3D Bioprinting Technologies for Hard Tissue and Organ Engineering
Abstract
:1. Introduction
2. 3D Printing Technologies
2.1. Classification of 3D Printing Technologies
2.1.1. Categories Divided in Working Principles
2.1.2. Categories Divided in Starting Material States
2.1.3. 3D Printing Categories in Energy Sources
2.1.4. Categories Divided in Biological Functions
2.2. Three Main 3D Bioprinting Technologies
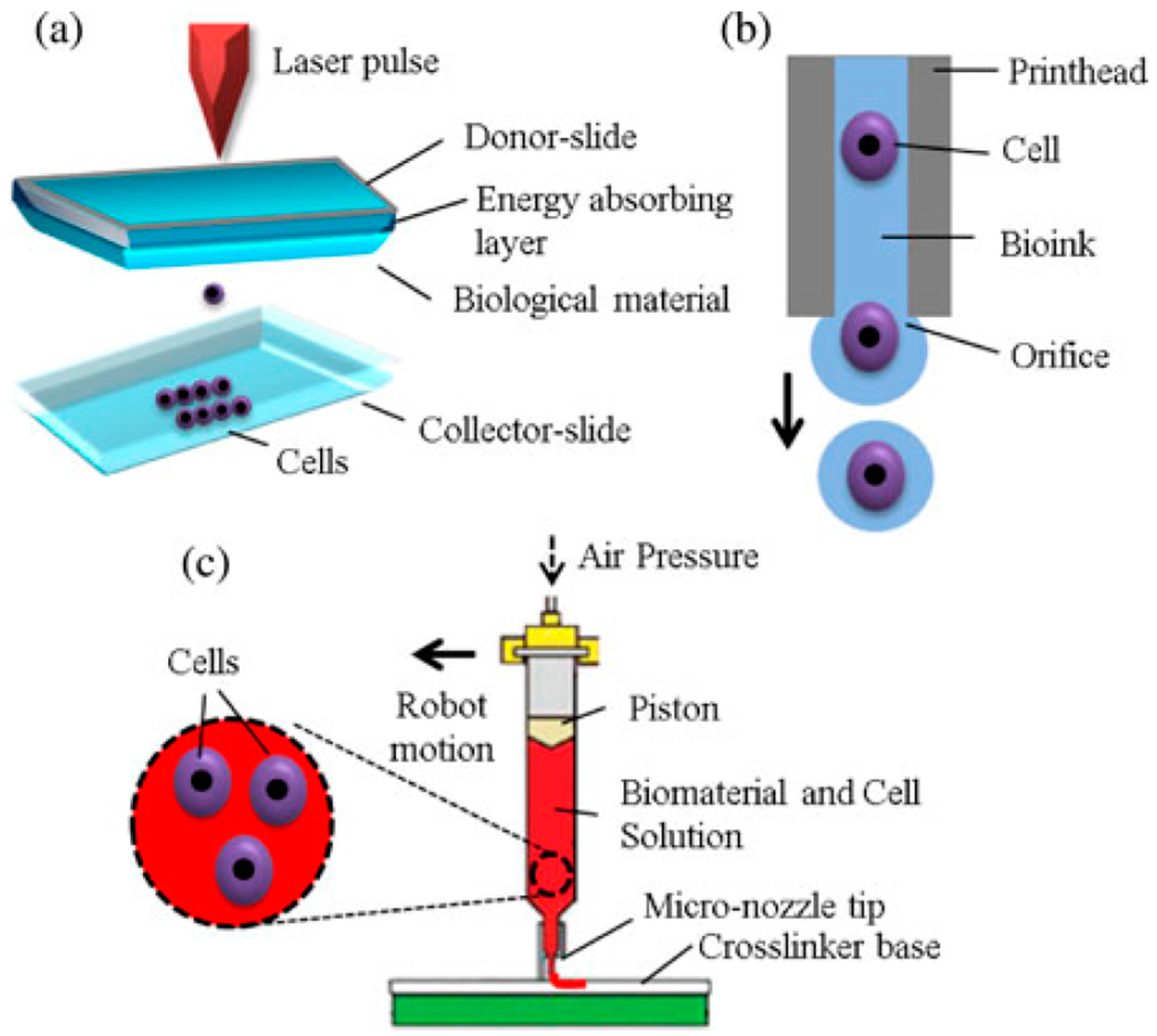
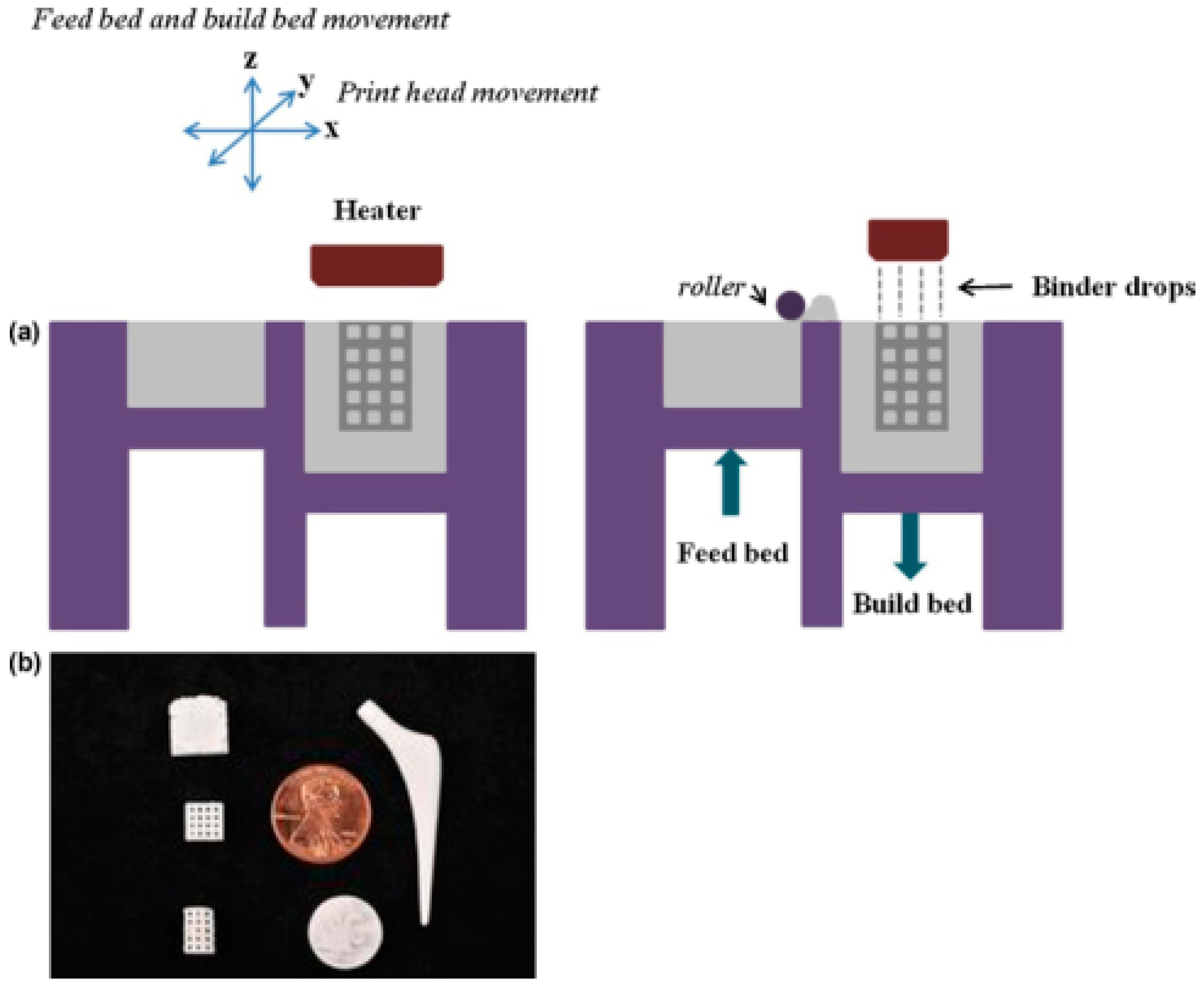
2.2.1. Inkjet-Based 3D Bioprinting
2.2.2. Laser-Based 3D Bioprinting
2.2.3. Extrusion-Based 3D Bioprinting
3. Examples of 3D Bioprinting Technologies for Hard Tissue and Organ Engineering
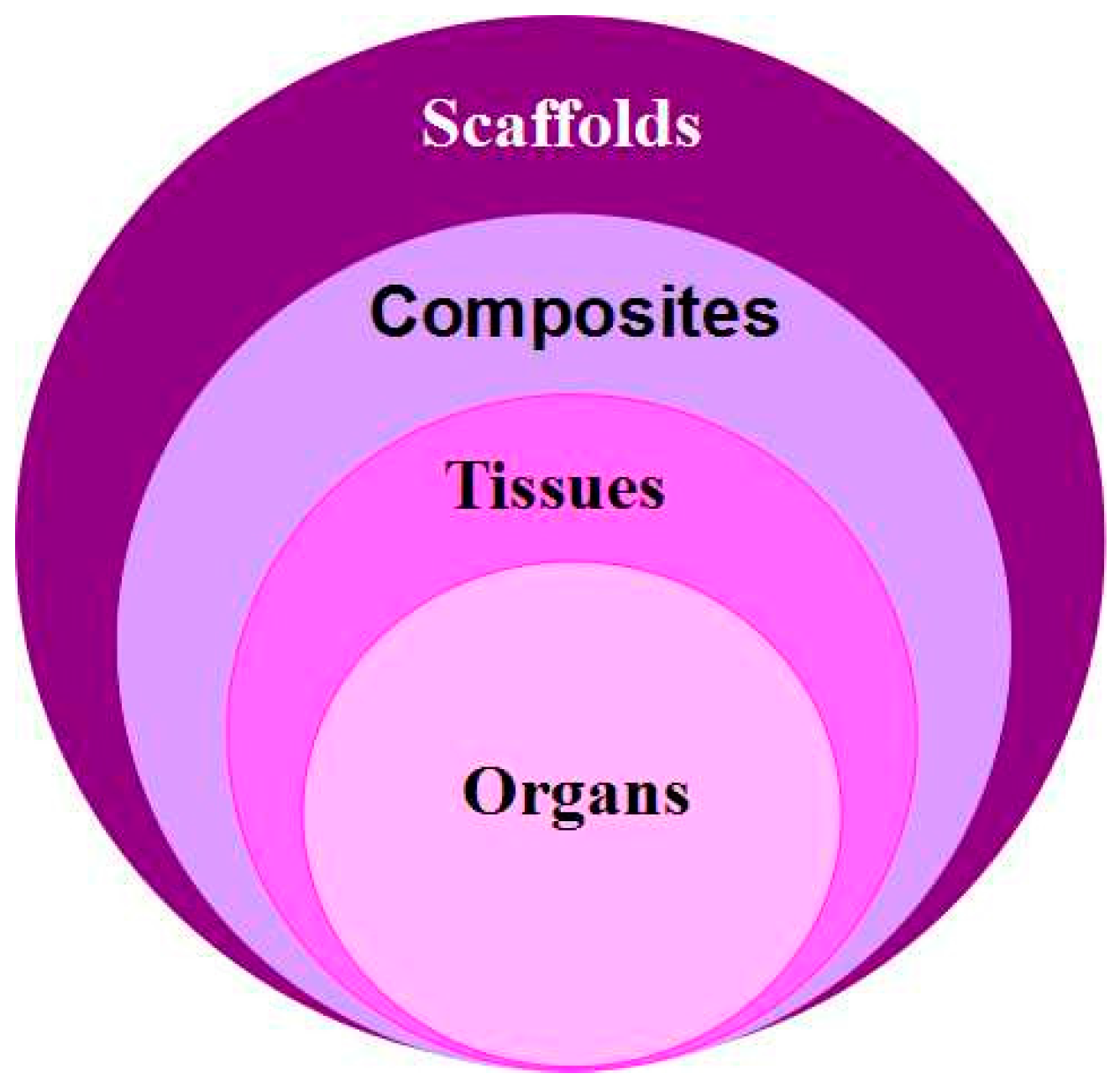
3.1. Hard Tissue Scaffolds Printing
3.2. Construction of Patient-Specific Tissues
3.3. Hard Organ Printing
4. Conclusions and Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, W.Y. The structure, development and health of the teeth. Bull. Biol. 1982, 8, 36–39. [Google Scholar]
- Eckstein, F.; Reiser, M.; Englmeier, K.H.; Putz, R. In vivo morphometry and functional analysis of human articular cartilage with quantitative magnetic resonance imaging—From image to data, from data to theory. Anat. Embryol. 2001, 203, 147–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Ma, J.B.; Wang, Y.N.; He, B.L. Progress in the research of bone substitutes. J. Biomed. Eng. 2001, 18, 647–652. [Google Scholar]
- Wang, X.; Ma, J.; Feng, Q.; Cui, F.Z. Skeletal repair in rabbits with calcium phosphate cements incorporated phosphorylated chitin. Biomaterials 2002, 23, 4591–4600. [Google Scholar] [CrossRef]
- Wang, X.; Ma, J.; Wang, Y.; He, B. Bone repair in radii and tibias of rabbits with phosphorylated chitosan reinforced calcium phosphate cements. Biomaterials 2002, 23, 4167–4176. [Google Scholar] [CrossRef]
- Wang, X.; Ma, J.; Wang, Y.; He, B. Structural characterization of phosphorylated chitosan and their applications as effective additives of calcium phosphate cements. Biomaterials 2001, 22, 2247–2255. [Google Scholar] [CrossRef]
- Wang, X.H.; Ma, J.B.; Wang, Y.N.; He, B.L. Reinforcement of calcium phosphate cements with phosphorylated chitin. Chin. J. Polym. Sci. 2002, 4, 325–332. [Google Scholar]
- Wang, X.H.; Ma, J.B.; Feng, Q.L.; Cui, F.Z. In vivo evaluation of S-chitosan enhanced calcium phosphate cements. J. Bioact. Compat. Polym. 2003, 18, 259–271. [Google Scholar] [CrossRef]
- Wang, X.H.; Feng, Q.L.; Cui, F.Z.; Ma, J.B. The effects of S-chitosan on the physical properties of calcium phosphate cements. J. Bioact. Compat. Polym. 2003, 18, 45–57. [Google Scholar] [CrossRef]
- Bruder, S.P.; Kraus, K.H.; Goldberg, V.M.; Kadiyala, S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J. Bone Jt. Surg. Am. 1998, 80, 985–996. [Google Scholar]
- Seitz, H.; Rieder, W.; Irsen, S.; Leukers, B.; Tille, C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tuomi, J.; Mäkitie, A.A.; Poloheimo, K.-S.; Partanen, J.; Yliperttula, M. The integrations of biomaterials and rapid prototyping techniques for intelligent manufacturing of complex organs. In Advances in Biomaterials Science and Applications in Biomedicine; Lazinica, R., Ed.; In Tech: Rijeka, Croatia, 2013; pp. 437–463. [Google Scholar]
- Wang, X. Overview on biocompatibilities of implantable biomaterials. In Advances in Biomaterials Science and Biomedical Applications in Biomedicine; Lazinica, R., Ed.; In Tech: Rijeka, Croatia, 2013; pp. 111–155. [Google Scholar]
