1. Introduction
Solid oxide fuel cells (SOFCs) transform chemical energy from fuels, such as natural gas, humidified hydrogen, into electrical energy with high conversion efficiency and low pollution. An SOFC includes three principal components: the electrolyte, the cathode and the anode. Each part of the SOFC needs to be compatible both physically and chemically with one another to minimize interfacial reactions. A dense electrolyte is needed to prevent gas mixing, whereas the cathode and the anode must be porous to allow gas transport to the reaction sites. SOFCs generate electricity through the oxidation of fuel at anode and the reduction of oxygen at cathode. To provide a high voltage and power output, interconnects are used to connect the cells in series.
Lanthanum chromite (LaCrO
3) based perovskite oxides have been widely investigated as the ceramic interconnect for SOFCs due to their fairly high electrical conductivity and excellent thermodynamic stability at higher temperature [
1,
2]. La
0.7Ca
0.3Cr
0.5Co
0.5O
3 was reported with very high conductivity 85 Scm
–1 at 700 °C. However, its thermal expansion coefficient (TEC) increases from 11.12 × 10
–6 to 19 × 10
–6 K
–1 due to the Co addition. This is much larger than acceptable values 11 × 10
–6–12 × 10
–6 K
–1 for SOFC’s design [
1,
3,
4,
5]. Dense LaCrO
3 based ceramics are not easy to prepare, besides, stoichiometric pellets are difficult to form because the high vapor pressure of constituent chromium at high temperature. To improve the sinterability of LaCrO
3, some methods such as adding sintering aid, using a reducing atmosphere, and substituting lanthanum with other elements were tried by many researchers [
6,
7,
8,
9,
10,
11,
12]. Chick
et al. found La
0.7Ca
xCrO
3 with Ca deficiency, x = 0.28 and 0.29, never attained 60% density, even at 1550 °C sintering. In contrast, the samples with Ca enrichment, x = 0.31 and 0.32, attained densities over 90% at 1400 °C sintering [
12]. Mori
et al. reported La
0.8Sr
0.2CrO
3 having an average linear TEC of 9.9 × 10
–6 K
–1 in air and 12.2 × 10
–6 K
–1 in H
2 atmosphere, in the temperature range from 50 to 1000 °C [
13]. Yang and co-workers investigated La
1–xSr
xCrO
3 (x = 0–0.3) powder materials synthesized by the glycine-nitrate-process and found the maximum electrical conductivity 14.7 Scm
–1 at 1000 °C in La
0.8Sr
0.2CrO
3 sintered at 1550 °C/2 h [
14]. Properties for LaCrO
3 based oxides have been improved when part of La is substituted by Ca or Sr.
Cr-free oxides were also investigated as interconnect for SOFCs. Taguchi
et al. reported perovskite-type (La
0.1Ca
0.9)(Mn
1–xTi
x)O
3 (0 ≤ x ≤ 0.9) are n-type semiconductors at low temperature. At high temperature, the manganates exhibit a metal–insulator transition in the range 0 ≤ x ≤ 0.3 [
15]. Vashook
et al. investigated perovskite-type compounds La
1–xCa
xTiO
3 (x = 0.2–1.0) and La
2(1–x)/3Ca
xTiO
3 (x = 0, 0.1, 0.4 and 0.8). The crystal structures of the La
1–xCa
xTiO
3 compounds at room temperature were as orthorhombic in space group Pbnm (samples with 0.7 ≤ x ≤ 1) and as rhombohedral (x = 0.6). At room temperature three different perovskite-like structures has been found for A-deficient La
2(1–x)/3Ca
xTiO
3 compound: orthorhombic Pbnm structure for x = 0.8, orthorhombic Imma structure for x = 0.4, and monoclinic P2/m (or possibly orthorhombic Cm2m) structure for x = 0.1 [
16]. Kobayashi
et al. reported the crystal structures of Ca
0.5R
0.5Mn
0.5Ti
0.5O
3 (R = La, Nd, Eu) and Ca
0.67R
0.33Mn
0.33Ti
0.67O
3 (R = Y, Gd, Dy, Ho, Yb) are orthorhombic Pnma (No. 62) corresponding to GdFeO
3 type perovskite structure. The cell parameters b, c and cell volume increase with increasing ionic radius of rare earth element. The decrease in ionic radius of rare earth ions in these compounds makes increase distortion from ideal cubic perovskite [
17]. Hosseini
et al. fabricated lanthanum–manganese-doped CaTiO
3 perovskite oxides La
0.4Ca
0.6Ti
1–xMn
xO
3–δ (x = 0.0, 0.2, 0.4, 0.6) powders using an EDTA-citrate method and co-sintered as an interconnect material on an extruded porous anode substrate in a flat-tubular solid oxide fuel cell. The highest electrical conductivity occurs when x = 0.6; at 12.20 S cm
–1 and 2.70 S cm
–1 under oxidizing and reducing conditions [
18]. Y
3+ has typically been used to replace La
3+ in ceramics containing lanthanum to improve their properties. We try to investigate the possibility of using (YCa)(TiMn)O
3−δ ceramics as interconnect for SOFCs.
