Synthesis and Characterization of Nanofibrous Polyaniline Thin Film Prepared by Novel Atmospheric Pressure Plasma Polymerization Technique
Abstract
:1. Introduction
2. Results and Discussion
2.1. Detailed iPC-APPJ Device and Optical, Electronical, and Discharge Characteristics

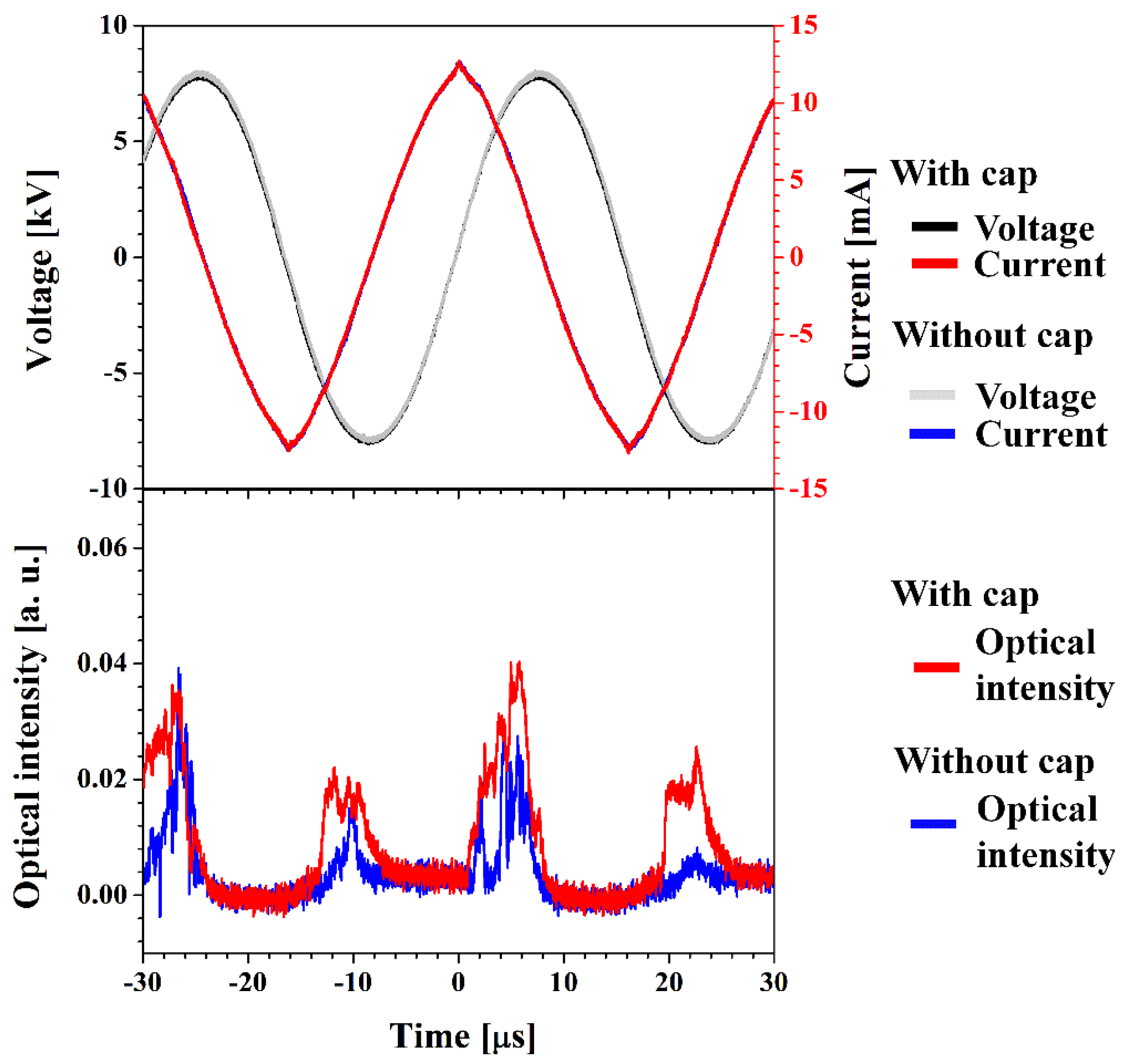
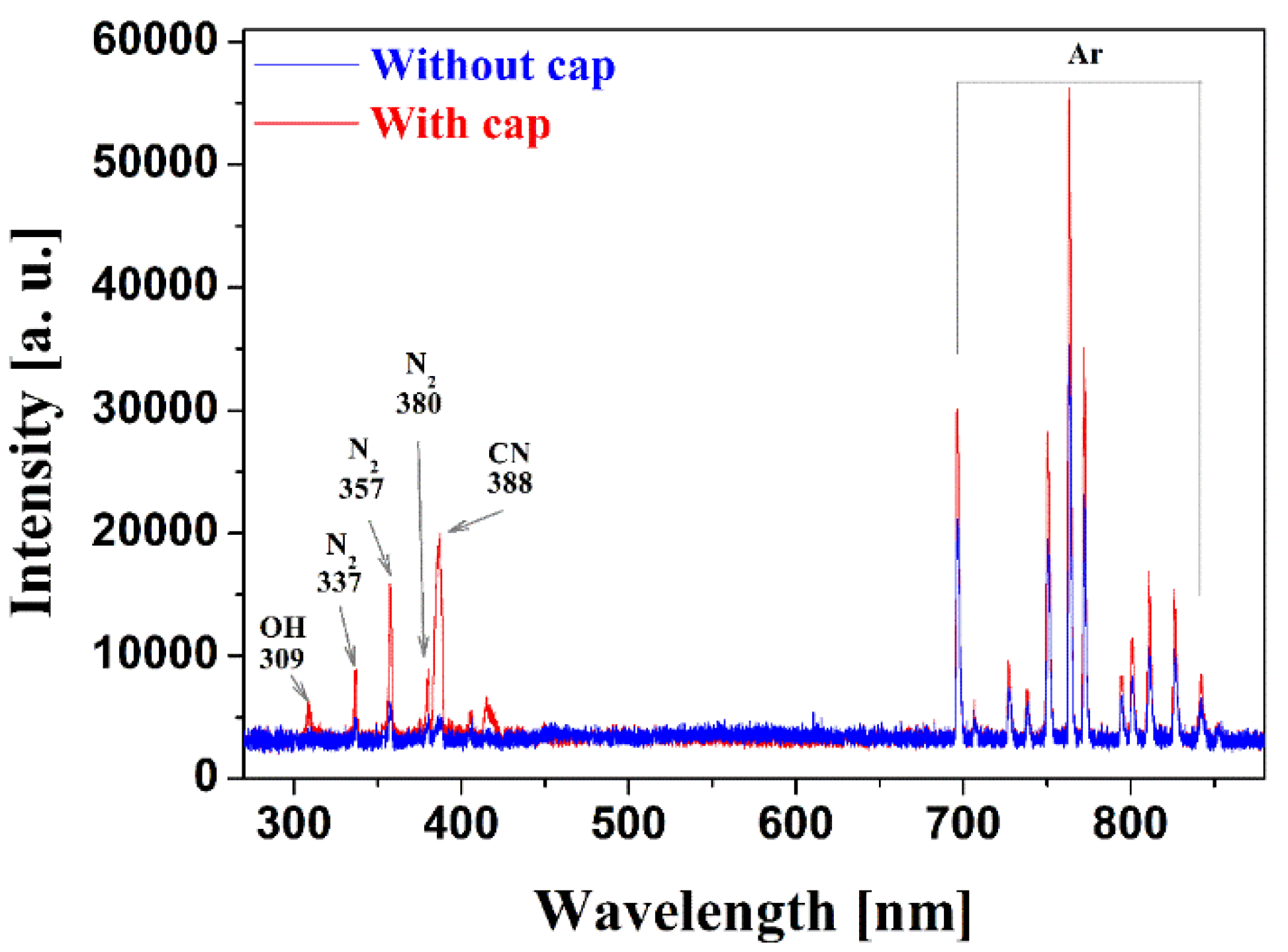
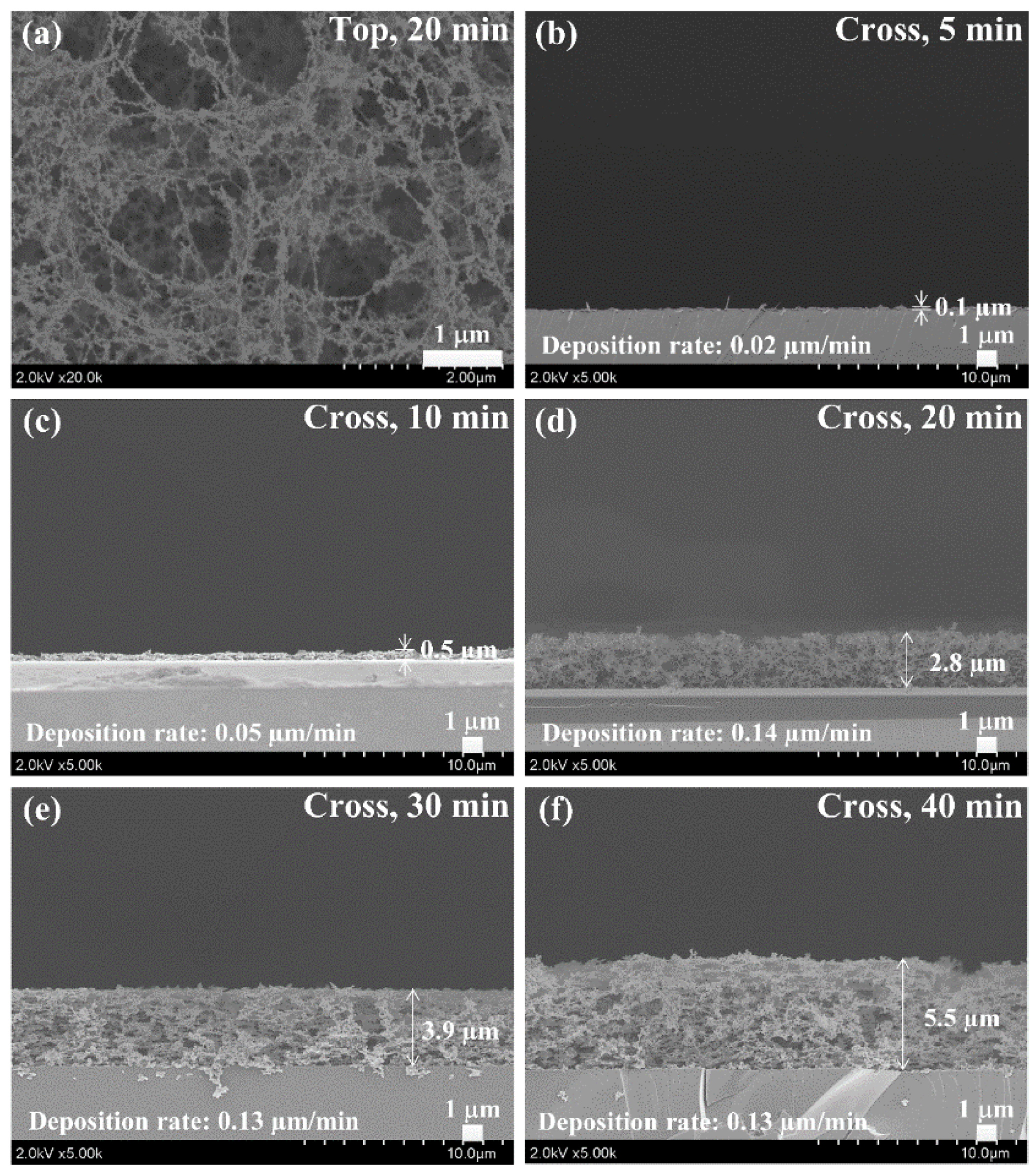
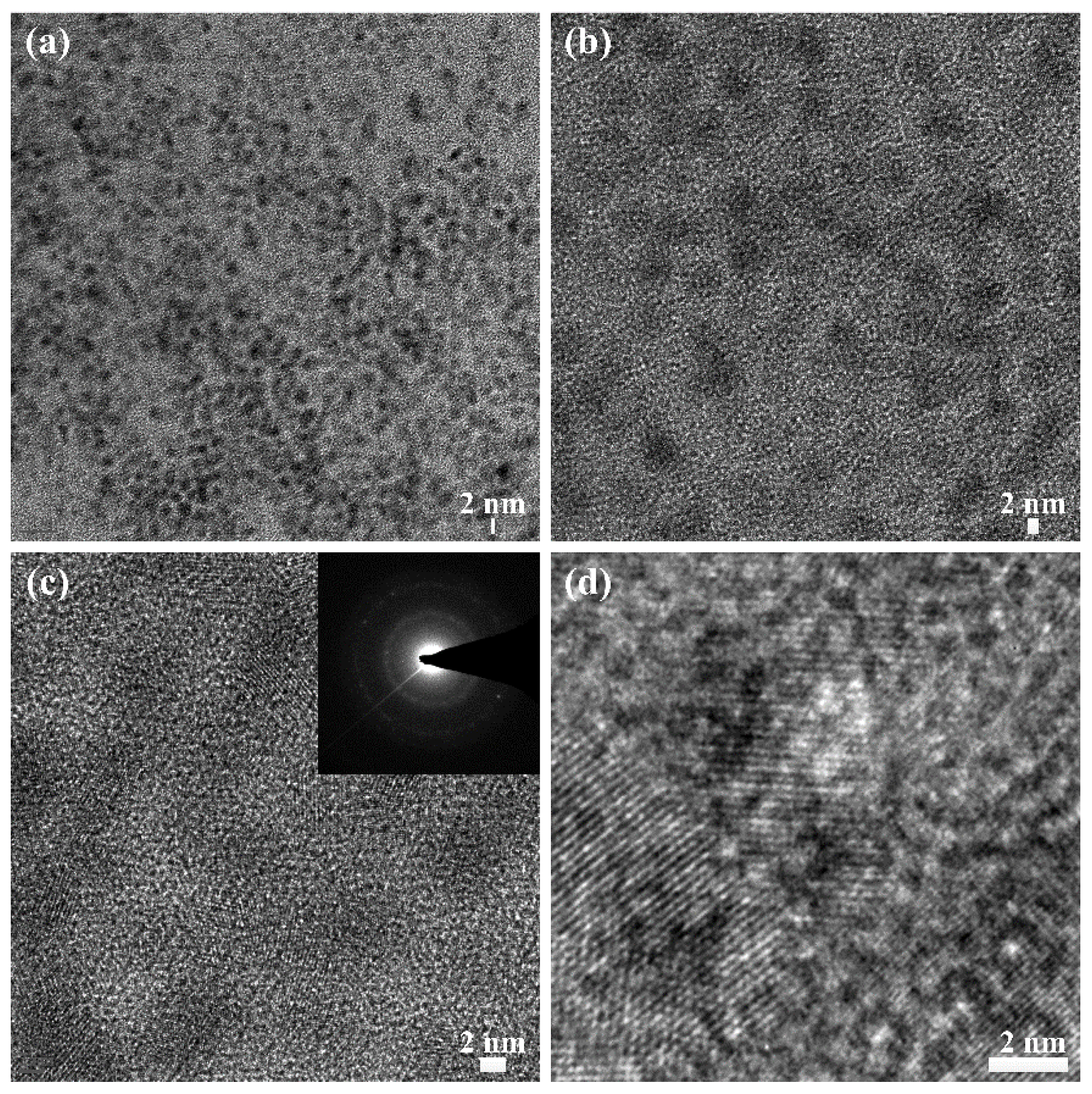
2.2. Functionalization and Characterization of Newly Synthesized pPANI Nanoparticles and Nanofibers Using iPC-APPJ
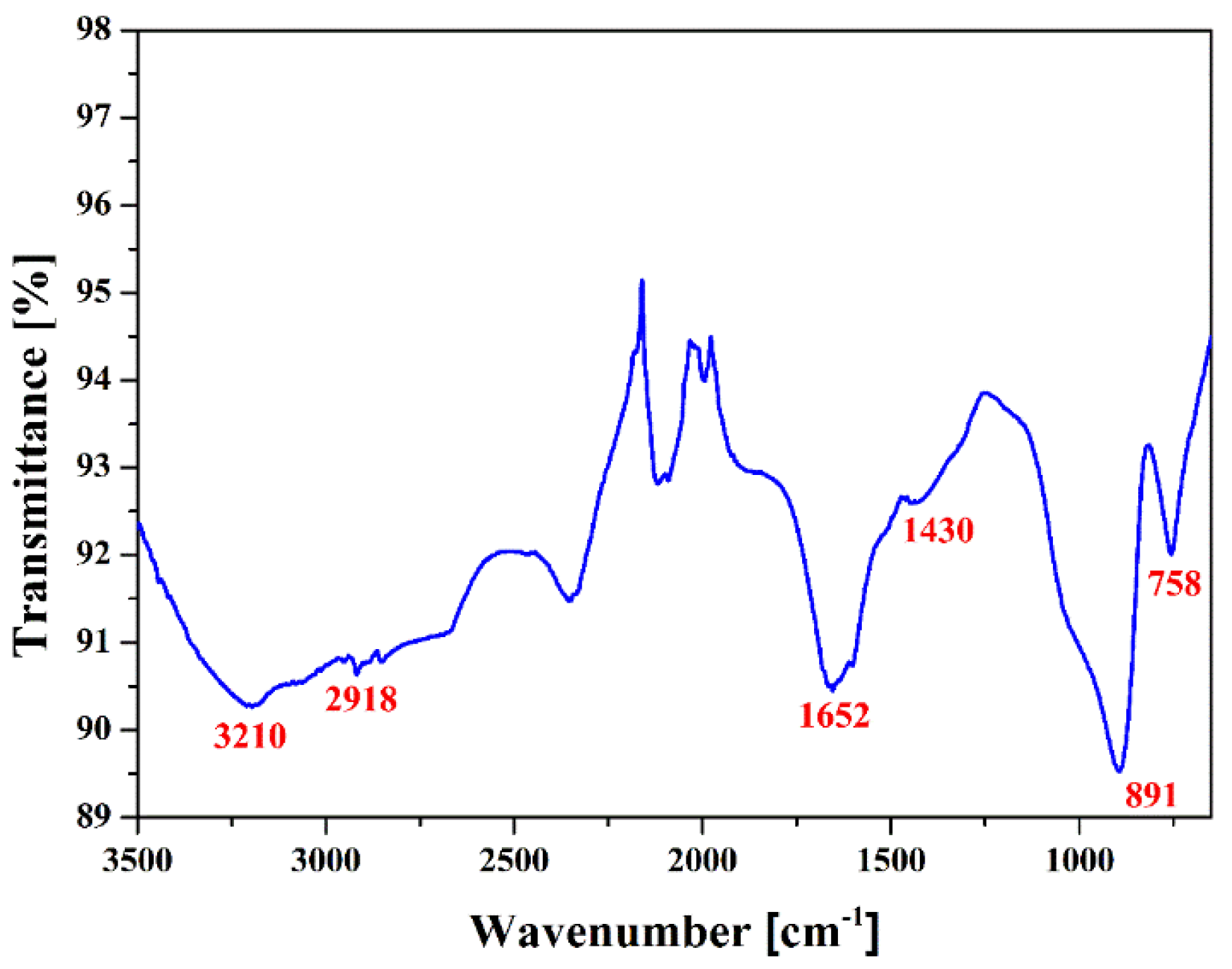
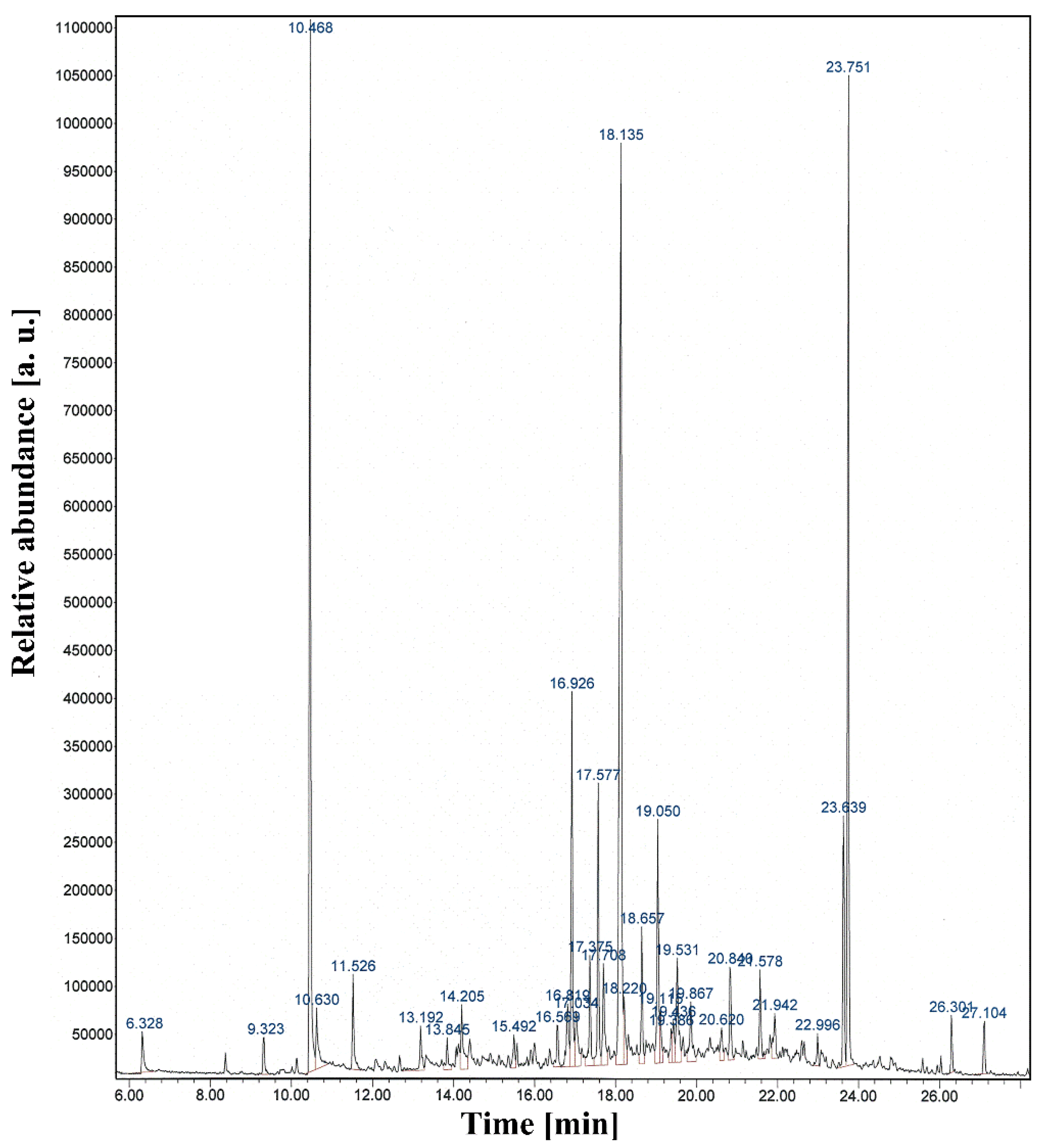
| Samples | Mw (kDa) | Mn (kDa) | Polydispersity Index (PDI) |
|---|---|---|---|
| Aniline monomer (Aldrich) | 0.093 | - | - |
| pPANI | 533.2 | 283.0 | 1.9 |
3. Experimental Section
3.1. Plasma Polyaniline Synthesis
3.2. Optical and Electronical Measurement
3.3. Field Emission Scanning Electron Microscopy
3.4. Transmission Electron Microscopy
3.5. Fourier Transform Infrared Spectroscopy
3.6. Gas Chromatography-Mass Spectrometry
3.7. Gel Permeation Chromatography
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johansson, P.; Jimbo, R.; Kozai, Y.; Sakurai, T.; Kjellin, P.; Currie, F.; Wennerberg, A. Nanosized hydroxyapatite coating on PEEK implants enhances early bone formation: A histological and three-dimensional investigation in rabbit bone. Materials 2015, 8, 3815–3830. [Google Scholar] [CrossRef]
- Wei, M.; Wang, Y. Surface attachment of gold nanoparticles guided by block copolymer micellar films and its application in silicon etching. Materials 2015, 8, 3793–3805. [Google Scholar] [CrossRef]
