Plasma-Induced Wafer-Scale Self-Assembly of Silver Nanoparticles and Application to Biochemical Sensing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Wafer-Level Low-Dimensional Structures
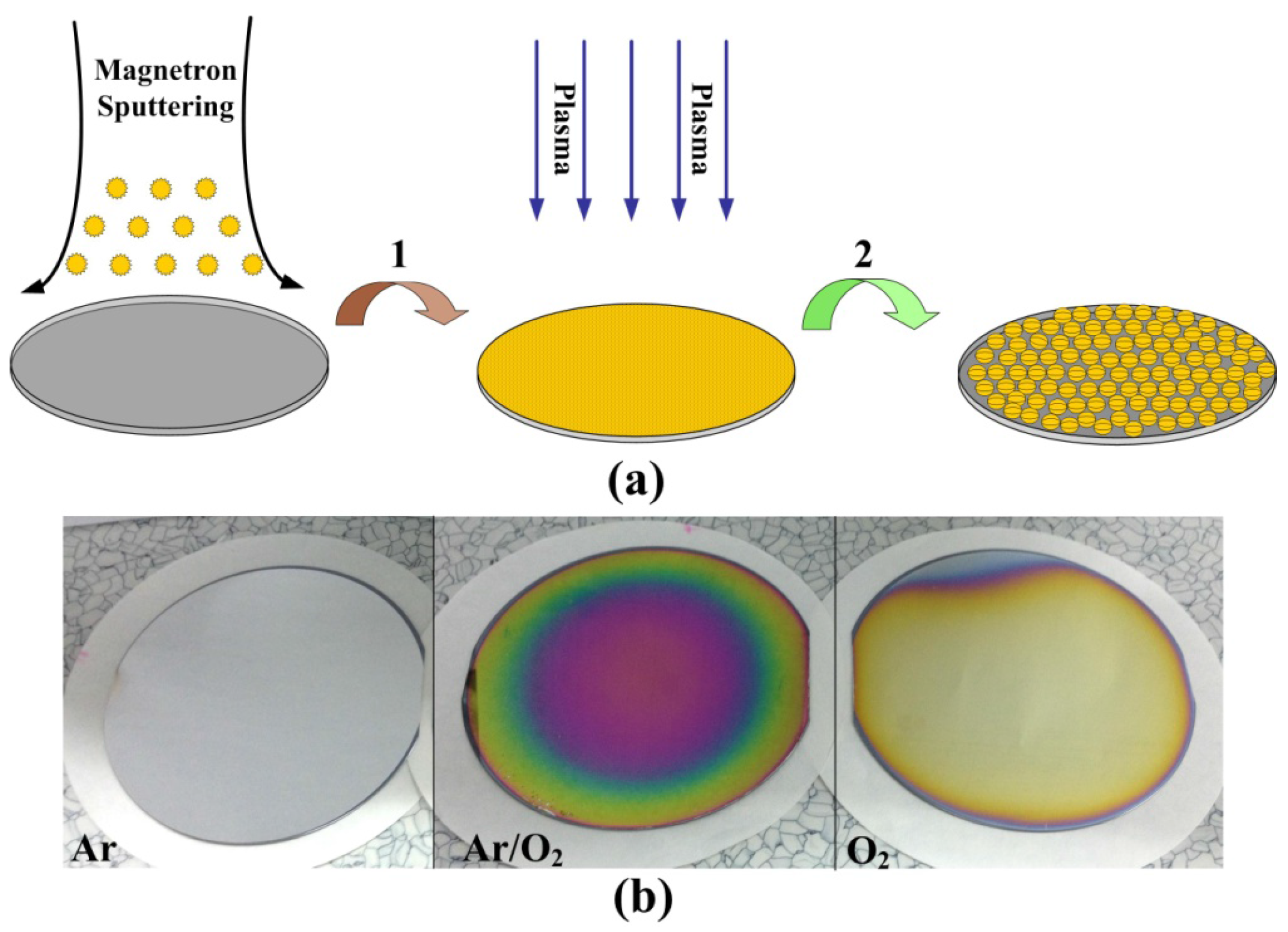
2.2. Ordered Controlled Preparation of Low-Dimensional Structures
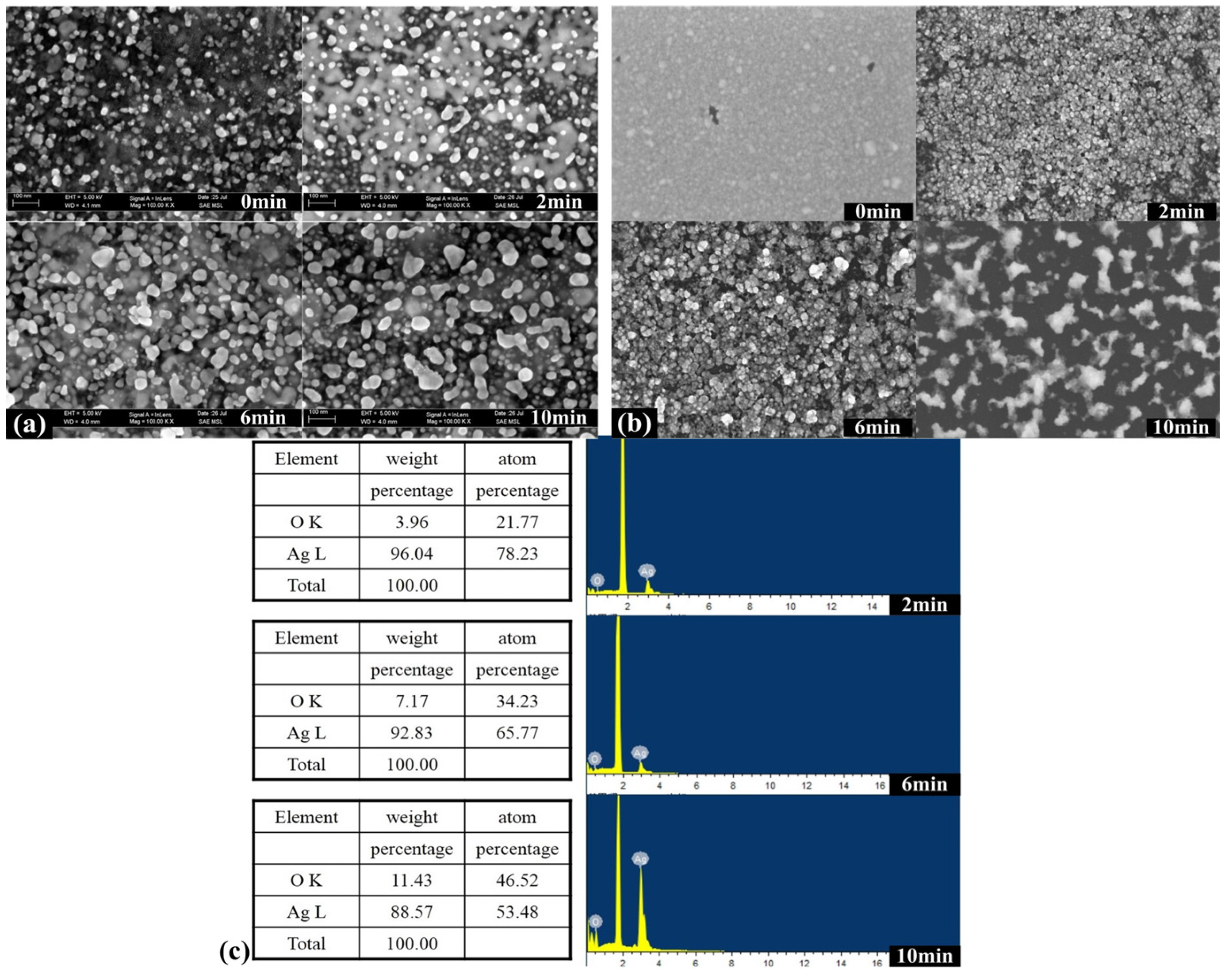

2.3. Wafer-Scale Low-Dimensional Ordered Structures
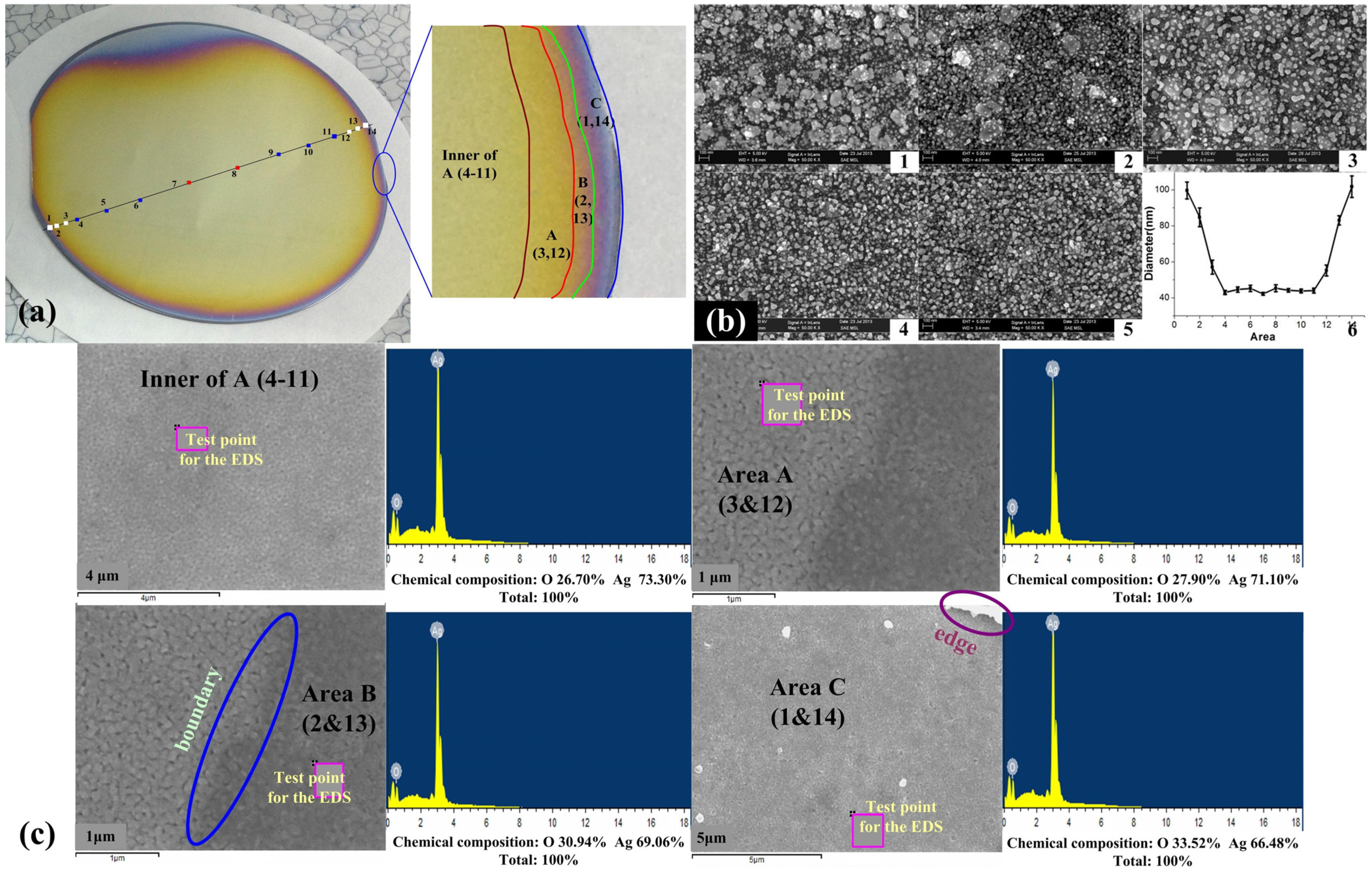
2.4. Surface Raman Enhancement and Surface Fluorescence Enhancement

3. Experimental
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, R.B.; Li, B.X.; Fang, C.H.; Wang, J.F. Metal/Semiconductor hybrid nanostructures for plasmon-enhanced applications. Adv. Mater. 2014, 26, 5274–5309. [Google Scholar] [CrossRef] [PubMed]
- Vigderman, L.; Khanal, B.P.; Zubarev, E.R. Functional gold nanorods: Synthesis, self-assembly, and sensing applications. Adv. Mater. 2012, 24, 4811–4841. [Google Scholar] [CrossRef] [PubMed]
- Kubackova, J.; Fabriciova, G.; Miskovsky, P.; Jancura, D.; Sanchez-Cortes, S. Sensitive surface-enhanced raman spectroscopy (SERS) detection of organochlorine pesticides by alkyl dithiol-functionalized metal nanoparticles-induced plasmonic hot spots. Anal. Chem. 2015, 87, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Skotadis, E.; Stathopoulos, S.; Roussi, V.; Tsouti, V.; Tsoukalas, D. PHEMA functionalization of gold nanoparticles for vapor sensing: Chemi-resistance, chemi-capacitance and chemi-impedance. Sens. Actuators B Chem. 2012, 170, 129–136. [Google Scholar] [CrossRef]
