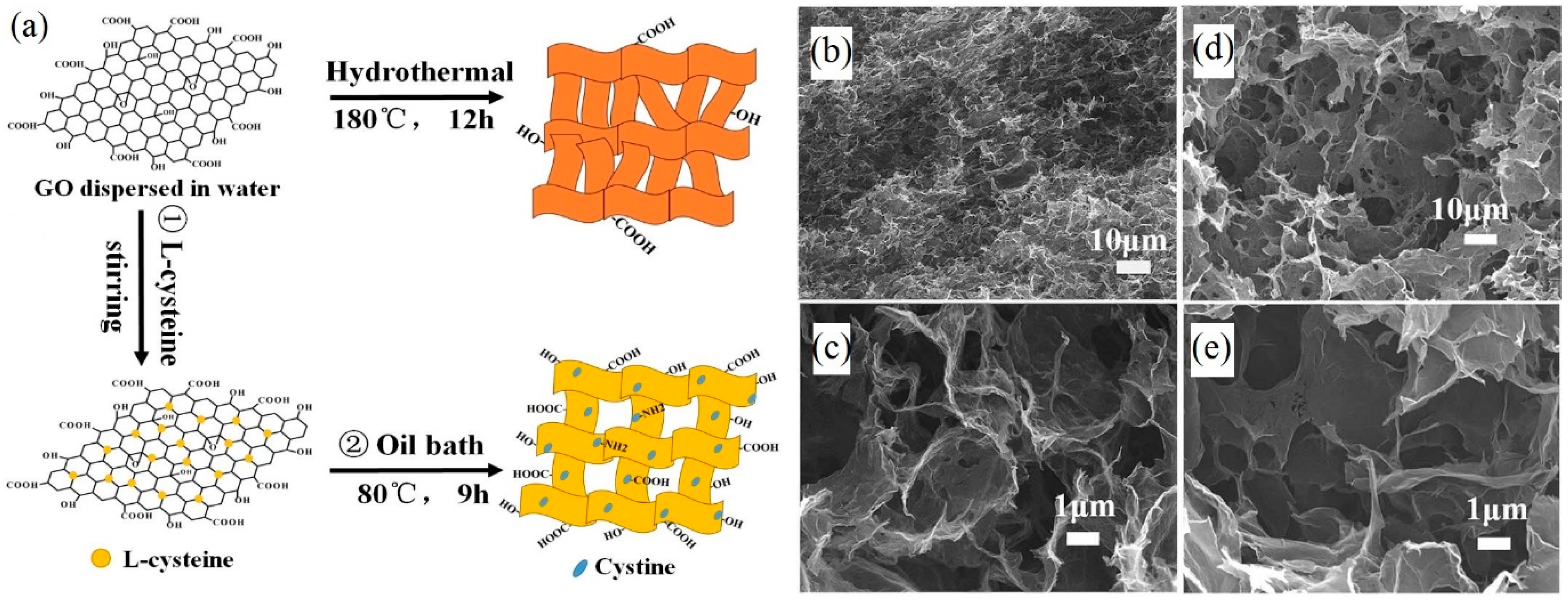
- Qiao, Y.; Wen, G.-Y.; Wu, X.-S.; Zou, L. l-cysteine tailored porous graphene aerogel for enhanced power generation in microbial fuel cells. RSC Adv. 2015, 5, 58921–58927. [Google Scholar] [CrossRef]
- Quan, X.C.; Mei, Y.; Xu, H.D.; Sun, B.; Zhang, X. Optimization of Pt-Pd alloy catalyst and supporting materials for oxygen reduction in air-cathode microbial fuel cells. Electrochim. Acta 2015, 165, 72–77. [Google Scholar] [CrossRef]
- Chen, Y.; Gai, P.P.; Zhang, J.R.; Zhu, J.J. Design of an enzymatic biofuel cell with large power output. J. Mater. Chem. A 2015, 3, 11511–11516. [Google Scholar] [CrossRef]
- Mink, J.E.; Qaisi, R.M.; Hussain, M.M. Graphene-based flexible micrometer-sized microbial fuel cell. Energy Technol. 2013, 1, 648–652. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, X.; Li, H.; Xiong, G.; Hu, H.; Fisher, T.S. Mechanically robust honeycomb graphene aerogel multifunctional polymer composites. Carbon 2015, 93, 659–670. [Google Scholar] [CrossRef]
- Angosto, J.M.; Fernandez-Lopez, J.A.; Godinez, C. Brewery and liquid manure wastewaters as potential feedstocks for microbial fuel cells: A performance study. Environ. Sci. Technol. 2015, 36, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Dunaj, S.J.; Vallino, J.J.; Hines, M.E.; Gay, M.; Kobyljanec, C.; Rooney-Varga, J.N. Relationships between soil organic matter, nutrients, bacterial community structure, and the performance of microbial fuel cells. Environ. Sci. Technol. 2012, 46, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Ito, T.; Kawano, Y.; Iguchi, A.; Miyahara, M.; Suzuki, Y.; Watanabe, K. Electricity generation from cattle manure slurry by cassette-electrode microbial fuel cells. J. Biosci. Bioeng. 2013, 116, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Nimje, V.R.; Chen, C.Y.; Chen, H.R.; Chen, C.C.; Huang, Y.M.; Tseng, M.J.; Cheng, K.C.; Chang, Y.F. Comparative bioelectricity production from various wastewaters in microbial fuel cells using mixed cultures and a pure strain of Shewanella oneidensis. Bioresour. Technol. 2012, 104, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Ieropoulos, I.A.; Ledezma, P.; Stinchcombe, A.; Papaharalabos, G.; Melhuish, C.; Greenman, J. Waste to real energy: The first MFC powered mobile phone. Phys. Chem. Chem. Phys. 2013, 15, 15312–15316. [Google Scholar] [CrossRef] [PubMed]
- Ieropoulos, I.A.; Stinchcombe, A.; Gajda, I.; Forbes, S.; Merino-Jimenez, I.; Pasternak, G.; Sanchez-Herranz, D.; Greenman, J. Pee power urinal-microbial fuel cell technology field trials in the context of sanitation. Environ Sci. Water Res. Technol. 2016, 2, 336–343. [Google Scholar] [CrossRef]
- Kim, K.Y.; Chae, K.J.; Choi, M.J.; Ajayi, F.F.; Jang, A.; Kim, C.W.; Kim, I.S. Enhanced coulombic efficiency in glucose-fed microbial fuel cells by reducing metabolite electron losses using dual-anode electrodes. Bioresour. Technol. 2011, 102, 4144–4149. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Yang, W.; Logan, B.E. Impact of electrode configurations on retention time and domestic wastewater treatment efficiency using microbial fuel cells. Water Res. 2015, 80, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hatzell, M.C.; Hutchinson, A.J.; Logan, B.E. Capturing power at higher voltages from arrays of microbial fuel cells without voltage reversal. Energy Environ. Sci. 2011, 4, 4662–4667. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Hsu, W.-H.; Huang, Y.-C. Characterization of carbon nanotube/craphene on carbon cloth as an electrode for air-cathode microbial fuel cells. J. Nanomater. 2015, 2015, 686891. [Google Scholar] [CrossRef]
- Song, T.S.; Wang, D.B.; Wang, H.Q.; Li, X.X.; Liang, Y.Y.; Xie, J.J. Cobalt oxide/nanocarbon hybrid materials as alternative cathode catalyst for oxygen reduction in microbial fuel cell. Int. J. Hydrogen Energy 2015, 40, 3868–3874. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Ghangrekar, M.M.; Pradhan, D.; Das, D. Graphene oxide-impregnated PVA-STA composite polymer electrolyte membrane separator for power generation in a single-chambered microbial fuel cell. Ind. Eng. Chem. Res. 2013, 52, 11597–11606. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Shi, F.; Zhang, J.; Zhu, J.J. High biocurrent generation in Shewanella-inoculated microbial fuel cells using ionic liquid functionalized graphene nanosheets as an anode. Chem. Commun. 2013, 49, 6668–6670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.E.; Wang, W.J.; Sun, D.; Wang, X.; Zhang, J.R.; Zhu, J.J. Nanostructured graphene/TiO2 hybrids as high-performance anodes for microbial fuel cells. Chem. Eur. J. 2014, 20, 7091–7097. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhou, S.; Zhao, B.; Zhuang, L.; Wang, Y. Microbially-reduced graphene scaffolds to facilitate extracellular electron transfer in microbial fuel cells. Bioresour. Technol. 2012, 116, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, A.; Gadhamshetty, V.; Mukherjee, R.; Chen, Z.; Ren, W.; Cheng, H.M.; Koratkar, N. Passivation of microbial corrosion using a graphene coating. Carbon 2013, 56, 45–49. [Google Scholar] [CrossRef]