- Miao, M.; Chen, Q.; Zhang, C.; Cao, X.; Zhou, W.; Qiu, Q.; An, Z. Nanoprecipitation of PMMA stabilized by core cross-linked star polymers. Macromol. Chem. Phys. 2013, 214, 1158–1164. [Google Scholar] [CrossRef]
- Dan, L.I.; Huang, J.; Kaner, R.B. Polyaniline nanofibers: A unique polymer nanostructure for versatile applications. Acc. Chem. Res. 2009, 42, 135–145. [Google Scholar]
- Bagheri, H.; Babanezhad, E.; Es-haghi, A. An aniline-based fiber coating for solid phase microextraction of polycyclic aromatic hydrocarbons from water followed by gas chromatography-mass spectrometry. J. Chromatogr. A 2007, 1152, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Deng, J.; Yang, Y.; Yang, W. Synthesis of large-area three-dimensional polyaniline nanowire networks using a “soft template”. Macromol. Rapid Commun. 2005, 26, 395–400. [Google Scholar] [CrossRef]
- Jing, X.; Wang, Y.; Wu, D.; She, L.; Guo, Y. Polyaniline nanofibers prepared with ultrasonic irradiation. J. Polym. Sci. Part A Ploym. Chem. 2006, 44, 1014–1019. [Google Scholar] [CrossRef]
- Wu, C.G.; Bein, T. Conducting polyaniline filaments in a mesoporous channel host. Science 1994, 264, 1757–1759. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kaner, R.B. A general chemical route to polyaniline nanofibers. J. Am. Chem. Soc. 2004, 126, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Goux, W.J.; Manohar, S.K. Synthesis of polyaniline nanofibers by “nanofiber seeding”. J. Am. Chem. Soc. 2004, 126, 4502–4503. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, Y.; Wei, Y.; Macdiarmid, A.G.; Lelkes, P.I. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 2006, 27, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, C.; Srinivas, D.; Kavitha, B.; Narsimlu, N.; Kumar, K.S. Synthesis and characterization of nano synthesis and characterization of nano size conducting polyaniline. IOSR J. Appl. phys. 2012, 1, 12–15. [Google Scholar] [CrossRef]