- Li, L.; Steiner, U.; Mahajan, S. Single nanoparticle SERS probes of ion intercalation in metal-oxide electrodes. Nano Lett. 2014, 14, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Pilo-Pais, M.; Watson, A.; Demers, S.; LaBean, T.H.; Finkelstein, G. Surface-enhanced raman scattering plasmonic enhancement using DNA origami-based complex metallic nanostructures. Nano Lett. 2014, 14, 2099–2104. [Google Scholar] [CrossRef] [PubMed]
- Byeon, J.H.; Roberts, J.T. Photoionization of nanosized aerosol gold agglomerates and their deposition to form nanoscale islands on substrates. Langmuir 2014, 30, 8770–8775. [Google Scholar] [CrossRef] [PubMed]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.Y.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y.N. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Matveeva, E.; Gryczynski, I.; Leonenko, Z.; Lakowicz, J.R. Metal-enhanced fluoroimmunoassay on a silver film by vapor deposition. J. Phys. Chem. B 2005, 109, 7969–7975. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Photopoulos, P.; Tserepi, A.; Tsoukalas, D. Two-dimensional nanoparticle self-assembly using plasma-induced Ostwald ripening. Nanotechnology 2011, 22, 235306. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Han, Y.H.; Lee, B.C.; Lee, J.C. One-pot fabrication of various silver nanostructures on substrates using electron beam irradiation. Nanotechnology 2010, 21, 075302. [Google Scholar] [CrossRef] [PubMed]
- Kharissova, O.V.; Dias, H.V.R.; Kharisov, B.I.; Pérez, B.O.; Pérez, V.M.J. The greener synthesis of nanoparticles. Trends Biotechnol. 2013, 31, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.R.; Lim, D.W.; Suh, M.P. Fabrication of metal nanoparticles in metal-organic frameworks. Chem. Soc. Rev. 2013, 42, 1807–1824. [Google Scholar] [CrossRef] [PubMed]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Clair, T.P.S.; Goodman, D.W. Oxygen-induced morphological changes of Ag nanoclusters supported on TiO2 (110). Faraday Discuss. 1999, 114, 279–284. [Google Scholar] [CrossRef]
- Lai, X.; Goodman, D.W. Structure–reactivity correlations for oxide-supported metal catalysts: New perspectives from STM. J. Mol. Catal. A Chem. 2000, 162, 33–50. [Google Scholar] [CrossRef]
- Tang, J.; Guo, H.; Chen, M.; Yang, J.T.; Tsoukalas, D.; Zhang, B.Z.; Liu, J.; Xue, C.Y.; Zhang, W.D. Wrinkled Ag nanostructured gratings towards single molecule detection by ultrahigh surface raman scattering enhancement. Sens. Actuators B Chem. 2015, 218, 145–151. [Google Scholar]