In our previous studies, Pb(Mg
1/3Nb
2/3)O
3 (PMN) and Pb(Fe
1/2Nb
1/2)O
3 (PFN) ceramics had been prepared via a simple and effective reaction-sintering process [
19,
20]. Dense PMN ceramics (8.09 g/cm
3, 99.5% of theoretic density 8.13 g/cm
3) with maximum dielectric constant 19,900 at 1 kHz were obtained. Other Pb-based complex perovskite ceramics were also successfully produced by this reaction-sintering process. In our recent investigations, some microwave dielectric ceramics such as BaTi
4O
9, Ba
5Nb
4O
15, Sr
5Nb
4O
15, CaNb
2O
6, NiNb
2O
6, Zn
0.5Ti
0.5NbO
4 and Ni
0.5Ti
0.5NbO
4 were also prepared via this simple and effective reaction-sintering process [
21,
22,
23,
24,
25,
26]. In this study, preparation and properties of (YCa)(TiMn)O
3−δ ceramics via a reaction-sintering process were investigated.
2. Results and Discussion
The XRD profiles for the 2 h sintered YTiO
3 (YT), Y
0.6Ca
0.4Ti
0.6Mn
0.4O
3−δ (YCTM4) and Y
0.6Ca
0.4Ti
0.4Mn
0.6O
3−δ (YCTM6) ceramics are illustrated in
Figure 1. The reflections for YT in
Figure 1a match well with those of Y
2Ti
2O
7 (ICDD PDF # 00-042-0413) instead those of YTiO
3 (ICDD PDF # 00-027-1481). This implies that Y
2Ti
2O
7 formed more easily than YTiO
3 as the raw materials with Y/Ti ratio 1/1 were heated in air. Weak peaks (+) around 30° and 50.4° 2θ for YTiO
2.085 are seen in
Figure 1a. Gill
et al. prepared Y
2Ti
2O
7 from Y
2O
3 and Ti
2O
3. After 800 °C/12 h calcining, Y
2Ti
2O
7 phase along with weak YTiO
2.085 phase was detected for pellets sintered at 1500 °C/12 h. Almost monophasic YTiO
2.085 phase was detected for pellets sintered at 1550 °C/12 h [
27]. The reflections for YCTM4 in
Figure 1b show that a phase with similar crystal structure of YTiO
3 formed as the major phase and Y
2Ti
2O
7 phase still formed in YCTM4. Weak peaks for YTiO
2.085 and some unknown phases are seen in
Figure 1b. The reflections for major phase are also similar to those for Y
0.33Ca
0.67Ti
0.67Mn
0.33O
3 reported by Kobayashi
et al. [
17]. The crystal structure for YCTM4 is different from YT as Ca and Mn were added. The reflections for YCTM6 in
Figure 1c show that only a phase with similar crystal structure of YTiO
3 and Y
0.33Ca
0.67Ti
0.67Mn
0.33O
3 reported by Kobayashi
et al. [
17] formed. Y
2Ti
2O
7 phase is not detected. More Mn addition inhibited the formation of Y
2Ti
2O
7, YTiO
2.085, and unknown phases. The reaction-sintering process is proved to be a simple and effective process to obtain YT, YCTM4 and YCTM6 ceramics. The calcination step of the conventional mixed oxide route was performed during the heating up period.