- Abdolahi, A.; Hamzah, E.; Ibrahim, Z.; Hashim, S. Synthesis of uniform polyaniline nanofibers through interfacial polymerization. Materials 2012, 5, 1487–1494. [Google Scholar] [CrossRef]
- Cruz, G.J.; Morales, J.; Castillo-Ortega, M.M.; Olayo, R. Synthesis of polyaniline films by plasma polymerization. Synth. Met. 1997, 88, 213–218. [Google Scholar] [CrossRef]
- Arasi, A.Y.; Jeyakumari, J.J.L.; Sundaresan, B.; Dhanalakshmi, V.; Anbarasan, R. The structural properties of poly(aniline)—Analysis via FTIR spectroscopy. Spectrochim. Acta Part Mol. Biomol. Spectrosc. A 2009, 74, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Ghassempour, A.; Najafi, N.M.; Mehdinia, A.; Davarani, S.S.H.; Fallahi, M.; Nakhshab, M. Analysis of anatoxin-a using polyaniline as a sorbent in solid-phase microextraction coupled to gas chromatography–mass spectrometry. J. Chromatogr. A 2005, 1078, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Lu, W.; Goering, R.; Mattes, B.R. Investigation of polyaniline processibility using GPC/UV–vis analysis. Synth. Met. 2009, 159, 666–674. [Google Scholar] [CrossRef]
- Goktas, H.; Demircioglu, Z.; Sel, K.; Gunes, T.; Kaya, I. The optical properties of plasma polymerized polyaniline thin films. Thin Solid Films 2013, 548, 81–85. [Google Scholar] [CrossRef]
- Tiwari, A.; Kumar, R.; Prabaharan, M.; Pandey, R.R.; Kumari, P.; Chaturvedi, A.; Mishra, A.K. Nanofibrous polyaniline thin film prepared by plasma-induced polymerization technique for detection of NO2 gas. Polym. Adv. Technol. 2010, 21, 615–620. [Google Scholar] [CrossRef]