- Wang, X.; Yan, Y.; Zhang, R. Gelatin-based hydrogels for controlled cell assembly. In Biomedical Applications of Hydrogels Handbook; Ottenbrite, R.M., Ed.; Springer: New York, NY, USA, 2010; pp. 269–284. [Google Scholar]
- Wang, X. 3D printing of tissue/organ analogues for regenerative medicine. In Handbook of Intelligent Scaffolds for Regenerative Medicine, 2nd ed.; Pan Stanford Publishing: Palo Alto, CA, USA, 2016; in press. [Google Scholar]
- Wang, X.; Zhang, Q. Overview on “Chinese-Finnish workshop on biomanufacturing and evaluation techniques”. Artif. Org. 2011, 35, E191–E193. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Wang, X. Biodegradable polymers and stem cells for bioprinting. Molecules 2016, 21, 539. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, X. Organ manufacturing. In Organ Manufacturing; Wang, X.H., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2015; pp. 1–28. [Google Scholar]
- Liu, L.; Wang, X. Hared tissue and organ manufacturing. In Organ Manufacturing; Wang, X.H., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2015; pp. 301–333. [Google Scholar]
- Wang, X.; Wang, J. Vascularization and adipogenesis of a spindle hierarchical adipose-derived stem cell/collagen/alginate-PLGA construct for breast manufacturing. IJITEE 2015, 4, 1–8. [Google Scholar]
- Schrepfer, I.; Wang, X.H. Progress in 3D printing technology in health care. In Organ Manufacturing; Wang, X.H., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2015; pp. 29–74. [Google Scholar]
- Ozbolat, I.T.; Yu, Y. Bioprinting toward organ fabrication: Challenges and future trends. IEEE Trans. Biomed. Eng. 2013, 60, 691–699. [Google Scholar]
- Henzler, T.; Chai, L.; Wang, X.H. Integrated model for organ manufacturing: A systematic approach from medical imaging to rapid prototyping. In Organ Manufacturing; Wang, X.H., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2015; pp. 171–200. [Google Scholar]
- Azari, A.; Nikzad, S. The evolution of rapid prototyping in dentistry: A review. Rapid Prototyp. J. 2009, 15, 216–225. [Google Scholar] [CrossRef]
- Melchels, F.P.; Domingos, M.A.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Wang, X. Intelligent freeform manufacturing of complex organs. Artif. Org. 2012, 36, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, Y.; Zhang, R. Recent trends and challenges in complex organ manufacturing. Tissue Eng. Part B 2010, 16, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping as tool for manufacturing bioartificial livers. Trends Biotechnol. 2007, 25, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Markillie, P. A third industrial revolution. Integr. Biol. 2009, 1, 148–149. [Google Scholar]
- Schieker, M.; Seitz, H.; Drosse, I.; Seitz, S.; Mutschler, W. Biomaterials as scaffold for bone tissue engineering. Eur. J. Trauma 2006, 32, 114–124. [Google Scholar] [CrossRef]
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 98, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Fastermann, P. 3D Printing: How Additive Manufacturing Technique Works (3D-Drucken: Wie Die Generative Fertigungstechnik Funktioniert); Technik im Fokus—Springer: Berlin, Germany, 2014. [Google Scholar]