Relative density of YT, YCTM4 and YCTM6 ceramics sintered at various temperatures and soak time is shown in
Figure 2. A low density, 69.1% of the theoretical density 4.98 g/cm
3 for Y
2Ti
2O
7, was found for 1400 °C/2 h sintering YT pellets. Density of YT increased with sintering temperature and soak time. Dense YT with 91.4% of the theoretical density for Y
2Ti
2O
7 was observed after being sintered at 1500 °C/6 h. Gill
et al. prepared Y
2Ti
2O
7 and obtained a low density 73% of the theoretical density after 800 °C/12 h calcining and 1500 °C/12 h sintering [
27]. YCTM4 pellets show lower densities. YCTM4 with 77.9% of the theoretical density ~4.97 g/cm
3 for Y
0.6Ca
0.4Ti
0.6Mn
0.4O
3−δ was observed even after sintering at 1500 °C/6 h. Lower densities may be caused by the unknown phases, pores, and YTiO
2.085 with a low density 4.28 g/cm
3. A higher sintering temperature or a prolonged sintering period is suggested for obtaining dense YCTM4. YCTM6 pellets show higher densities. Dense YCTM6 with 93.6% of the theoretical density ~5.01 g/cm
3 for Y
0.6Ca
0.4Ti
0.4Mn
0.6O
3−δ was observed after being sintered at 1500 °C/4 h. The reaction-sintering process is proved to be effective at obtaining YT and YCTM6 ceramics with a relative density higher than 90%.
Figure 1.
XRD patterns of the 2 h sintered (a) YTiO3 (YT); (b) Y0.6Ca0.4Ti0.6Mn0.4O3−δ(YCTM4); (c) Y0.6Ca0.4Ti0.4Mn0.6O3−δ (YCTM6) ceramics. +: YTiO2.085; ?: unknown phases.
Figure 1.
XRD patterns of the 2 h sintered (a) YTiO3 (YT); (b) Y0.6Ca0.4Ti0.6Mn0.4O3−δ(YCTM4); (c) Y0.6Ca0.4Ti0.4Mn0.6O3−δ (YCTM6) ceramics. +: YTiO2.085; ?: unknown phases.
Figure 2.
Relative density of YT, YCTM4, and YCTM6 ceramics sintered at various temperatures and soak time.
Figure 2.
Relative density of YT, YCTM4, and YCTM6 ceramics sintered at various temperatures and soak time.
SEM photographs of as-fired YT ceramics sintered at various temperatures and soak time are presented in