- Zaitsev, A.; Poncin-Epaillard, F.; Lacoste, A.; Debarnot, D. A bottom-up and templateless process for the elaboration of plasma-polymer nanostructures. Plasma Process. Polym. 2015. [Google Scholar] [CrossRef]
- Michel, M.; Bour, J.; Petersen, J.; Arnoult, C.; Ettingshausen, F.; Roth, C.; Ruch, D. Atmospheric plasma deposition: A new pathway in the design of conducting polymer-based anodes for hydrogen fuel cells. Fuel Cells 2010, 10, 932–937. [Google Scholar] [CrossRef]
- MacKinnon, M.; Reuben, B.G. The synthesis of boron carbide in an RF plasma. J. Electrochem Soc. 1975, 122, 806–811. [Google Scholar] [CrossRef]
- Hallabaugh, C.M.; Hull, D.E.; Newkirk, L.R.; Petrovic, J.J. R.F.-plasma system for the production of ultrafine, ultrapure silicon carbide powder. J. Mater. Sci. 1983, 18, 3190–3194. [Google Scholar] [CrossRef]
- Yoshida, T.; Kawasaki, A.; Nakagawa, K.; Akashi, K. The synthesis of ultrafine titanium nitride in an r.f. plasma. J. Mater. Sci. 1979, 14, 1624–1630. [Google Scholar] [CrossRef]
- Chou, C.H.; Phillips, J. Plasma production of metallic nanoparticles. J. Mat. Res. 1992, 7, 2107–2113. [Google Scholar] [CrossRef]
- Brenner, J.R.; Harkness, J.B.L.; Knickelbein, M.B.; Krumdick, G.K.; Marshall, C.L. Microwave plasma synthesis of carbon-supported ultrafine metal particles. Nanostruct. Mater. 1997, 8, 1–17. [Google Scholar] [CrossRef]
- Girshick, S.L.; Chin, C.-P.; Muno, R.; Wu, C.Y.; Yang, L.; Singh, S.K.; McMurry, P.H. Thermal plasma synthesis of ultrafine iron particles. J. Aerosol. Sci. 1993, 24, 367–382. [Google Scholar] [CrossRef]