- He, Y.R.; Xiao, X.; Li, W.W.; Sheng, G.P.; Yan, F.F.; Yu, H.Q.; Yuan, H.; Wu, L.J. Enhanced electricity production from microbial fuel cells with plasma-modified carbon paper anode. Phys. Chem. Chem. Phys. 2012, 14, 9966–9971. [Google Scholar] [CrossRef] [PubMed]
- Baranitharan, E.; Khan, M.R.; Prasad, D.M.R.; Bin Salihon, J. Bioelectricity Generation from palm oil mill effluent in microbial fuel cell using polacrylonitrile carbon felt as electrode. Water Air Soil Pollut. 2013, 224, 1–11. [Google Scholar] [CrossRef]
- Chen, S.L.; He, G.H.; Carmona-Martinez, A.A.; Agarwal, S.; Greiner, A.; Hou, H.Q.; Schroder, U. Electrospun carbon fiber mat with layered architecture for anode in microbial fuel cells. Electrochem. Commun. 2011, 13, 1026–1029. [Google Scholar] [CrossRef]
- Taskan, E.; Hasar, H. Comprehensive comparison of a new tin-coated copper mesh and a graphite plate electrode as an anode material in microbial fuel cell. Appl. Biochem. Biotechnol. 2015, 175, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dustin Lee, J.H.; Xia, Q.; Ma, Y.; Yu, Y.; Lanry Yung, L.Y.; Xie, J.; Ong, C.N.; Vecitis, C.D.; Zhou, Z. A graphene-based electrochemical filter for water purification. J. Mater. Chem. A 2014, 2, 16554–16562. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yu, F.; Ma, J.; Chen, J. Graphene as a template and structural scaffold for the synthesis of a 3D porous bio-adsorbent to remove antibiotics from water. RSC Adv. 2015, 5, 27964–27969. [Google Scholar] [CrossRef]
- Scott, S.M.; Hu, T.; Yao, T.K.; Xin, G.Q.; Lian, J. Graphene-based sorbents for iodine-129 capture and sequestration. Carbon 2015, 90, 1–8. [Google Scholar] [CrossRef]
- Yang, H.; Gong, J.; Wen, X.; Xue, J.; Chen, Q.; Jiang, Z.; Tian, N.; Tang, T. Effect of carbon black on improving thermal stability, flame retardancy and electrical conductivity of polypropylene/carbon fiber composites. Compos. Sci. Technol. 2015, 113, 31–37. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.; Peng, S.; Jiang, H.; Zhang, Y.; Deng, W.; Tan, Y.; Ma, M.; Xie, Q. Facile fabrication of graphene-containing foam as a high-performance anode for microbial fuel cells. Chem. Eur. J. 2015, 21, 10634–30638. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, S.; Chen, C.; Yan, L. Self-Assembly and Embedding of Nanoparticles by in situ reduced graphene for preparation of a 3D graphene/nanoparticle aerogel. Adv. Mater. 2011, 23, 5679–5683. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yan, L. In situ self-assembly of mild chemical reduction graphene for three-dimensional architectures. Nanoscale 2011, 3, 3132–3137. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-C.; Xu, H.; Wang, X.-W.; Huang, Y.-X.; Chan-Park, M.B.; Zhang, H.; Wang, L.-H.; Huang, W.; Chen, P. 3D graphene-cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ding, C.M.; Liu, H.; Zhu, Y.; Jiang, L. Shewanella-mediated synthesis of reduced graphene oxide film for enhanced extracellular electron transfer. Chem. J. Chin. Univ. 2014, 35, 2201–2206. [Google Scholar]
- Bi, H.; Xie, X.; Yin, K.; Zhou, Y.; Wan, S.; He, L.; Xu, F.; Banhart, F.; Sun, L.; Ruoff, R.S. Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents. Adv. Funct. Mater. 2012, 22, 4421–4425. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.X.; Liu, Z.L.; Zhang, P.Y. A new method for fabrication of graphene/polyaniline nanocomplex modified microbial fuel cell anodes. J. Power Sources 2013, 224, 139–144. [Google Scholar] [CrossRef]
- Kumar, G.G.; Kirubaharan, C.J.; Udhayakumar, S.; Ramachandran, K.; Karthikeyan, C.; Renganathan, R.; Nahrn, K.S. Synthesis, structural, and morphological characterizations of reduced graphene oxide-supported polypyrrole anode catalysts for improved microbial fuel cell performances. ACS Sustain. Chem. Eng. 2014, 2, 2283–2290. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, L.; Ong, C.N.; Xie, J. Nitrogen-doped graphene nanosheets as reactive water purification membranes. Nano Res. 2016, 9, 1983–1993. [Google Scholar] [CrossRef]
- Fang, X.Y.; Yu, X.X.; Zheng, H.M.; Jin, H.B.; Wang, L.; Cao, M.S. Temperature- and thickness-dependent electrical conductivity of few-layer graphene and graphene nanosheets. Phys. Lett. A 2015, 379, 2245–2251. [Google Scholar] [CrossRef]
- Zhang, Y.; Mo, G.; Li, X.; Ye, J. Iron tetrasulfophthalocyanine functionalized graphene as a platinum-free cathodic catalyst for efficient oxygen reduction in microbial fuel cells. J. Power Sources 2012, 197, 93–96. [Google Scholar] [CrossRef]
