Ionic Liquid-Doped Gel Polymer Electrolyte for Flexible Lithium-Ion Polymer Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrolyte
| Compound | GPE Composition (vol%) | |||||||
|---|---|---|---|---|---|---|---|---|
| EMI-Tf | 0% | 25% | 30% | 40% | 50% | 60% | 75% | 100% |
| EC | 50% | 37.5% | 35% | 30% | 25% | 20% | 12.5% | 0% |
| PC | 50% | 37.5% | 35% | 30% | 25% | 20% | 12.5% | 0% |
2.3. Membrane Synthesis and Activation
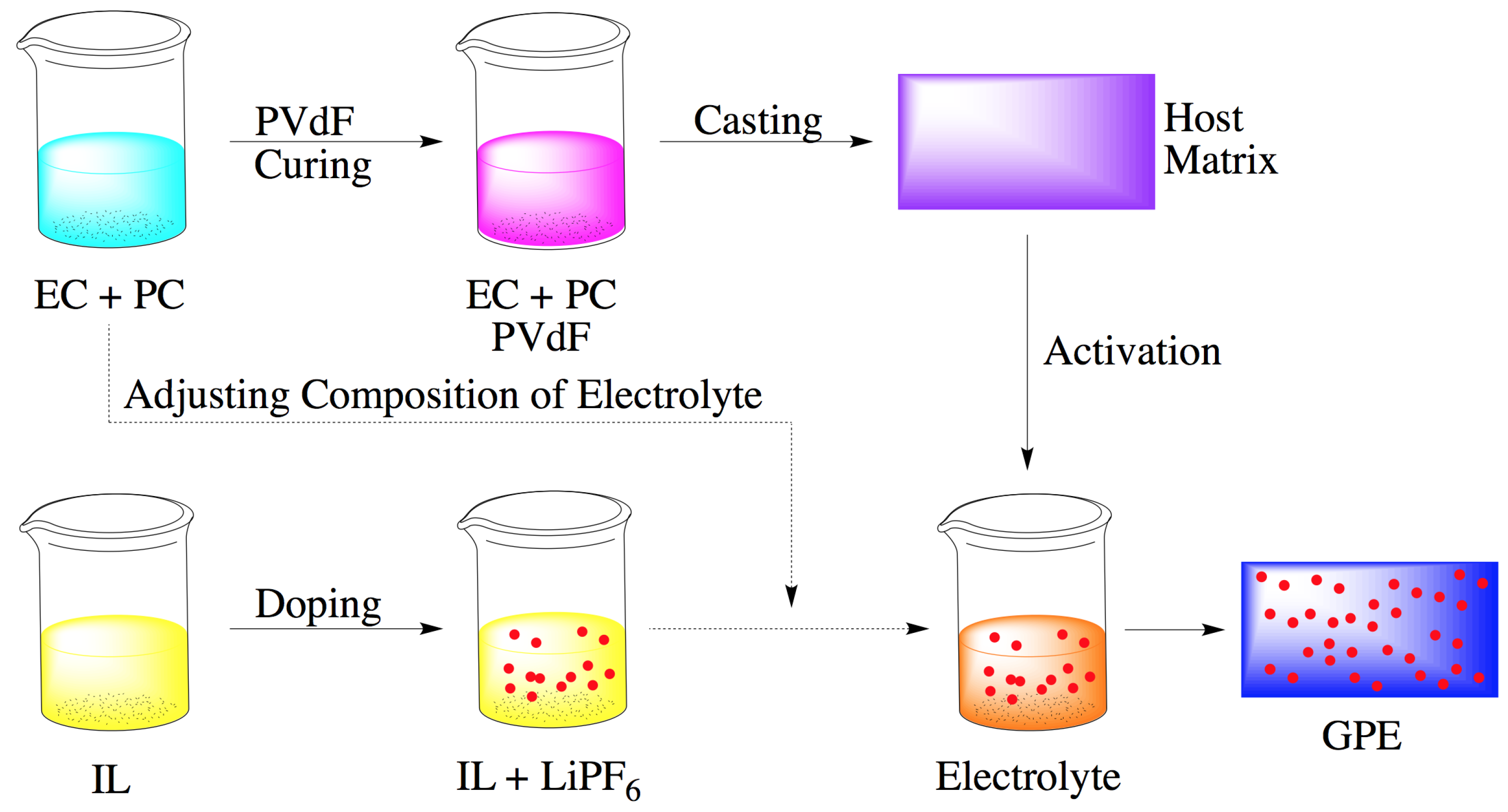
2.4. Full Cell Assembly

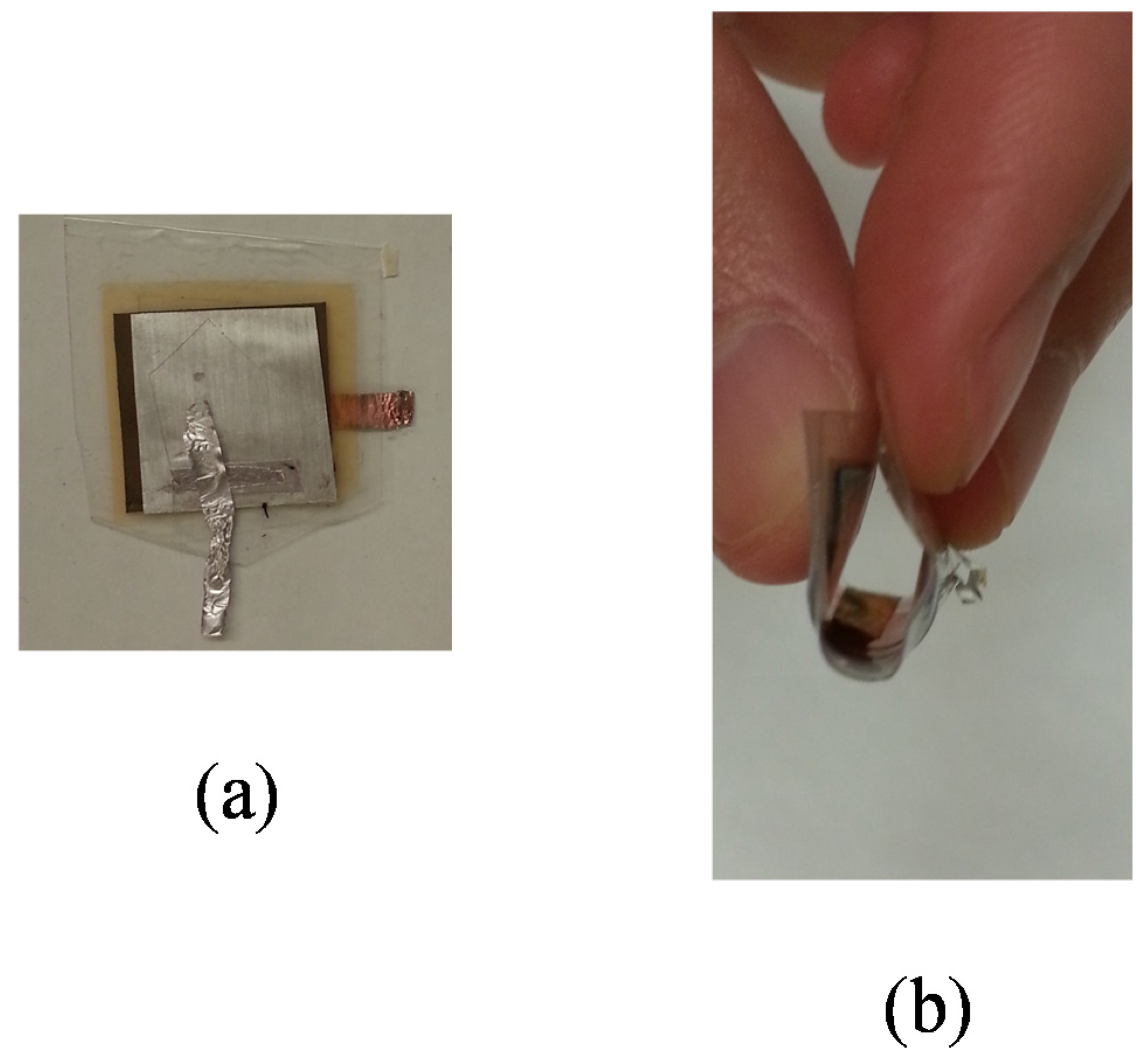
2.5. Measurement
3. Results and Discussion
3.1. Ionic Conductivity

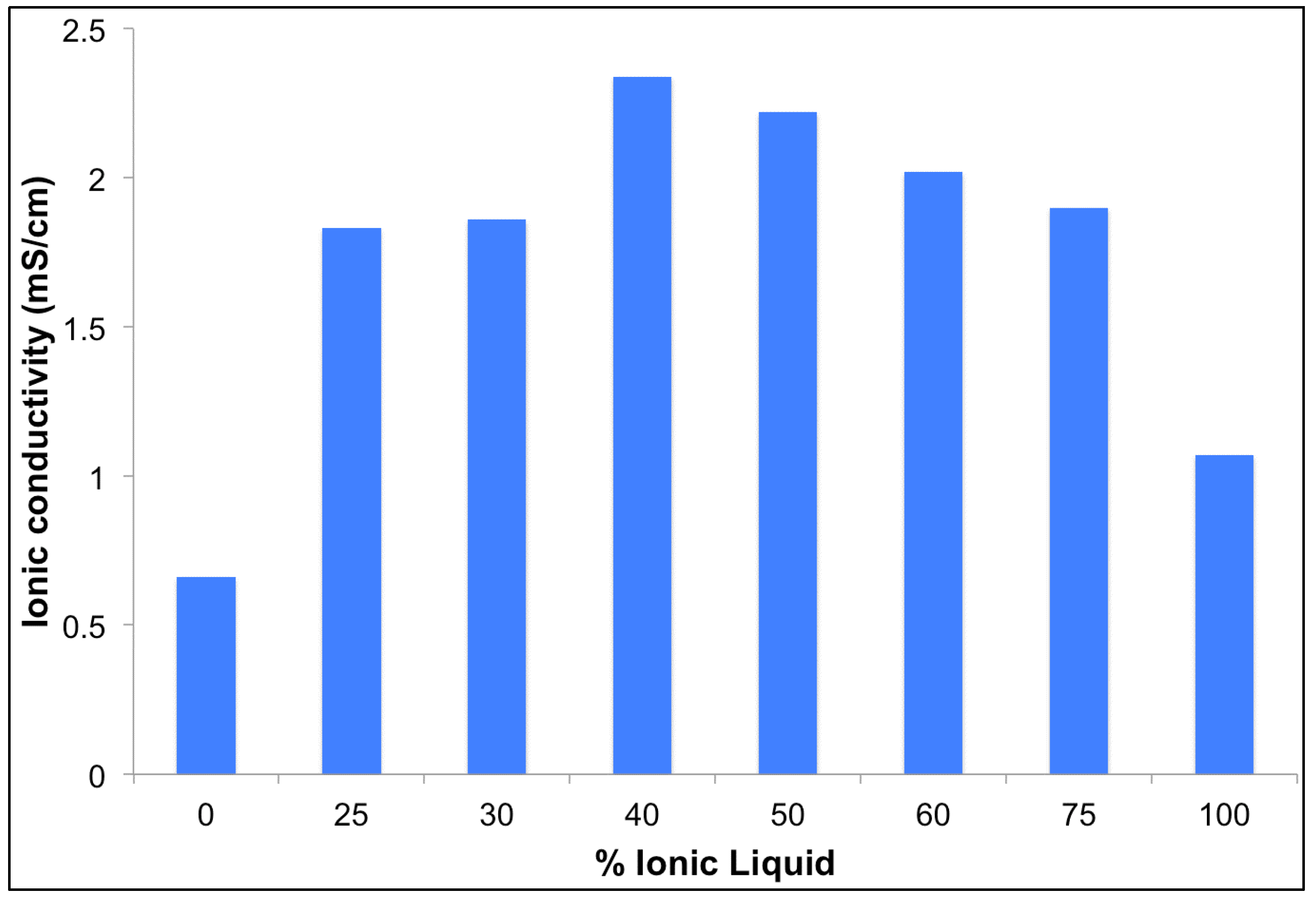