- Weigle, J.C.; Luhrs, C.C.; Chen, C.K.; Perry, W.L.; Mang, J.T.; Nemer, M.B.; Lopez, G.P.; Phillips, J. Generation of aluminum nanoparticles using an atmospheric pressure plasma torch. J. Phys. Chem. B 2004, 108, 18601–18607. [Google Scholar] [CrossRef]
- Matsoukas, T.; Russell, M. Particle charging in low-pressure plasmas. J. Appl. Phys. 1995, 77, 4285–4292. [Google Scholar] [CrossRef]
- Phillips, J.; Luhrs, C.C.; Richard, M. Review: Engineering particles using the aerosol-through-plasma method. IEEE. Trans. Plasma Sci. 2009, 37, 726–739. [Google Scholar] [CrossRef]
- Dato, A.; Radmilovic, V.; Lee, Z.; Phillips, J.; Frenklach, M. Substrate-free gas-phase synthesis of graphene sheets. Nano Lett. 2008, 8, 2012–2016. [Google Scholar] [CrossRef] [PubMed]
- Tatarova, E.; Dias, A.; Henriques, J.; Botelho do Rego, A.M.; Ferraria, A.M.; Abrashev, M.V.; Luhrs, C.C.; Phillips, J.; Dias, F.M.; Ferreira, C.M. Microwave plasmas applied for the synthesis of free standing graphene sheets. J. Phys. D Appl. Phys. 2014, 47, 385501. [Google Scholar] [CrossRef]
- Tsyganov, D.; Bundaleska, N.; Tatarova, E.; Dias, A.; Henriques, J.; Rego, A.; Ferraria, A.; Abrashev, M.V.; Dias, F.M.; Luhrs, C.C.; Phillips, J. On the plasma-based growth of ‘flowing’ graphene sheets at atmospheric pressure conditions. Plasma Sources Sci. Technol. 2016, 25, 015013. [Google Scholar] [CrossRef]
- Morales, J.; Olayo, M.G.; Cruz, G.J.; Castillo-Ortega, M.M.; Olayo, R. Electronic conductivity of pyrrole and aniline thin films polymerized by plasma. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 3247–3255. [Google Scholar] [CrossRef]