- Mao, S.; Lu, G.; Chen, J. Three-dimensional graphene-based composites for energy applications. Nanoscale 2015, 7, 6924–6943. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, G.Q.; Chen, J.; Zhou, Y.F.; Yuan, G.E.; Yang, F.L. Spontaneous redox synthesis of Prussian blue/graphene nanocomposite as a non-precious metal catalyst for efficient four-electron oxygen reduction in acidic medium. J. Power Sources 2013, 240, 101–108. [Google Scholar] [CrossRef]
- Li, D.; Muller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Gnana Kumar, G.; Awan, Z.; Suk Nahm, K.; Xavier, J.S. Nanotubular MnO2/graphene oxide composites for the application of open air-breathing cathode microbial fuel cells. Biosens. Bioelectron. 2014, 53, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Wang, C.; Hou, S.X.; Yang, N. Sustainable energy recovery in wastewater treatment by microbial fuel cells: Stable power generation with nitrogen-doped graphene cathode. Environ. Sci. Technol. 2013, 47, 13889–13895. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Pan, D.; Li, X.; Fu, F.; Zhao, Y.; Wang, X. Effect of polyaniline-graphene nanosheets modified cathode on the performance of sediment microbial fuel cell. J. Chem. Technol. Biotechnol. 2013, 88, 1946–1950. [Google Scholar] [CrossRef]
- Xiao, L.; Damien, J.; Luo, J.; Jang, H.D.; Huang, J.; He, Z. Crumpled graphene particles for microbial fuel cell electrodes. J. Power Sources 2012, 208, 187–192. [Google Scholar] [CrossRef]
- Wen, Q.; Wang, S.; Yan, J.; Cong, L.; Pan, Z.; Ren, Y.; Fan, Z. MnO2–graphene hybrid as an alternative cathodic catalyst to platinum in microbial fuel cells. J. Power Sources 2012, 216, 187–191. [Google Scholar] [CrossRef]
- Chen, J.; Deng, F.; Hu, Y.; Sun, J.; Yang, Y. Antibacterial activity of graphene-modified anode on Shewanella oneidensis MR-1 biofilm in microbial fuel cell. J. Power Sources 2015, 290, 80–86. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, Y.; Guo, C.X.; Lim, S.; Song, H.; Li, C.M. Graphene/carbon cloth anode for high-performance mediatorless microbial fuel cells. Bioresour. Technol. 2012, 114, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Chen, Y.; Wei, H.; Li, F.; Hu, Y.; Wei, C.; Feng, C. One-step electrosynthesis of polypyrrole/graphene oxide composites for microbial fuel cell application. Electrochim. Acta 2013, 111, 366–373. [Google Scholar] [CrossRef]
- Zhao, C.; Gai, P.; Liu, C.; Wang, X.; Xu, H.; Zhang, J.; Zhu, J.-J. Polyaniline networks grown on graphene nanoribbons-coated carbon paper with a synergistic effect for high-performance microbial fuel cells. J. Mater. Chem. A 2013, 1, 12587–12594. [Google Scholar] [CrossRef]
- Zou, L.; Qiao, Y.; Wu, X.-S.; Ma, C.-X.; Li, X.; Li, C.M. Synergistic effect of titanium dioxide nanocrystal/reduced graphene oxide hybrid on enhancement of microbial electrocatalysis. J. Power Sources 2015, 276, 208–214. [Google Scholar] [CrossRef]
- Li, R.; Chen, C.B.; Li, J.; Xu, L.M.; Xiao, G.Y.; Yan, D.Y. A facile approach to superhydrophobic and superoleophilic graphene/polymer aerogels. J. Mater. Chem. A 2014, 2, 3057–3064. [Google Scholar] [CrossRef]
- Zhuang, L.; Yuan, Y.; Yang, G.; Zhou, S. In situ formation of graphene/biofilm composites for enhanced oxygen reduction in biocathode microbial fuel cells. Electrochem. Commun. 2012, 21, 69–72. [Google Scholar] [CrossRef]
- Erbay, C.; Yang, G.; de Figueiredo, P.; Sadr, R.; Yu, C.H.; Han, A. Three-dimensional porous carbon nanotube sponges for high-performance anodes of microbial fuel cells. J. Power Sources 2015, 298, 177–183. [Google Scholar] [CrossRef]
- Ma, J.; Yang, M.; Yu, F.; Zheng, J. Water-enhanced removal of ciprofloxacin from water by porous graphene hydrogel. Sci. Rep. 2015, 5, 13578. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Lin, Z.Y.; Huang, X.Q.; Wang, Y.; Huang, Y.; Duan, X.F. Functionalized graphene hydrogel-based high-performance supercapacitors. Adv. Mater. 2013, 25, 5779–5784. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Cui, Y.R.; Song, H.; Sun, J.H. Layer-by-layer construction of graphene-based microbial fuel cell for improved power generation and methyl orange removal. Bioproc. Biosyst. Eng. 2014, 37, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-J.; Zhao, H.-Z.; Yang, Q.-Z.; Song, J.; Xue, A. A novel layer-by-layer self-assembled carbon nanotube-based anode: Preparation, characterization, and application in microbial fuel cell. Electrochim. Acta 2010, 55, 3041–3047. [Google Scholar] [CrossRef]
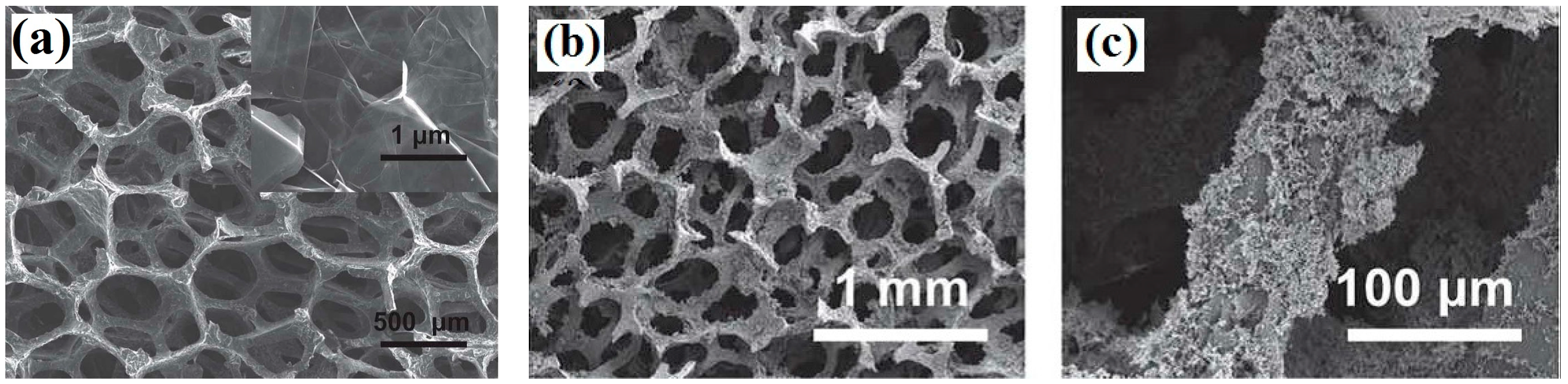
- Xie, X.; Yu, G.H.; Liu, N.; Bao, Z.N.; Criddle, C.S.; Cui, Y. Graphene-sponges as high-performance low-cost anodes for microbial fuel cells. Energy Environ. Sci. 2012, 5, 6862–6866. [Google Scholar] [CrossRef]