- Merlen, A.; Lagugné-Labarthet, F.; Harté, E. Surface-enhanced raman and fluorescence spectroscopy of dye molecules deposited on nanostructure gold surfaces. J. Phys. Chem. C 2010, 114, 12878–12884. [Google Scholar] [CrossRef]
- Campion, A.; Kambhampati, P. Surface-enhanced raman scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafer, J.H. Locazied surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.H.; Ren, X.J. Effects of localized surface plasmons on the photoluminescence properties of Au-coated ZnO films. Opt. Express 2009, 17, 8735–8740. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Guo, H.; Yang, J.; Zhao, M.; Liu, J.; Xue, C.; Tang, J. Plasma-Induced Wafer-Scale Self-Assembly of Silver Nanoparticles and Application to Biochemical Sensing. Materials 2015, 8, 3806-3814. https://doi.org/10.3390/ma8073806
Shi Y, Guo H, Yang J, Zhao M, Liu J, Xue C, Tang J. Plasma-Induced Wafer-Scale Self-Assembly of Silver Nanoparticles and Application to Biochemical Sensing. Materials. 2015; 8(7):3806-3814. https://doi.org/10.3390/ma8073806
Chicago/Turabian StyleShi, Yunbo, Hao Guo, Jiangtao Yang, Miaomiao Zhao, Jun Liu, Chenyang Xue, and Jun Tang. 2015. "Plasma-Induced Wafer-Scale Self-Assembly of Silver Nanoparticles and Application to Biochemical Sensing" Materials 8, no. 7: 3806-3814. https://doi.org/10.3390/ma8073806