- Ameen, S.; Song, M.; Kim, D.-G.; Im, Y.-B.; Seo, H.-K.; Kim, Y.S.; Shin, H.-S. Iodine doped polyaniline thin film for heterostructure devices via PECVD technique: morphological, structural, and electrical properties. Macromol. Res. 2012, 20, 30–36. [Google Scholar] [CrossRef]
- Debarnot, D.; Mérian, T.; Poncin-Epaillard, F. Film chemistry control and growth kinetics of pulsed plasma-polymerized aniline. Plasma Chem. Plasma Process. 2011, 31, 217–231. [Google Scholar] [CrossRef]
- Chen, J.-Z.; Hsu, C.-C.; Wang, C.; Liao, W.-Y.; Wu, C.-H.; Wu, T.-J.; Liu, H.-W.; Chang, H.; Lien, S.-T.; Li, H.-C.; et al. Rapid atmospheric-pressure-plasma-jet processed porous materials for energy harvesting and storage devices. Coatings 2015, 5, 26–38. [Google Scholar] [CrossRef]
- Shashurin, A.; Keidar, M. Experimental approaches for studying non-equilibrium atmospheric plasma jets. Phys. Plasmas 2015, 22, 122002. [Google Scholar] [CrossRef]
- Ishaq, M.; Bazaka, K.; Ostrikov, K. Intracellular effects of atmospheric-pressure plasmas on melanoma cancer cells. Phys. Plasmas 2015, 22, 122003. [Google Scholar] [CrossRef]
- Urabe, K.; Sands, B.L.; Ganguly, B.N.; Sakai, O. Temporally and spectrally resolved observation of a crossed-flow DBD plasma jet using pure helium and argon/acetone mixed gases. Plasma Sources Sci. Technol. 2012, 21, 034004. [Google Scholar] [CrossRef]
- Chen, K.-S.; Liao, S.-C.; Lin, S.-W.; Tsao, S.-H.; Ting, T.H.; Inagaki, N.; Wu, H.-M.; Chen, W.-Y. The film deposition via atmospheric pressure plasma from ethanol and He mixing gases. Surf. Coat. Technol. 2013, 231, 408–411. [Google Scholar] [CrossRef]
- Descamps, P.; Asad, S.S.; Wilde, J.D. Atmospheric-pressure plasma enhanced chemical deposition: role of the reactor flow dynamics. J. Phys. D Appl. Phys. 2013, 46, 365201. [Google Scholar] [CrossRef]