- Mehdinia, A.; Ziaei, E.; Jabbari, A. Facile microwave-assisted synthesized reduced graphene oxide/tin oxide nanocomposite and using as anode material of microbial fuel cell to improve power generation. Int. J. Hydrogen Energy 2014, 39, 10724–10730. [Google Scholar] [CrossRef]
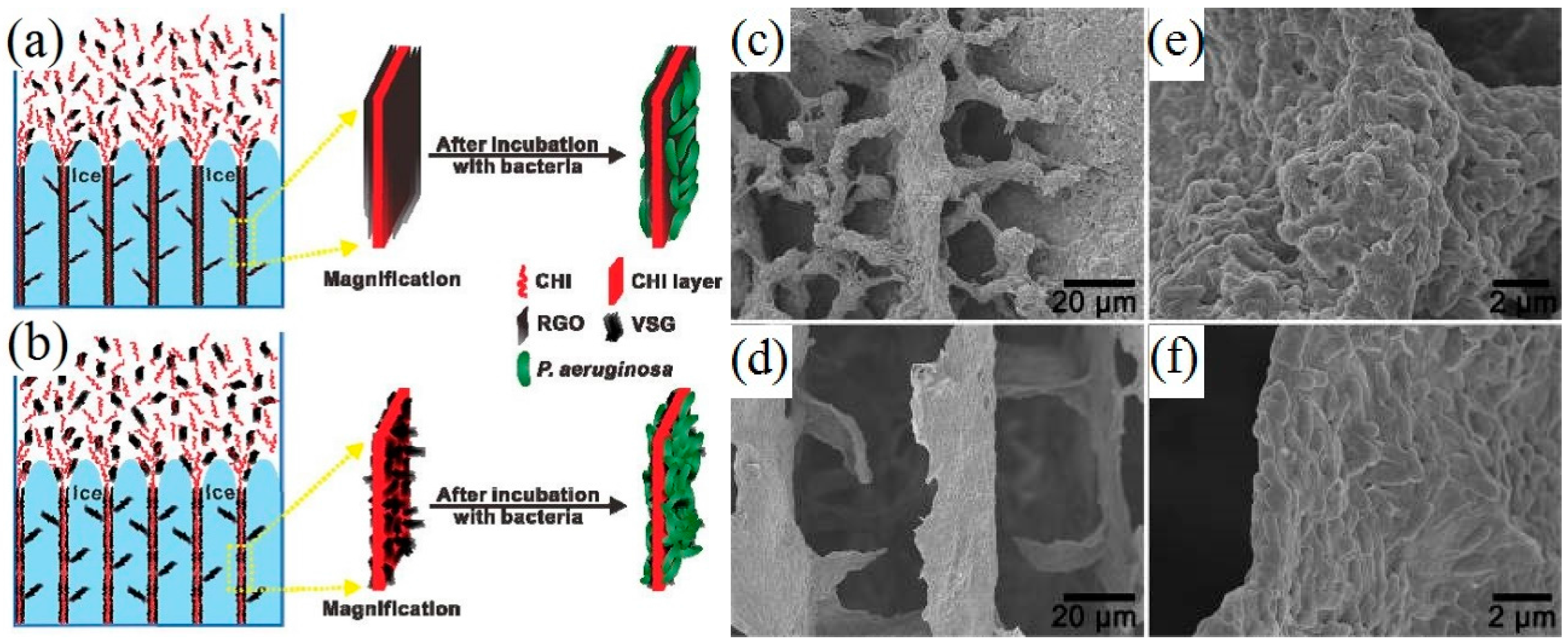
- He, Z.M.; Liu, J.; Qiao, Y.; Li, C.M.; Tan, T.T.Y. Architecture engineering of hierarchically porous chitosan/vacuum-stripped graphene scaffold as bioanode for high performance microbial fuel cell. Nano Lett. 2012, 12, 4738–4741. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.C.; Dong, X.C.; Chan-Park, M.B.; Song, H.; Chen, P. Macroporous and monolithic anode based on polyaniline hybridized three-dimensional graphene for high-performance microbial fuel cells. ACS Nano 2012, 6, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Y.; Chen, L. Easy-to-operate and low-temperature synthesis of gram-scale nitrogen-doped graphene and its application as cathode catalyst in microbial fuel cells. ACS Nano 2011, 5, 9611–9618. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Bonanni, A.; Sofer, Z.; Pumera, M. Large-scale quantification of CVD graphene surface coverage. Nanoscale 2013, 5, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.H.; Fu, P.; Wen, D.H.; Yuan, Y.; Zhou, S.G. Self-constructed carbon nanoparticles-coated porous biocarbon from plant moss as advanced oxygen reduction catalysts. Appl. Catal. B Environ. 2016, 181, 635–643. [Google Scholar] [CrossRef]
- Yang, T.; Liu, J.; Zhou, R.; Chen, Z.; Xu, H.; Qiao, S.Z.; Monteiro, M.J. N-doped mesoporous carbon spheres as the oxygen reduction reaction catalysts. J. Mater. Chem. A 2014, 2, 18139–18146. [Google Scholar] [CrossRef]
- Kim, S.Y.; Suh, W.H.; Choi, J.H.; Yi, Y.S.; Lee, S.K.; Stucky, G.D.; Kang, J.K. Template-free synthesis of high surface area nitrogen-rich carbon microporous spheres and their hydrogen uptake capacity. J. Mater. Chem. A 2014, 2, 2227–2232. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, X.J.; Dionysiou, D.D.; Liu, H.; Huang, Y.M. Homogeneous deposition-assisted synthesis of iron nitrogen composites on graphene as highly efficient non-precious metal electrocatalysts for microbial fuel cell power generation. J. Power Sources 2015, 278, 773–781. [Google Scholar] [CrossRef]
- Gong, X.B.; You, S.J.; Wang, X.H.; Zhang, J.N.; Gan, Y.; Ren, N.Q. A novel stainless steel mesh/cobalt oxide hybrid electrode for efficient catalysis of oxygen reduction in a microbial fuel cell. Biosens. Bioelectron. 2014, 55, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Wu, X.-S.; Ma, C.-X.; He, H.; Li, C.M. A hierarchical porous graphene/nickel anode that simultaneously boosts the bio- and electro-catalysis for high-performance microbial fuel cells. RSC Adv. 2014, 4, 21788–21793. [Google Scholar] [CrossRef]
- Wen, Q.; Wang, S.; Yan, J.; Cong, L.; Chen, Y.; Xi, H. Porous nitrogen-doped carbon nanosheet on graphene as metal-free catalyst for oxygen reduction reaction in air-cathode microbial fuel cells. Bioelectrochemistry 2014, 95, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Li, W.W.; Yu, H.Q.; He, Z. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 2014, 7, 911–924. [Google Scholar] [CrossRef]
- kumar, G.G.; Sarathi, V.G.; Nahm, K.S. Recent advances and challenges in the anode architecture and their modifications for the applications of microbial fuel cells. Biosens. Bioelectron. 2013, 43, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Baranitharan, E.; Khan, M.R.; Prasad, D.M.R.; Teo, W.F.A.; Tan, G.Y.A.; Jose, R. Effect of biofilm formation on the performance of microbial fuel cell for the treatment of palm oil mill effluent. Bioproc. Biosyst. Eng. 2015, 38, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mo, G.; Li, X.; Zhang, W.; Zhang, J.; Ye, J.; Huang, X.; Yu, C. A graphene modified anode to improve the performance of microbial fuel cells. J. Power Sources 2011, 196, 5402–5407. [Google Scholar] [CrossRef]
- Chen, M.Q.; Zeng, Y.X.; Zhao, Y.T.; Yu, M.H.; Cheng, F.L.; Lu, X.H.; Tong, Y.X. Monolithic three-dimensional graphene frameworks derived from inexpensive graphite paper as advanced anodes for microbial fuel cells. J. Mater. Chem. A 2016, 4, 6342–6349. [Google Scholar] [CrossRef]
- Tang, J.H.; Yuan, Y.; Liu, T.; Zhou, S.G. High-capacity carbon-coated titanium dioxide core-shell nanoparticles modified three dimensional anodes for improved energy output in microbial fuel cells. J. Power Sources 2015, 274, 170–176. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Wang, C.H.; Zhai, Y.; Zhang, R.Q.; Van Hove, M.A. Selective adsorption of L-serine functional groups on the anatase TiO2(101) surface in benthic microbial fuel cells. Phys. Chem. Chem. Phys. 2014, 16, 20806–20817. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Huang, H.X.; Li, B.; Li, W.S. Novelly developed three-dimensional carbon scaffold anodes from polyacrylonitrile for microbial fuel cells. J. Mater. Chem. A 2015, 3, 5110–5118. [Google Scholar] [CrossRef]