- Gausemeier, J.; Echterhoff, N.; Kokoschka, M.; Wall, M. Thinking Ahead of the Future of Additive Manufacturing. Future Applications; University of Paderborn: Paderborn, Germany, 2011. [Google Scholar]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Krishna, B.V.; Bandyopadhyay, A.; Bose, S. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater. 2007, 3, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 4,575,330, 11 March 1986. [Google Scholar]
- Wilson, C.E.; de Bruijn, J.D.; van Blitterswijk, C.A.; Verbout, A.J.; Dhert, W.J. Design and fabrication of standardized hydroxyapatite scaffolds with a defined macro-architecture by rapid prototyping for bone-tissue-engineering research. J. Biomed. Mater. Res. A 2004, 68, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Committee F42 on Additive Manufacturing Technologies. Terminology for Additive Manufacturing Technologies; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Chua, C.K.; Yeong, W.Y. Bioprinting: Principles and Applications; World Scientific Publishing Co.: Singapore, 2015; p. 296. [Google Scholar]
- Doyle, K. Bioprinting: From patches to parts. Gen. Eng. Biotechnol. News 2014, 34, 34–35. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Lam, C.X.F.; Mo, X.M.; Teoh, S.H.; Hutmacher, D.W. Scaffold development using 3D printing with a starch-based polymer. Mater. Sci. Eng. 2002, 20, 49–56. [Google Scholar] [CrossRef]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.; Hutmacher, D.W.; Melchels, F.P.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Yeong, W.Y.; Chua, C.K.; Leong, K.F.; Chandrasekaran, M.; Lee, M.W. Indirect fabrication of collagen scaffold based on inkjet printing technique. Rapid Rrot. J. 2006, 12, 229–237. [Google Scholar] [CrossRef]
- Philippi, J.A.; Miller, E.; Weiss, L.; Huard, J.; Waggoner, A.; Campbell, P. Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle- and bone-like subpopulations. Stem Cells 2008, 26, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Sears, N.A.; Seshadri, D.R.; Dhavalikar, P.S.; Cosgriff-Hernandez, E. A review of three-dimensional printing in tissue engineering. Tissue Eng. B 2016, 22, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Odde, D.J.; Renn, M.J. Laser-guided direct writing of living cells. Biotechnol. Bioeng. 2000, 67, 312–318. [Google Scholar] [CrossRef]
- Odde, D.J.; Renn, M.J. Laser-guided direct writing for applications in biotechnology. Trends Biotechnol. 1999, 17, 385–389. [Google Scholar] [CrossRef]
- Murphy, M.; Lee, C.; Steen, W.M. Studies in Rapid Prototyping by Laser Surface Cladding Conference (LIA); LIA Laser Institute of America: Orlando, FL, USA, 1994. [Google Scholar]
- Cheah, C.M.; Chua, C.K.; Lee, C.W.; Feng, C.; Totong, K. Rapid prototyping and tooling techniques: A review of applications for rapid investment casting. Int. J. Adv. Manuf. Technol. 2005, 25, 308–320. [Google Scholar] [CrossRef]
- Lander, R.; Pfister, A.; Hubner, U.; John, H.; Schmelzeisen, R.; Mulhaupt, R. Fabrication of soft tissue engineeering scaffolds by means of rapid prototyping techniques. J. Mater. Sci. 2002, 37, 3107–3116. [Google Scholar] [CrossRef]
- Wang, W.; Li, G.; Huang, Y. Modeling of bubble expansion-induced cell mechanical profile in laser-assisted cell direct writing. J. Manuf. Sci. Eng. 2009, 131, 051013. [Google Scholar] [CrossRef]