- Uygun, A.; Oksuz, L.; Yavuz, A.G.; Guleç, A.; Sen, S. Characteristics of nanocomposite films deposited by atmospheric pressure uniform RF glow plasma. Curr. Appl. Phys. 2011, 11, 250–254. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.-J.; Park, C.-S.; Shin, B.J.; Seo, J.H.; Tae, H.-S. Atmospheric pressure plasma polymerization using double grounded electrodes with He/Ar mixture. AIP Adv. 2015, 5, 097137. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ballato, J.; Kim, S.-O. Intense and energetic atmospheric pressure plasma jet arrays. Plasma Process. Polym. 2012, 9, 253–260. [Google Scholar] [CrossRef]
- Moon, S.Y.; Choe, W. A comparative study of rotational temperatures using diatomic OH, O2 and N2+ molecular spectra emitted from atmospheric plasmas. Spectrochim. Acta Part Atomic Spectrosc. B 2003, 58, 249–257. [Google Scholar] [CrossRef]
- Phillips, D.M. Determination of gas temperature from unresolved bands in the spectrum from a nitrogen discharge. J. Phys. Part D Appl. Phys. 1975, 8, 507–521. [Google Scholar] [CrossRef]
- Laux, C.O.; Kruger, C.H. Arrays of radiative transition probabilities for the N2 first and second positive, no beta and gamma, N2+ first negative, and O2 Schumann-Runge band systems. J. Quant. Spectrosc. Radiat. Transf. 1992, 48, 9–24. [Google Scholar] [CrossRef]
- Zhuo, S.; Shao, M.; Lee, S.-T. Upconversion and downconversion fluorescent graphene quantum dots: ultrasonic preparation and photocatalysis. ACS Nano 2012, 6, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Baerlocher, C.; Gramm, F.; Massuger, L.; McCusker, L.B.; He, Z.; Hovmoller, S.; Zou, X. Structure of the polycrystalline zeolite catalyst IM-5 solved by enhanced charge flipping. Science 2007, 315, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Deka, S.; Joy, P.A. Electronic structure and ferromagnetism of polycrystalline Zn1-xCoxO (0 ≤ x ≤ 0.15). Solid State Commun. 