- Gadhamshetty, V.; Krishnamurthy, A.; Koratkar, N. Ultrathin graphene coating for passivating microbial corrosion in microbial fuel cells. In Proceedings of the 245th ACS National Meeting, New Orleans, LA, USA, 7–11 April 2013; p. 245.
- Feng, Y.J.; Yang, Q.; Wang, X.; Logan, B.E. Treatment of carbon fiber brush anodes for improving power generation in air-cathode microbial fuel cells. J. Power Sources 2010, 195, 1841–1844. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zeng, L.; Cui, D.; Xiang, X.; Li, W. Polyaniline/mesoporous tungsten trioxide composite as anode electrocatalyst for high-performance microbial fuel cells. Biosens. Bioelectron. 2013, 41, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.A.; Logan, B.E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007, 9, 492–496. [Google Scholar] [CrossRef]
- Zhao, F.; Rahunen, N.; Varcoe, J.R.; Chandra, A.; Avignone-Rossa, C.; Thumser, A.E.; Slade, R.C. Activated carbon cloth as anode for sulfate removal in a microbial fuel cell. Environ. Sci. Technol. 2008, 42, 4971–4976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Ye, D.D.; Zhu, X.; Liao, Q.; Zhang, B.A. Tubular bamboo charcoal for anode in microbial fuel cells. J. Power Sources 2014, 272, 277–282. [Google Scholar] [CrossRef]
- Han, T.H.; Sawant, S.Y.; Hwang, S.J.; Cho, M.H. Three-dimensional, highly porous N-doped carbon foam as microorganism propitious, efficient anode for high performance microbial fuel cell. RSC Adv. 2016, 6, 25799–25807. [Google Scholar] [CrossRef]
- Goto, Y.; Yoshida, N.; Umeyama, Y.; Yamada, T.; Tero, R.; Hiraishi, A. Enhancement of electricity production by graphene oxide in soil microbial fuel cells and plant microbial fuel cells. Front. Bioeng. Biotechnol. 2015, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-E.; Gai, P.; Song, R.; Zhang, J.; Zhu, J.-J. Graphene/Au composites as an anode modifier for improving electricity generation in Shewanella-inoculated microbial fuel cells. Anal. Methods 2015, 7, 4640–4644. [Google Scholar] [CrossRef]
- Yuan, H.; He, Z. Graphene-modified electrodes for enhancing the performance of microbial fuel cells. Nanoscale 2015, 7, 7022–7029. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.E.; Wu, J.S.; Ding, Y.Z.; Wang, V.B.; Zhang, Y.D.; Kjelleberg, S.; Loo, J.S.C.; Cao, B.; Zhang, Q.C. Hybrid conducting biofilm with built-in bacteria for high-performance microbial fuel cells. ChemElectroChem 2015, 2, 654–658. [Google Scholar] [CrossRef]
- Wang, G.M.; Qian, F.; Saltikov, C.; Jiao, Y.Q.; Li, Y. Microbial reduction of graphene oxide by Shewanella. Nano Res. 2011, 4, 563–570. [Google Scholar] [CrossRef]
- Jain, A.; Gazzola, G.; Panzera, A.; Zanoni, M.; Marsii, E. Visible spectroelectrochemical characterization of Geobacter sulfurreducens biofilms on optically transparent indium tin oxide electrode. Electrochim. Acta 2011, 56, 10776–10785. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Li, X.; Ren, Y.; Wang, X. A monolithic three-dimensional macroporous graphene anode with low cost for high performance microbial fuel cells. RSC Adv. 2016, 6, 21001–21010. [Google Scholar] [CrossRef]
- Sruti, A.N.; Jagannadham, K. Electrical conductivity of graphene composites with In and In-Ga alloy. J. Electron. Mater. 2010, 39, 1268–1276. [Google Scholar] [CrossRef]
- Tang, J.; Chen, S.; Yuan, Y.; Cai, X.; Zhou, S. In situ formation of graphene layers on graphite surfaces for efficient anodes of microbial fuel cells. Biosens. Bioelectron. 2015, 71, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, X.P.; Lei, S.B.; Song, Y.H. Graphene-based polyaniline nanocomposites: Preparation, properties and applications. J. Mater. Chem. A 2014, 2, 4491–4509. [Google Scholar] [CrossRef]
- Liu, D.Q.; Jia, Z.; Wang, D.L. Preparation of hierarchically porous carbon nanosheet composites with graphene conductive scaffolds for supercapacitors: An electrostatic-assistant fabrication strategy. Carbon 2016, 100, 664–677. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.D.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Lai, J.P.; Zhang, N.; Liu, Y.B.; Liu, R.; Liu, X.Y. Tannic acid induced self-assembly of three-dimensional graphene with good adsorption and antibacterial properties. ACS Sustain. Chem. Eng. 2016, 4, 1404–1413. [Google Scholar] [CrossRef]
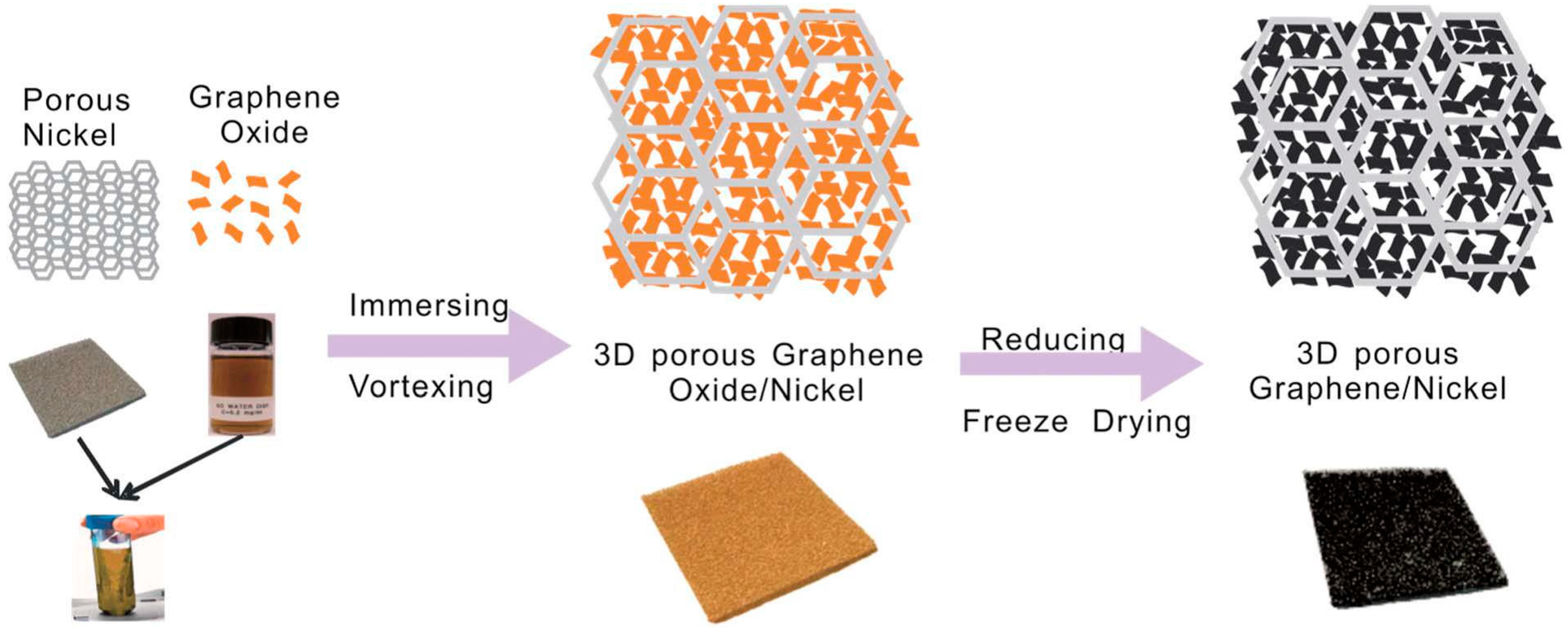
- Wang, H.; Wang, G.; Ling, Y.; Qian, F.; Song, Y.; Lu, X.; Chen, S.; Tong, Y.; Li, Y. High power density microbial fuel cell with flexible 3D graphene-nickel foam as anode. Nanoscale 2013, 5, 10283–10290. [Google Scholar] [CrossRef] [PubMed]