- Hopp, B.; Smausz, T.; Szabó, G.; Kolozsvári, L.; Kafetzopoulos, D.; Fotakis, C.; Nógrádi, A. Femtosecond laser printing of living cells using absorbing film-assisted laser-induced forward transfer. Opt. Eng. 2012, 51, 014312. [Google Scholar] [CrossRef]
- Sachs, E.; Cima, M.; Cornie, J. Three dimensional printing: Rapid tooling and prototypes directly from a CAD model. CIRP Ann. Manuf. Technol. 1990, 39, 201–204. [Google Scholar] [CrossRef]
- Li, W.; Sun, W.; Zhang, Y.; Wei, W.; Ambasudhan, R.; Xia, P.; Talantova, M.; Lin, T.; Kim, J.; Wang, X.; et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc. Natl. Acad. Sci. USA 2011, 108, 8299–8304. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Shim, J.-H.; Jang, J.; Kim, S.-W.; Cho, D.-W. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2013, 9, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Peltola, S.M.; Melchels, F.P.; Grijpma, D.W.; Kelloma’ki, M.A. Review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008, 40, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Duarte Campos, D.F.; Blaeser, A.; Korsten, A.; Neuss, S.; Jäkel, J.; Vogt, M.; Fischer, H. The stiffness and structure of three-dimensional printed hydrogels direct the differentiation of mesenchymal stromal cells toward adipogenic and osteogenic lineage. Tissue Eng. Part A 2014, 21, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M.; Yang, S. Extrusion-based additive manufacturing of PEEK for biomedical applications. Virtual Phys. Prototyp. 2015, 10, 123–135. [Google Scholar] [CrossRef]
- Yan, Y.; Cui, F.; Zhang, R.; Hu, Y. Rapid prototyping manufacturing for artificial human bone. Mater. Rev. 2000, 14, 11–13. [Google Scholar]
- Xiong, Z.; Yan, Y.; Wang, S.; Zhang, R.; Zhang, C. Fabrication of porous scaffolds for bone tissue engineering via low-temperation deposition. Scr. Mater. 2002, 46, 771–776. [Google Scholar] [CrossRef]
- Yan, Y.; Xiong, Z.; Hu, Y.; Wang, S.; Zhang, R.; Zhang, C. Layered manufacturing of tissue engineering scaffolds via multi-nozzle deposition. Mater. Lett. 2003, 57, 2623–2628. [Google Scholar] [CrossRef]
- He, K.; Wang, X.; Kumta, S.; Qin, L.; Yan, Y.N.; Zhang, R.; Wang, X. Fabrication of a two-level tumor bone repair biomaterial based on a rapid prototyping technique. Biofabrication 2009, 1, 1–7. [Google Scholar]
- Xu, W.; Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping of polyurethane for the creation of vascular systems. J. Bioact. Compat. Polym. 2008, 23, 103–114. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Yan, Y.; Zhang, R. A polyurethane-gelatin hybrid construct for the manufacturing of implantable bioartificial livers. J. Bioact. Compat. Polym. 2008, 23, 409–422. [Google Scholar] [CrossRef]
- Cui, T.; Yan, Y.; Zhang, R.; Liu, L.; Xu, W.; Wang, X. Rapid prototyping of a double layer polyurethane-collagen conduit for peripheral nerve regeneration. Tissue Eng. Part C 2009, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping a new polyurethane-collagen conduit and its Schwann cell compatibility. J. Bioact. Compat. Polym. 2009, 24, 5–17. [Google Scholar]