Figure 3. Porous pellets with grains smaller than 3 μm are seen for 1400 °C/2 h sintering YT pellets. Grain size increased with sintering temperature and soak time. Grains > 10 μm are seen for 1500 °C/6 h sintering YT pellets. Ding
et al. prepared Y
2Ti
2O
7 with 1 mol% La
2O
3 and found grains smaller than 4 μm after 1450 °C/2–4 h sintering [
28]. SEM photographs of the YCTM4 ceramics sintered at various temperatures and soak time are presented in
Figure 4. Porous pellets with grains smaller than 8 μm are seen for 1400 °C/2 h sintering YCTM4 pellets. Grain size increased with sintering temperature and soak time. Pores disappeared and grains >10 μm are seen for 1500 °C/6 h sintering YCTM4 pellets. Grain growth increased as Ca and Mn were added into YT. Grains <4 μm are seen for La
0.4Ca
0.6Ti
0.6Mn
0.4O
3–δ via an EDTA-citrate method after 950 °C/5 h calcining and 1400 °C/10 h sintering [
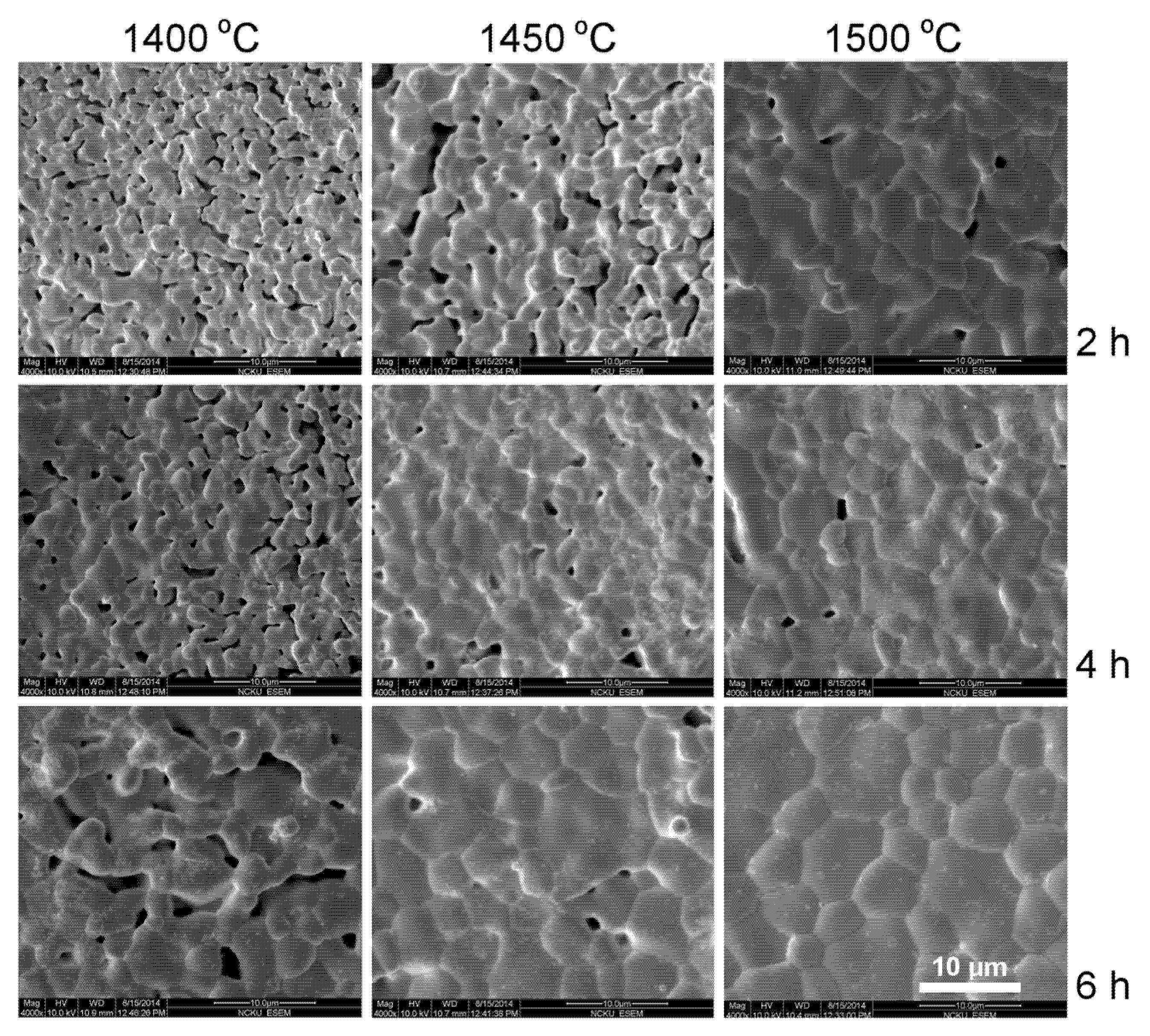
18]. Therefore, the reaction-sintering process is proved to be effective for grain growth in YT and YCTM4 ceramics. The calcinations stage and the following pulverization for the conventional solid–state reaction route could be bypassed. SEM photographs of the YCTM6 ceramics sintered at various temperatures and soak time are presented in
Figure 5. Porous pellets with grains smaller than 6 μm are seen for 1400 °C/2 h sintering YCTM6 pellets. Grain size increased with sintering temperature and soak time. Pores disappeared and grains > 20 μm are seen for 1500 °C/6 h sintering YCTM6 pellets. More Mn addition increased the grain growth in YCTM6 pellets than in YCTM4 pellets. Grains <4 μm are seen for La
0.4Ca
0.6Ti
0.4Mn
0.6O
3–δ via an EDTA-citrate method after 950 °C/5 h calcining and 1400 °C/10 h sintering [
18]. It is noted some cracks are seen in YCTM6 pellets. These cracks propagated not only along the grain boundaries but also through the grains. Besides, the amount and the size of cracks increased with sintering temperature and soak time. Sintering at temperatures below 1450 °C for a prolonged period or adding sintering aids are suggested for obtaining dense YCTM6 pellets without cracks.