- Najafabadi, A.T.; Ng, N.; Gyenge, E. Electrochemically exfoliated graphene anodes with enhanced biocurrent production in single-chamber air-breathing microbial fuel cells. Biosens. Bioelectron. 2016, 81, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Cao, C.L.; Zheng, Y.; Chen, S.L.; Zhao, F. Abiotic oxygen reduction reaction catalysts used in microbial fuel cells. Chemelectrochemstry 2014, 1, 1813–1821. [Google Scholar] [CrossRef]
- Ben Liew, K.; Daud, W.R.W.; Ghasemi, M.; Leong, J.X.; Lim, W.S.; Ismail, M. Non-Pt catalyst as oxygen reduction reaction in microbial fuel cells: A review. Int. J. Hydrogen Energy 2014, 39, 4870–4883. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Ghangrekar, M.M.; Das, D.; Pradhan, D. Graphene supported alpha-MnO2 nanotubes as a cathode catalyst for improved power generation and wastewater treatment in single-chambered microbial fuel cells. RSC Adv. 2013, 3, 7902–7911. [Google Scholar] [CrossRef]
- Lu, Z.J.; Bao, S.J.; Gou, Y.T.; Cai, C.J.; Ji, C.C.; Xu, M.W.; Song, J.; Wang, R.Y. Nitrogen-doped reduced-graphene oxide as an efficient metal-free electrocatalyst for oxygen reduction in fuel cells. RSC Adv. 2013, 3, 3990–3995. [Google Scholar] [CrossRef]
- Qu, L.T.; Liu, Y.; Baek, J.B.; Dai, L.M. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.V.; Kumar, G.G. Current status, key challenges and its solutions in the design and development of graphene based ORR catalysts for the microbial fuel cell applications. Biosens. Bioelectron. 2016, 77, 1208–1220. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Lin, J.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, Q.; Dai, L. Highly efficient metal-free growth of nitrogen-doped single-walled carbon nanotubes on plasma-etched substrates for oxygen reduction. J. Am. Chem. Soc. 2010, 132, 15127–15129. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Z.; Hu, Y.Y.; Xu, Q.; Sun, J.; Hou, B.; Zhang, Y.P. Iron- and nitrogen-functionalized graphene as a non-precious metal catalyst for enhanced oxygen reduction in an air-cathode microbial fuel cell. J. Power Sources 2012, 213, 265–269. [Google Scholar] [CrossRef]
- Feng, Y.; Shi, X.; Wang, X.; Lee, H.; Liu, J.; Qu, Y.; He, W.; Kumar, S.M.S.; Kim, B.H.; Ren, N. Effects of sulfide on microbial fuel cells with platinum and nitrogen-doped carbon powder cathodes. Biosens. Bioelectron. 2012, 35, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Chatenet, M.; Genies-Bultel, L.; Aurousseau, M.; Durand, R.; Andolfatto, F. Oxygen reduction on silver catalysts in solutions containing various concentrations of sodium hydroxide–comparison with platinum. J. Appl. Electrochem. 2002, 32, 1131–1140. [Google Scholar] [CrossRef]
- Feng, C.; Lv, Z.; Yang, X.; Wei, C. Anode modification with capacitive materials for a microbial fuel cell: An increase in transient power or stationary power. Phys. Chem. Chem. Phys. 2014, 16, 10464–10472. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Yu, H.; Wang, X. Catalysis kinetics and porous analysis of rolling activated carbon-PTFE air-cathode in microbial fuel cells. Environ. Sci. Technol. 2012, 46, 13009–13015. [Google Scholar] [CrossRef] [PubMed]
- Gangadharan, P.; Nambi, I.M.; Senthilnathan, J. Liquid crystal polaroid glass electrode from e-waste for synchronized removal/recovery of Cr+6 from wastewater by microbial fuel cell. Bioresour. Technol. 2015, 195, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Woods, M.P.; Biddinger, E.J.; Matter, P.H.; Mirkelamoglu, B.; Ozkan, U.S. Correlation between oxygen reduction reaction and oxidative dehydrogenation activities over nanostructured carbon catalysts. Catal. Lett. 2010, 136, 1–8. [Google Scholar] [CrossRef]
- Mustakeem. Electrode materials for microbial fuel cells: Nanomaterial approach. Mater. Renew. Sustain. Energy 2015, 4, 22. [Google Scholar]
- Ci, S.; Cai, P.; Wen, Z.; Li, J. Graphene-based electrode materials for microbial fuel cells. Sci. Chin. Mater. 2015, 58, 496–509. [Google Scholar] [CrossRef]
- Dai, Y.; Chan, Y.; Jiang, B.; Wang, L.; Zou, J.; Pang, K.; Fu, H. Bifunctional Ag/Fe/N/C catalysts for enhancing oxygen reduction via cathodic biofilm inhibition in microbial fuel cells. ACS Appl. Mater. Interfaces 2016, 8, 6992–7002. [Google Scholar] [CrossRef] [PubMed]
- Khomenko, V.G.; Barsukov, V.Z.; Katashinskii, A.S. The catalytic activity of conducting polymers toward oxygen reduction. Electrochim. Acta 2005, 50, 1675–1683. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Liu, Y.; Pu, L.; Chen, Z.; Deng, S. The high-performance and mechanism of P-doped activated carbon as a catalyst for air-cathode microbial fuel cells. J. Mater. Chem. A 2015, 3, 21149–21158. [Google Scholar] [CrossRef]