- Wang, X.; Yan, Y.; Pan, Y.; Xiong, Z.; Liu, H.; Cheng, J.; Liu, F.; Lin, F.; Wu, R.; Zhang, R.; et al. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 2006, 12, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, X.; Pan, Y.; Liu, H.; Cheng, J.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials 2005, 26, 5864–5871. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, X.; Xiong, Z.; Liu, H.; Liu, F.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Direct construction of a three-dimensional structure with cells and hydrogel. J. Bioact. Compat. Polym. 2005, 20, 259–269. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X.; Yan, Y.; Yao, R.; Ge, Y. A cell-assembly derived physiological 3D model of the metabolic syndrome, based on adipose-derived stromal cells and a gelatin/alginate/fibrinogen matrix. Biomaterials 2010, 31, 3868–3877. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yan, Y.; Liu, H.; Yao, Y.; Wang, X. Control adipose-derived stromal cells differentiation into adipose and endothelial cells in a 3-D structure established by cell-assembly technique. J. Bioact. Compat. Polym. 2009, 24, 31–47. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, Y.; Wang, X.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R. Three-dimensional gelatin and gelatin/hyaluronan hydrogel structures for traumatic brain injury. J. Bioact. Compat. Polym. 2007, 22, 19–29. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Yan, Y.; Zheng, W.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R. Rapid prototyping three-dimensional cell/gelatin/fibrinogen constructs for medical regeneration. J. Bioact. Compat. Polym. 2007, 22, 363–377. [Google Scholar] [CrossRef]
- Nakamura, M.; Kobayashi, A.; Takagi, F.; Watanabe, A.; Hiruma, Y.; Ohuchi, K.; Iwasaki, Y.; Horie, M.; Morita, I.; Takatani, S. Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue Eng. 2005, 11, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Farzadi, A.; Hashjin, M.S.; Eydivand, M.A.; Osman, N.A. Effect of layer thickness and printing orientation on mechanical properties and dimensional accuracy of 3D printed porous samples for bone tissue engineering. PLoS ONE 2014, 9, e108252. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Qian, Z.J.; Ryu, B.; Kumar, N.A.; Kim, S.K. Preparation and characterization of carbon nanotube-grafted-chitosan–natural hydroxyapatite composite for bone tissue engineering. Carbohydr. Polym. 2011, 83, 569–577. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Gruene, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Hofmann, N.; Chichkov, B. Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng. Part C Methods 2010, 17, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Gima, M.J.; Sachs, E.; Cima, L.G.; Yoo, J.; Khanuja, S.; Borland, S.W.; Wu, B.; Giordan, R.A. Computer-driven microstructures by 3D printing: Bio- and structural materials. In Presented at the ’94 SFF, Austin, TX, USA, 8–10 August 1994; pp. 181–190.
- Wu, B.M.; Borland, S.W.; Giordano, R.A.; Cima, L.G.; Sachs, E.M.; Cima, M.J. Solid free form fabrication of drug delivery devices. J. Control. Release 1996, 40, 77–87. [Google Scholar] [CrossRef]
- Cima, L.G.; Gima, M.J. Preparation of Medical Devices by Solid Free-Form Fabrication Methods. U.S. Patent 5,490,962, 13 February 1996. [Google Scholar]
- Giordano, R.A.; Wu, B.M.; Borland, S.W.; Gima, L.G.; Sachs, E.M.; Cima, M.J. Mechanical properties of dense polylactic acid structures fabricated by three dimensional printing. J. Biomater. Sci. Polym. Ed. 1996, 8, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Barlow, J.W. Selective laser sintering of bioceramic materials for implants. In Presented at the ’93 SFF, Austin, TX, USA, 9–11 August 1993; pp. 376–380.