Figure 3.
SEM photographs of the YT ceramics sintered at various temperatures and soak time.
Figure 3.
SEM photographs of the YT ceramics sintered at various temperatures and soak time.
Figure 4.
SEM photographs of the YCTM4 ceramics sintered at various temperatures and soak time.
Figure 4.
SEM photographs of the YCTM4 ceramics sintered at various temperatures and soak time.
Figure 5.
SEM photographs of the YCTM6 ceramics sintered at various temperatures and soak time.
Figure 5.
SEM photographs of the YCTM6 ceramics sintered at various temperatures and soak time.
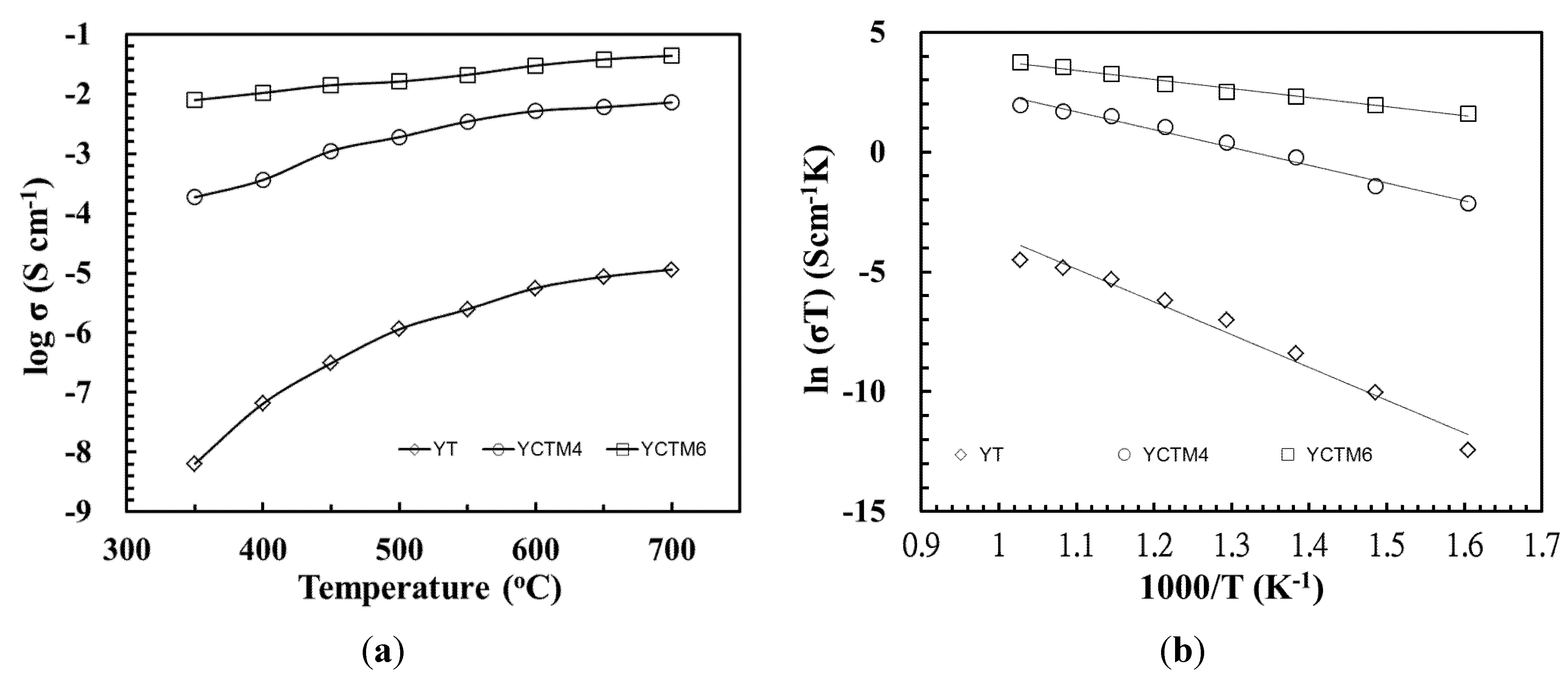
DC total conductivity of 1500 °C/6 h sintering YT, YCTM4, and YCTM6 ceramics are shown in
Figure 6. Log σ is found from –8.19 Scm
–1 at 350 °C to –4.94 Scm
–1 at 700 °C for YT. Gill
et al. prepared Y
2Ti
2O
7 and obtained log σ about –7.8 Scm
–1 at 700 °C and –6.636 Scm
–1 at 900 °C after 800 °C/12 h calcining and 1500 °C/12 h sintering [
27]. The reaction-sintering process is proved to be effective at obtaining YT ceramics with a higher conductivity even the calcination was bypassed. Log σ is found from –3.73 Scm
–1 at 350 °C to –2.14 Scm
–1 at 700 °C for YCTM4. Conductivity increased as Ca and Mn were added into YT. σ about 1.5 Scm
–1 at 700 °C for La
0.4Ca
0.6Ti
0.6Mn
0.4O
3–δ via an EDTA-citrate method after 950 °C/5 h calcining and 1400 °C/10 h sintering was obtained [
18]. Log σ is found from –2.1 Scm
–1 at 350 °C to –1.36 Scm
–1 at 700 °C for YCTM6. σ about 7 Scm
–1 at 700 °C for La
0.4Ca
0.6Ti
0.4Mn
0.6O
3–δ via an EDTA-citrate method after 950 °C/5 h calcining and 1400 °C/10 h sintering was obtained [
18]. Conductivity further increased as more Mn was added into YCTM4. A similar tendency was also observed in the study of La
0.4Ca
0.6Ti
1–xMn
xO
3–δ by Hosseini
et al. [
18]. Conductivity increased as Mn content increased in La
0.4Ca
0.6Ti
1–xMn
xO
3–δ. According to the Arrhenius relationship [
29], the experimental activation energy E
a can be determined from the slope of the line when natural logarithm of conductivity (ln σ) is plotted against 1/T as shown in