- Jiang, H.; Halverson, L.J.; Dong, L. A miniature microbial fuel cell with conducting nanofibers-based 3D porous biofilm. J. Micromech. Microeng. 2015, 25, 125017–125032. [Google Scholar] [CrossRef]
- Ren, H.; Lee, H.S.; Chae, J. Miniaturizing microbial fuel cells for potential portable power sources: Promises and challenges. Microfluid. Nanofluid. 2012, 13, 353–381. [Google Scholar] [CrossRef]
- He, C.S.; Mu, Z.X.; Yang, H.Y.; Wang, Y.Z.; Mu, Y.; Yu, H.Q. Electron acceptors for energy generation in microbial fuel cells fed with wastewaters: A mini-review. Chemosphere 2015, 140, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, S.F.Y. Cathode reactions and applications in microbial fuel cells: A review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2504–2525. [Google Scholar] [CrossRef]
- Yan, Z.H.; Wang, M.; Huang, B.X.; Liu, R.M.; Zhao, J.S. Graphene Supported Pt-Co alloy nanoparticles as Cathode Catalyst for Microbial Fuel Cells. Int. J. Electrochem. Sci. 2013, 8, 149–158. [Google Scholar]
- Liu, X.; Yang, C.; Zhang, L.; Li, L.; Liu, S.; Yu, J.; You, L.; Zhou, D.; Xia, C.; Zhao, J.; et al. Metabolic profiling of cadmium-induced effects in one pioneer intertidal halophyte Suaeda salsa by NMR-based metabolomics. Ecotoxicology 2011, 20, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Chang, J.S.; Lai, J.Y. Microalgae-microbial fuel cell: A mini review. Bioresour. Technol. 2015, 198, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jiao, Y.; Lee, D.J. A lab-scale anoxic/oxic-bioelectrochemical reactor for leachate treatments. Bioresour. Technol. 2015, 186, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kundu, P.P. Biocatalysts in microbial fuel cells. Enzyme Microb. Technol. 2010, 47, 179–188. [Google Scholar] [CrossRef]
- Li, R.; Dai, Y.; Chen, B.; Zou, J.; Jiang, B.; Fu, H. Nitrogen-doped Co/Co9S8/partly-graphitized carbon as durable catalysts for oxygen reduction in microbial fuel cells. J. Power Sources 2016, 307, 1–10. [Google Scholar] [CrossRef]
- Hou, Y.; Yuan, H.; Wen, Z.; Cui, S.; Guo, X.; He, Z.; Chen, J. Nitrogen-doped graphene/CoNi alloy encased within bamboo-like carbon nanotube hybrids as cathode catalysts in microbial fuel cells. J. Power Sources 2016, 307, 561–568. [Google Scholar] [CrossRef]
- Ci, S.Q.; Wu, Y.M.; Zou, J.P.; Tang, L.H.; Luo, S.L.; Li, J.H.; Wen, Z.H. Nitrogen-doped graphene nanosheets as high efficient catalysts for oxygen reduction reaction. Chin. Sci. Bull. 2012, 57, 3065–3070. [Google Scholar] [CrossRef]
- Ansari, M.O.; Khan, M.M.; Ansari, S.A.; Amal, I.; Lee, J.; Cho, M.H. pTSA doped conducting graphene/polyaniline nanocomposite fibers: Thermoelectric behavior and electrode analysis. Chem. Eng. J. 2014, 242, 155–161. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, G.Q.; Chen, J.; Yuan, G.E.; Fu, L.; Yang, F.L. Prussian blue/graphene-modified electrode used as a novel oxygen reduction cathode in microbial fuel cell. J. Taiwan Inst. Chem. Eng. 2016, 58, 374–380. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Yang, F.; Ren, N. Performance of carbon fiber cathode membrane with C–Mn–Fe–O catalyst in MBR–MFC for wastewater treatment. J. Membr. Sci. 2015, 484, 27–34. [Google Scholar] [CrossRef]
- Wang, H.; Park, J.D.; Ren, Z.J. Practical energy harvesting for microbial fuel cells. Environ. Sci. Technol. 2015, 49, 3267–3277. [Google Scholar] [CrossRef] [PubMed]

| No. | S/D | Anode | Membranes | Inoculation | Cathode Electrode | RE/Ω | OCV/mV | Power Density/mW·m−2 | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Electrode | Modification | Volume/mL | |||||||||
| 1 | D | 3D GF | PANI | 150 | Nafion 117 | S. oneidensis MR-1 | Carbon cloth | 2000 | >250 | 768 | [70] |
| 2 | D | Graphite felt | PPy/GO | 25 | CEM | S. oneidensis MR-1 | Carbon felt | 500 | ~350 | 1326 | [56] |
| 3 | D | Carbon paper | GNRs/PANI | 100 | Nafion 211 | S. oneidensis MR-1 | Carbon paper | 1000 | - | 856 | [57] |
| 4 | D | Carbon paper | Graphene/Au | 100 | Nafion 211 | S. oneidensis MR-1 | Carbon paper | 1000 | - | 508 | [97] |
| 5 | D | Nickel foams | Graphene/TiO2 | 100 | Nafion 112 | S. oneidensis MR-1 | Carbon paper | - | - | 1060 | [22] |
| 6 | D | Carbon cloth | Graphene | 180 | Nafion 117 | P. aeruginosa | Carbon cloth | 1960 | ~700 | 52.5 | [55] |
| 7 | D | Carbon cloth | rGO-SnO2 | 75 | Nafion 117 | Escherichia coli | Pt rod | 550 | 830 | 1624 | [67] |
| 8 | D | Ni foam | rGO | 25 | - | S. oneidensis MR-1 | - | 100 | 620 | 661 W·m−3 | [109] |
| 9 | D | Carbon cloth | NGNS | 10 | Nafion 115 | Escherichia coli | Carbon cloth | 510 | ~350 | 1008 | [2] |
| 11 | S | Glassy carbon | Microbially reduced graphene | 10 | CEM | Anaerobic activated sludge | Carbon cloth/Pt | 1000 | >450 | 1905 | [23] |