- Langton, C.M.; Whitehead, M.A.; Langton, D.K.; Langley, G. Development of a cancellous bone structural model by stereolithography for ultrasound characterisation of the calcaneus. Med. Eng. Phys. 1997, 19, 599–604. [Google Scholar] [CrossRef]
- Chu, G.T.; Brady, G.A. Ceramic SFF by direct and indirect stereolithography. Mrs Online Proc. Libr. 1998, 542. [Google Scholar] [CrossRef]
- Steidle, C.; Klosterman, D.; Chartoff, R.; Graves, G.; Osborne, N. Automated fabrication of custom bone implants using rapid prototyping. In Presented at the 44th International SAMPE Symposium and Exhibition, Long Bach, CA, USA, 23–27 May 1999.
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef]
- Rayna, T.; Striukova, L. From rapid prototyping to home fabrication: How 3D printing is changing business model innovation. Technol. Forecast. Soc. Chang. 2016, 102. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Sittinger, M.; Risbud, M.V. Scaffold-based tissue engineering: Rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004, 22, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part II. Rapid prototyping techniques. Tissue Eng. 2002, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.K.; Leong, K.F. Rapid Prototyping: Principles and Applications in Manufacturing; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Ricci, J.L.; Clark, E.A.; Murriky, A.; Smay, J.E. Three-dimensional printing of bone repair and replacement materials: Impact on craniofacial surgery. J. Craniofac. Surg. 2012, 23, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Fierz, F.C.; Beckmann, F.; Huser, M.; Irsen, S.H.; Leukers, B.; Witte, F.; Degistirici, O.; Andronache, A.; Thie, M.; Muller, B. The morphology of anisotropic 3D-printed hydroxyapatite scaffolds. Biomaterials 2008, 29, 3799–3806. [Google Scholar] [CrossRef] [PubMed]
- Cazon, A.; Aizpurua, J.; Paterson, A.; Bibb, R.; Campbell, R.I. Customised design and manufacture of protective face masks combining a practitioner-friendly modelling approach and low-cost devices for digitising and additive manufacturing. Virtual Phys. Prototyp. 2014, 9, 251–261. [Google Scholar] [CrossRef]
- Lee, J.M.; Yeong, W.Y. A preliminary model of time-pressure dispensing system for bioprinting based on printing and material parameters. Virtual Phys. Prototyp. 2015, 10, 3–8. [Google Scholar] [CrossRef]
- Pardo, L.; Wilson, W.C.; Boland, T. Characterization of patterned self-assembled monolayers and protein arrays generated by he ink-jet method. Langimur 2003, 19, 1462–1466. [Google Scholar] [CrossRef]
- Wilson, W.C.; Boland, T. Cell and organ printing 1: Protein and cell printers. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 272, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Boland, T.; Mironov, V.; Gutowska, A.; Roth, E.A.; Markwald, R.R. Cell and organ printing 2: Fusion of cell aggregates in three-dimensional gels. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 272A, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003, 21, 157–161. [Google Scholar] [CrossRef]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, X. Creation of a vascular system for complex organ manufacturing. Int. J. Bioprint. 2015, 1, 77–86. [Google Scholar]
- Butscher, A.; Bohner, M.; Hofmann, S.; Gauckler, L.; Müller, R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater. 2011, 7, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Ringeisen, B.R.; Kim, H.; Barron, J.A.; Krizman, D.B.; Chrisey, D.B.; Jackman, S.; Auyeung, R.; Spargo, B.J. Laser printing of pluripotent embryonal carcinoma cells. Tissue Eng. 2004, 10, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Kuhn, S.; Sorg, H.; Gruene, M.; Schlie, S.; Gaebel, R.; Polchow, B.; Reimers, K.; Stoelting, S.; Ma, N.; et al. Laser pinting of skin cells and human stem cells. Tissue Eng. Part C Methods 2010, 16, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Diekman, B.O.; Christoforou, N.; Willard, V.P.; Suna, H.; Sanchez-Adamsa, J.; Leong, K.W.; Guilak, F. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2012, 109, 19172–19177. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; MartÍnez Ávila, H.; Hägg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lian, Q.; Li, D.; Wang, K.; Hao, D.; Bian, W.; He, J.; Jin, Z. Cartilage repair and subchondral bone migration using 3D printing osteochondral composites: A one-year-period study in rabbit trochlea. Biomed. Res. Int. 2014, 5, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; De Wijn, J.R.; Verbout, A.J.; Alblas, J.; Dhert, W.J. Three-dimensional fiber deposition of cell-laden, viable, patterned constructs for bone tissue. Tissue Eng. Part A 2008, 14, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Wijnberg, H.M.; Dhert, W.J.; Alblas, J. Distinct tissue formation by heterogeneous printing of osteo- and endothelial progenitor cells. Tissue Eng. Part A 2011, 17, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hong, J.M.; Ju, Y.M.; Jung, J.W.; Kang, H.-W.; Lee, S.J.; Yoo, J.J.; Kim, S.W.; Kim, S.H.; Cho, D.-W. A novel tissue-engineered trachea with a mechanical behavior similar to native trachea. Biomaterials 2015, 62, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, X. Artificial blood vessels and vascular systems. In Organ Manufacturing; Wang, X., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2015; pp. 75–99. [Google Scholar]
- Wang, J.; Wang, X. Development of a Combined 3D Printer and Its Application in Complex Tissue Construction. Master’s Thesis, Tsinghua University, Beijing, China, 2014. [Google Scholar]