Figure 6. E
a for YT, YCTM4, and YCTM6 were derived as 1.18, 0.64, and 0.33 eV, respectively. E
a of 1.87 eV for La
0.4Ca
0.6TiO
3–δ and E
a of 0.47 eV for La
0.4Ca
0.6Ti
0.8Mn
0.2O
3–δ was obtained via an EDTA-citrate method after 950 °C/5 h calcining and 1400 °C/10 h sintering [
18]. Hosseini
et al. thought increasing Mn content decreased E
a and increased electrical conductivity in air. Increasing Mn content causes the number of oxygen vacancies to increase and this in turn causes an increase in electron hole concentration [
18]. A similar tendency was also observed in this study.
Figure 6.
log σ and ln (σT) of 1500 °C/6 h sintering YT, YCTM4, and YCTM6 ceramics. (a) log σ; (b) ln (σT).
Figure 6.
log σ and ln (σT) of 1500 °C/6 h sintering YT, YCTM4, and YCTM6 ceramics. (a) log σ; (b) ln (σT).
3. Experimental Section
YTiO3 (YT), Y0.6Ca0.4Ti0.6Mn0.4O3−δ (YCTM4) and Y0.6Ca0.4Ti0.4Mn0.6O3−δ (YCTM6) samples in this study were prepared from reagent-grade powders: Y2O3 (99.9%, STREM CHEMICALS, Newburyport, MA, USA), CaCO3 (99%, SHOWA, Tokyo, Japan), TiO2 (99.9%, SHOWA, Tokyo, Japan), and MnO2 (99.9%, J.T. Baker, Phillipsburg, NJ, USA). Appropriate amounts of raw materials for each batch were weighed and put into a PE (Polyethylene) bottle. Zirconia balls with 5 and 10 mm diameters were used for milling the raw mother powders with de-ionized water for 12 h at a speed of 500 rpm. The dried and pulverized powders were pressed into pellets with 12 mm in diameter and 1–2 mm thick. The pellets were sintered at 1400–1500 °C at a rate 10 °C/min in a covered alumina crucible in air.
The reflections of various phases for the sintered pellets were analyzed by XRD. Microstructures were analyzed by scanning electron microscopy (SEM). The density of the sintered pellets was measured using the Archimedes method. Ag electrodes were formed on both sides of the sintered pellets. Agilent 34970A Data Acquisition was used for electrical resistivity measurements at 350–700 °C.