| 13 | S | Graphite block | Graphene | 100 | - | S. oneidensis MR-1 | Carbon paper | 500 | 150 | 102 | [54] |
| 14 | D | Carbon cloth | PANI-rGO | 40 | Nafion 117 | anaerobic sludge | Carbon felts | 500 | 770 | 1390 | [41] |
| 15 | D | Carbon cloth | TiO2/rGO | 100 | Nafion 117 | S. putrefaciens CN32 | Carbon fiber brush | 1500 | - | 3169 | [58] |
| 16 | D | CHI/VSG scaffolds | Nafion 117 | P. aeruginosa | Carbon cloth | 1960 | 910 | 1530 | [68] | ||
| 17 | D | Carbon cloth | Graphene | 20 | NO | Escherichia coli B | Carbon cloth | - | 900 | 2850 | [110] |
| 18 | S | 3D-Graphene | 28 | - | Previous reactor | Carbon cloth/Pt | 1000 | - | 1516 ± 87 | [102] | |
| 19 | D | 3D GS aerogels | 120 | CMI7000 | Anaerobic sludge | Carbon paper | 1000 | ~550 | 710 | [3] | |
| 20 | D | Carbon cloth | GA | 100 | Nafion 117 | S. putrefaciens CN32 | Carbon cloth | 1500 | ~700 | 679.7 | [4] |
| 21 | S | Carbon paper | Graphene with IL-NH2 | - | S. oneidensis MR-1 | - | - | - | 610 | [21] | |
| 22 | D | Stainless-steel mesh | Graphene-containing foam | - | Nafion 117 | S. putrefaciens | Carbon paper | - | ~600 | 768 | [33] |
| 23 | D | Carbon cloth | rGO/PPy | - | - | Escherichia coli | - | 1000 | 400 | 1068 | [42] |
| 24 | D | Carbon paper | Graphene | 140 | CMI-7000 | Anaerobic sludge | Carbon paper | 1000 | 580 | 368 | [64] |
| 25 | D | Polyurethane | GS | - | - | Previous reactor | Carbon cloth/Pt | 475 | - | 1570 | [66] |
| 26 | D | Stainless-steel mesh | Graphene | 115 | Nafion 112 | Escherichia coli | Carbon paper | - | 790 | 2668 | [84] |
| 27 | S | Ni foam | 3D rGO | 20 | - | Escherichia coli K12 | Carbon caper/Pt | 1000 | 623 | 897.1 | [85] |
| No. | S/D | Cathode | Membranes | Inoculation | Anode | External Resistor/Ω | OCV/mV | Power Density/mW·m−2 | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Electrode | Modification | Electrode | Volume/mL | ||||||||
| 1 | D | Carbon paper | NG | Nafion 117 | Activated sludge | Carbon cloth | 100 | 1000 | 650 | 776 ± 12 | [50] |
| 2 | D | Glassy carbon | Fe- and N-functionalized graphene | Nafion 117 | Previous reactor | Carbon felt | ~30 | 1000 | 570 | 885 | [76] |
| 3 | S | Carbon paper | MnO2-NTs/graphene | Nafion 117 | Previous reactor | Carbon cloth | 60 | 1000 | 613 | 4.68 W·m−3 | [113] |
| 4 | S | Carbon cloth | Pt-Co/G | NO | Previous reactor | Carbon cloth | 27 | 1000 | 710 | 1378 | [135] |
| 5 | D | Carbon cloth | Graphene/biofilm | Nafion 117 | Anaerobic activated sludge | Carbon cloth | 10 | 1000 | 390 | 323.2 ± 21 | [60] |
| 6 | D | Carbon cloth | NG | NO | Previous reactor | Carbon fiber brush | 20 | 1000 | 555 | 1350 ± 15 | [71] |
| 7 | S | Stainless steel net | NG | - | Anaerobic sludge | Carbon brush | 200 | 1500 | 243 | 1159.34 | [79] |
| 8 | S | Carbon Paper | Fe-NG | CEM | Anaerobic activated sludge | Carbon felt | 40 | 500 | 242 | 1149.8 | [119] |
| 9 | S | Stainless steel net | MnO2/GNS | - | Anaerobic sludge | Carbon felt | 200 | 1500 | 771 | 2083 | [53] |
| 10 | S | Carbon cloth | α-MnO2/GO | Nafion 117 | Sewage sludge | Carbon cloth | 25 | - | 710 | 3359 | [49] |
| 11 | S | Stainless steel mesh | Cobalt sulfides/GO | NO | Previous reactor | Graphite fiber | 28 | 1000 | 620 | 1156 ± 18 | [140] |
| 12 | D | Carbon cloth | NG/CoNi-alloy | CEM | - | Carbon brush | 140 | 1000 | ~700 | 2000 | [141] |
| 13 | S | PANI | Graphene | NO | Residual sludge | Graphite | 1800 | 500 | 640 | 99 | [51] |
| 14 | D | Carbon cloth | rGO particles | Ultex CMI 7000 | Anaerobic sludge | Carbon brush | 120 | 1000 | 650 | 3.3 W·m−3 | [52] |
| 15 | D | Carbon paper | Graphene with Iron tetrasulfophthalocyanine. | Nafion 112 | Escherichia coli | Carbon paper | 115 | - | - | 817 | [45] |
| 16 | S | Carbon cloth | Graphene/Pt | NO | Escherichia coli | Carbon cloth | 75 | 1000 | ~260 | 0.159 | [18] |
| 17 | D | Carbon cloth | NG | - | - | Carbon fiber brush | 120 | - | 840 | 4.06 W·m−3 | [142] |
| 18 | D | Graphene-Au-laccase hybrid | PFSA NRE-211 | Trametes versicolor | Graphene-Au | - | - | 1160 | 1.96 mW·cm−2 | [6] | |
| 19 | S | Carbon paper | Graphene/PANI | - | - | - | - | - | 593 | 17.95 | [143] |
| 20 | D | Graphite rods | Prussian blue/graphene | Nafion 117 | Previous reactor | Graphite rods | 80 | 1000 | 530 | 15.63 W·m−3 | [144] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, F.; Wang, C.; Ma, J. Applications of Graphene-Modified Electrodes in Microbial Fuel Cells. Materials 2016, 9, 807. https://doi.org/10.3390/ma9100807
Yu F, Wang C, Ma J. Applications of Graphene-Modified Electrodes in Microbial Fuel Cells. Materials. 2016; 9(10):807. https://doi.org/10.3390/ma9100807
Chicago/Turabian StyleYu, Fei, Chengxian Wang, and Jie Ma. 2016. "Applications of Graphene-Modified Electrodes in Microbial Fuel Cells" Materials 9, no. 10: 807. https://doi.org/10.3390/ma9100807