2005, 134, 665–669. [Google Scholar] [CrossRef]
- Kim, K.; Lee, Z.; Regan, W.; Kisielowski, C.; Crommie, M.F.; Zettl, A. Grain boundary mapping in polycrystalline graphene. ACS Nano 2011, 5, 2142–2146. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.M.; Predeep, P. Plasma-polymerized elastomer/conducting polymer composite: Structural and optical characterization. Polym. Compos. 2013, 34, 1091–1098. [Google Scholar] [CrossRef]
- Barman, T.; Pal, A.R. Contradictory ageing behaviour and optical property of iodine doped and H2SO4 doped pulsed DC plasma polymerized aniline thin films. Solid State Sci. 2013, 24, 71–78. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.-S.; Kim, D.H.; Shin, B.J.; Tae, H.-S. Synthesis and Characterization of Nanofibrous Polyaniline Thin Film Prepared by Novel Atmospheric Pressure Plasma Polymerization Technique. Materials 2016, 9, 39. https://doi.org/10.3390/ma9010039
Park C-S, Kim DH, Shin BJ, Tae H-S. Synthesis and Characterization of Nanofibrous Polyaniline Thin Film Prepared by Novel Atmospheric Pressure Plasma Polymerization Technique. Materials. 2016; 9(1):39. https://doi.org/10.3390/ma9010039
Chicago/Turabian StylePark, Choon-Sang, Dong Ha Kim, Bhum Jae Shin, and Heung-Sik Tae. 2016. "Synthesis and Characterization of Nanofibrous Polyaniline Thin Film Prepared by Novel Atmospheric Pressure Plasma Polymerization Technique" Materials 9, no. 1: 39. https://doi.org/10.3390/ma9010039