- Sui, S.; Wang, X.; Liu, P.; Yan, Y.; Zhang, R. Cryopreservation of cells in 3D constructs based on controlled cell assembly processes. J. Bioact. Compat. Polym. 2009, 24, 473–487. [Google Scholar] [CrossRef]
- Wang, X.; Paloheimo, K.-S.; Xu, H.; Liu, C. Cryopreservation of cell/hydrogel constructs based on a new cell-assembling technique. J. Bioact. Compat. Polym. 2010, 25, 634–653. [Google Scholar] [CrossRef]
- Wang, X.; Xu, H. Incorporation of DMSO and dextran-40 into a gelatin/alginate hydrogel for controlled assembled cell cryopreservation. Cryobiology 2010, 61, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cui, T.; Yan, Y.; Zhang, R. Peroneal nerve regeneration along a new polyurethane-collagen guide conduit. J. Bioact. Compat. Polym. 2009, 24, 109–127. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Z.; Wang, X.; Yan, Y.; Liu, H.; Zhang, R. Direct fabrication of a hybrid cell/hydrogel construct by a double-nozzle assembling technology. J. Bioact. Compat. Polym. 2009, 24, 249–265. [Google Scholar]
- Li, S.; Yan, Y.; Xiong, Z.; Weng, C.; Zhang, R.; Wang, X. Gradient hydrogel construct based on an improved cell assembling system. J. Bioact. Compat. Polym. 2009, 24, 84–99. [Google Scholar]
- Yao, R.; Zhang, R.; Wang, X. Design and evaluation of a cell microencapsulating device for cell assembly technology. J. Bioact. Compat. Polym. 2009, 24, 48–62. [Google Scholar]
- Yao, R.; Zhang, R.; Yan, Y.; Wang, X. In vitro angiogenesis of 3D tissue engineered adipose tissue. J. Bioact. Compat. Polym. 2009, 24, 5–24. [Google Scholar]
- Xu, Y.; Wang, X. Fluid and cell behaviors along a 3D printed alginate/gelatin/fibrin channel. Bioeng. Biotech. 2015, 112, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Wang, X. Rapid prototyping of tubular polyurethane and cell/hydrogel constructs. J. Bioact. Compat. Polym. 2011, 26, 363–374. [Google Scholar]
- Wang, X.; Sui, S.; Yan, Y.; Zhang, R. Design and fabrication of PLGA sandwiched cell/fibrin constructs for complex organ regeneration. J. Bioact. Compat. Polym. 2010, 25, 229–240. [Google Scholar] [CrossRef]
- Wang, X.; He, K.; Zhang, W. Optimizing the fabrication processes for manufacturing a hybrid hierarchical polyurethane-cell/hydrogel construct. J. Bioact. Compat. Polym. 2013, 28, 303–319. [Google Scholar] [CrossRef]
- Huang, Y.; He, K.; Wang, X. Rapid Prototyping of a hybrid hierarchical polyurethane-cell/hydrogel construct for regenerative medicine. Mater. Sci. Eng. C 2013, 33, 3220–3229. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, Y.; Liu, C. A combined rotational mold for manufacturing a functional liver system. J. Bioact. Compat. Polym. 2015, 39, 436–451. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L.; Wang, J.; Xu, Y.F.; Zhang, W.M.; Khang, G.; Wang, X. In vitro vascularization of a combined system based on a 3D bioprinting technique. J. Tissue Eng. Regen. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X. Application of 3D biomimetic models for drug delivery and regenerative medicine. Curr. Pharm. Des. 2015, 21, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, X.; Xu, Y.; Zhang, W.; Liu, C.-H.; Wang, X. Controlled release of growth factors for regenerative medicine. Curr. Pharm. Des. 2015, 21, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Editorial: Drug delivery design for regenerative medicine. Curr. Pharm. Des. 2015, 21, 1503–1505. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Rijff, B.L.; Khang, G. A building block approach into 3D printing a multi-channel organ regenerative scaffold. J. Stem Cell Res. Ther. 2015. [Google Scholar] [CrossRef]
- Wang, X. Spatial effects of stem cell engagement in 3D printing constructs. J. Stem Cells Res. Rev. Rep. 2014, 1, 5–9. [Google Scholar]
- Zhou, X.; Liu, C.; Wang, X. A 3D bioprinting liver tumor model for drug screening. World J. Pharm. Pharm. Sci. 2016. [Google Scholar] [CrossRef]
- Zhao, X.; Du, S.; Chai, L.; Xu, Y.; Liu, L.; Zhou, X.; Wang, J.; Zhang, W.; Liu, C.-H.; Wang, X. Anti-cancer drug screening based on an adipose-derived stem cell/hepatocyte 3D printing technique. J. Stem Cell Res. Ther. 2015, 5. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X. Preparation of an adipose-derived stem cell/fibrin-poly(dl-lactic-co-glycolic acid) construct based on a rapid prototyping technique. J. Bioact. Compat. Polym. 2013, 28, 191–203. [Google Scholar]
- Poldervaart, M.T.; Wang, H.; van der Stok, J.; Weinans, H.; Leeuwenburgh, S.C.G.; Öner, F.C.; Cumhur Öner, F.; Dhert, W.J.A.; Alblas, J. Sustained release of BMP-2 in bioprinted alginate for osteogenicity in mice and rats. PLoS ONE 2013, 8, e72610. [Google Scholar] [CrossRef] [PubMed]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Albrecht, L.D.; Sawyer, S.W.; Soman, P. Developing 3D scaffolds in the field of tissue engineering to treat complex bone defects. 3D Print. Addit. Manuf. 2016, 3, 106–112. [Google Scholar] [CrossRef]
- Schantz, J.T.; Brandwood, A.; Hutmacher, D.W.; Khor, H.L.; Bittner, K. Osteogenic differentiation of mesenchymal progenitor cells in computer designed fibrin-polymer-ceramic scaffolds manufactured by fused deposition modeling. J. Mater. Sci. Mater. Med. 2005, 16, 807–819. [Google Scholar] [CrossRef] [PubMed]



| Technique | Working principle | Main starting biomaterials | Advantages | Disadvantages | Morphology | References |
|---|---|---|---|---|---|---|
| Extrusion-based rapid prototyping (RP) | Fluidic material is forced through a piston nozzle at a low temperature (≤−20 °C) | Natural or synthetic polymer solutions | A wide range of materials can be used; high accuracy; flexible; reproducible; scalable; growth factors can be incorporated; constructs with high mechanical properties can be obtained | Organic solvents are needed for synthetic polymer deposition; cells are difficult to be incorporated |  | [59] |
| Pneumatic extrusion-based bioplotter | Polymer strands stabilized layer-by-layer in a liquid medium | Natural polymer solutions, such as alginate and proteins, cells and growth factors can be incorporated | Good biocompatibilities | Low cell survival rate; weak mechanical properties; fragile |  | [141] |
| Fused deposition modeling (FDM) | Strands of heated polymers extruded through nozzles | Synthetic polymers, such as acrylonitrile butadiene styrene (ABS), poly lactic acid (PLA), polyvinyl alcohol (PVA) | Automated; controllable; fast; sophisticated; accurate; reproducible; scalable | Limited materials can be used; cells cannot be incorporated directly |  | [142] |
| FDM | Strands of polymer composite extruded through a commercial FDM (MakerBot) | Hydroxyapatite (HA) incorporated polycaprolactone (PCL) | Automated; controllable; fast; sophisticated; accurate; reproducible; scalable | Limited materials can be used; cells cannot be incorporated directly |  | [143] |
| Indirect 3D bio-printing | Fibrin-polymer–ceramic scaffolds manufactured by fused deposition modeling | Calcium phosphate modified PCL (PCL-CaP) and treated with fibrinogen | A wide range of biomaterials can be used; cells and bioactive agents can be incorporated | Low accuracy of the final structures; complex processing procedures |  | [144] |
| Indirect micro-stereolithography (mSTL) | Tracheal cartilage regeneration on an indirect printed gelatin sponge | Poly-(l-Lactide-co-ε-caprolactone)/gelatin, heparin, transforming growth factor-β1, chondrocytes | A wide range of biomaterials can be used; bioactive agents can be incorporated | Low accuracy of the final structures; complex processing procedures; limited mechanical properties |  | [111] |
| Laser-based stereolithography (SLA) | A small-spot of laser is used for solid polymers | Synthetic polymers | High resolution; cells can be incorporated | Limited materials; low throughput |  | [54,85] |
| Thermal inkjet-based AM | Collagen was dissolved into phosphoric acid-based binder solution to fabricate collagen-calcium phosphate composites | Collagen solutions | The fabrication temperature can be reduced | Low accuracy; low mechanical properties; cells cannot be incorporated |  | [113] |
| Extrusion-based RP | Pneumatic forced nozzles for fluidic materials | Natural or synthetic polymer solutions | A wide range of biomaterials can be used; cells, bioactive agents can be incorporated | Nozzle easily clogging; harms to cells |  | [35] |
| Inkjet-based RP | Fluidic material is forced through an orifice | Hyaluronic acid (HA) improved gelatin-methacrylamide (gelMA) hydrogels | High mechanical properties; cells, bioactive agents can be incorporated | Limited biomaterials can be used; limited height of the construct |  | [144] |
| Direct write (DW) RP | 3D ink writing (or robocasting) in an oil bath | A concentrated colloidal gel (typically 50% HA particles suspended in an aqueous medium) | Two materials can be printed in a construct | Limited biomaterials can be used; limited height of the construct |  | [95] |
| Double nozzle extrusion-based RP | Fluidic materials are forced through two piston nozzles at a temperature about 10 °C | Natural polymer hydrogels, such as gelatin, gelatin/alginate, and gelatin/alginate/fibrinogen | A wide range of biomaterials can be used; cells, bioactive agents can be incorporated; branched vascular systems can be easily created; excellent biocompatibilities | Weak mechanical properties; high concentration of hydrogels affects cell–cell interactions; easily being biodegraded under in vivo conditions |  | [120,121] |
| Double nozzle low-temperature extrusion-based RP | Fluidic materials are forced through two piston nozzles at a temperature ≤−20 °C | Natural and synthetic polymer solutions | A wide range of biomaterials can be used; cells, growth factors, cytokines, chemicals, genes can be incorporated; branched vascular systems can be easily created; high mechanical properties; stable; fast; controllable; sophisticated; accurate; scalable; reproducible | High concentration of natural hydrogels affects cell–cell interactions; organic solvents are needed for synthetic polymer dissolution and to be removed after printing |  | [61,62,127,128] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Wei, Y.; Hou, W.; Tong, H.; Bai, S. 3D Bioprinting Technologies for Hard Tissue and Organ Engineering. Materials 2016, 9, 802. https://doi.org/10.3390/ma9100802
Wang X, Ao Q, Tian X, Fan J, Wei Y, Hou W, Tong H, Bai S. 3D Bioprinting Technologies for Hard Tissue and Organ Engineering. Materials. 2016; 9(10):802. https://doi.org/10.3390/ma9100802
Chicago/Turabian StyleWang, Xiaohong, Qiang Ao, Xiaohong Tian, Jun Fan, Yujun Wei, Weijian Hou, Hao Tong, and Shuling Bai. 2016. "3D Bioprinting Technologies for Hard Tissue and Organ Engineering" Materials 9, no. 10: 802. https://doi.org/10.3390/ma9100802
APA StyleWang, X., Ao, Q., Tian, X., Fan, J., Wei, Y., Hou, W., Tong, H., & Bai, S. (2016). 3D Bioprinting Technologies for Hard Tissue and Organ Engineering. Materials, 9(10), 802. https://doi.org/10.3390/ma9100802