| A (cm2) | IL (vol %) | R (Ω) | t (cm) | σ (mS·cm−1) |
|---|---|---|---|---|
| 1.89 | 0 | 11.2379 | 0.014 | 0.66 |
| 1.89 | 25 | 3.7595 | 0.013 | 1.83 |
| 1.89 | 30 | 3.4118 | 0.012 | 1.86 |
| 1.89 | 40 | 3.1770 | 0.014 | 2.34 |
| 1.89 | 50 | 3.8264 | 0.016 | 2.22 |
| 1.89 | 60 | 3.6806 | 0.014 | 2.02 |
| 1.89 | 75 | 3.9111 | 0.014 | 1.90 |
| 1.89 | 100 | 5.9292 | 0.012 | 1.07 |
3.2. Interfacial Properties
| ILs (vol %) | RS (Ω) | RCT (Ω) | CDL (F) | σS |
|---|---|---|---|---|
| 0 | 9.983 | 98.940 | 3.99 × 10−4 | 78.814 |
| 25 | 7.475 | 216.578 | 3.03 × 10−4 | 95.976 |
| 30 | 5.517 | 177.381 | 4.25 × 10−4 | 99.088 |
| 40 | 3.785 | 136.122 | 4.43 × 10−4 | 113.52 |
| 50 | 2.849 | 190.677 | 4.80 × 10−4 | 97.602 |
| 60 | 3.968 | 189.382 | 4.43 × 10−4 | 95.877 |
| 75 | 3.065 | 155.311 | 5.36 × 10−4 | 84.32 |
| 100 | 2.931 | 154.235 | 6.54 × 10−4 | 98.015 |
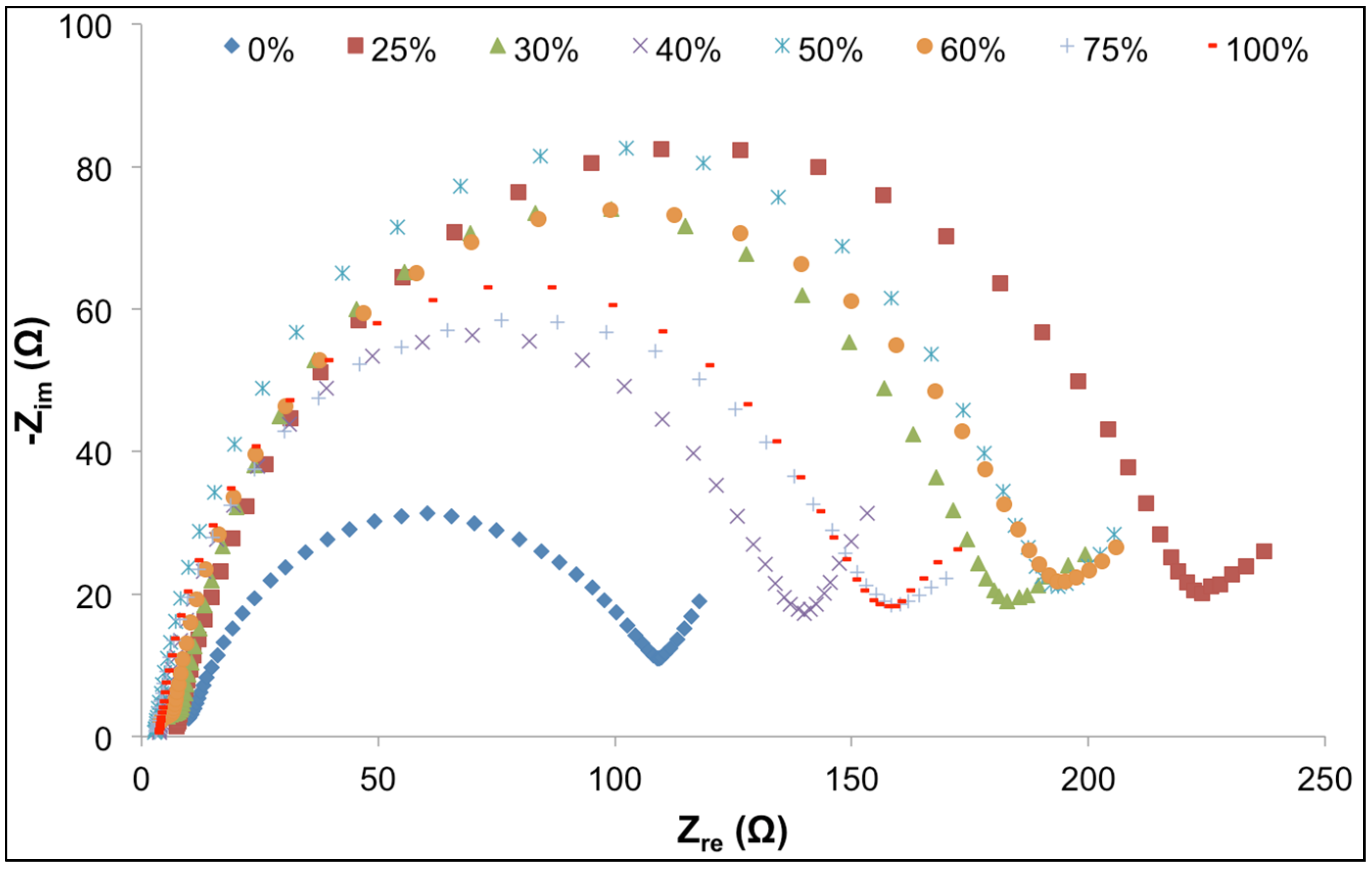
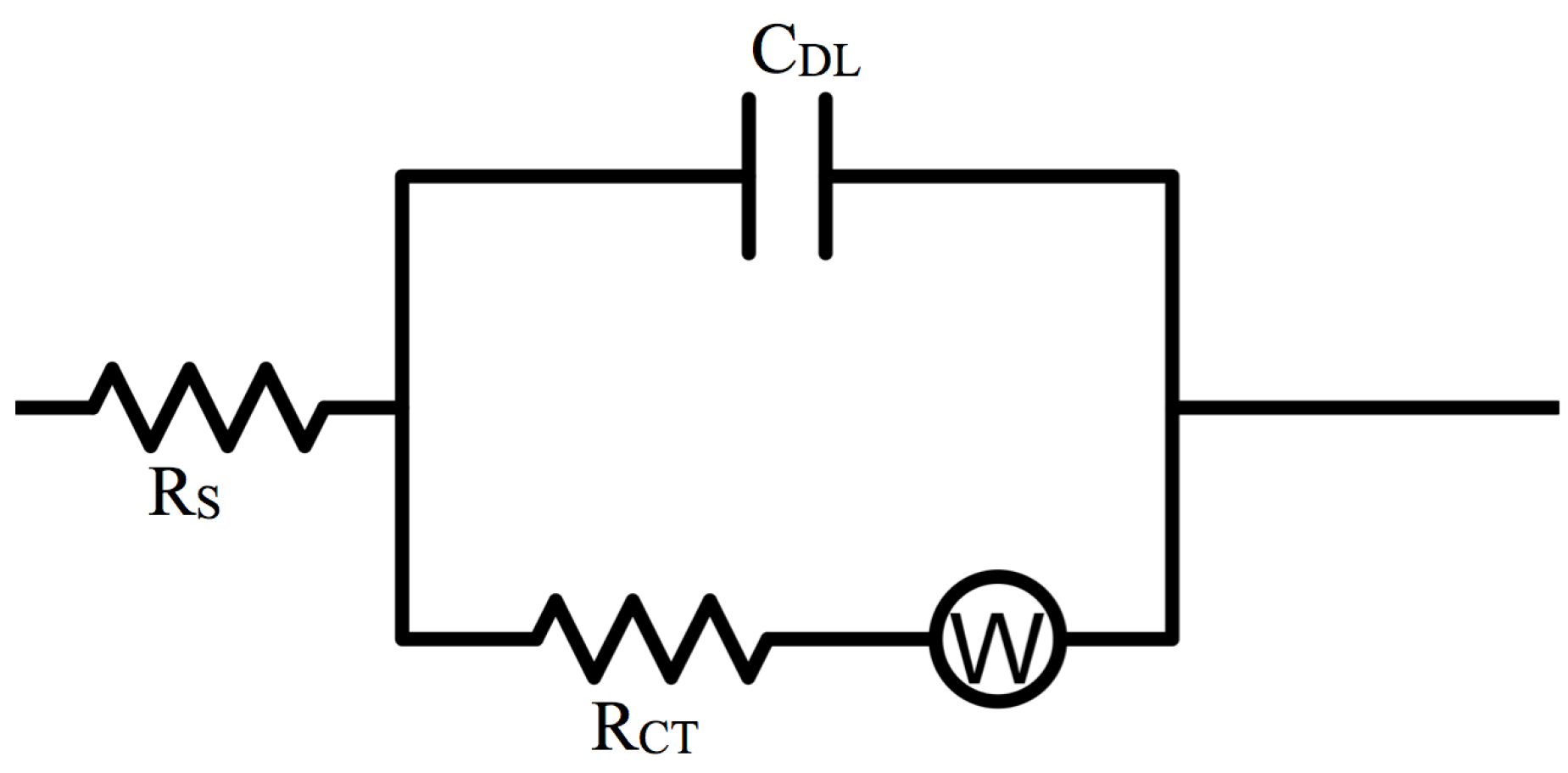

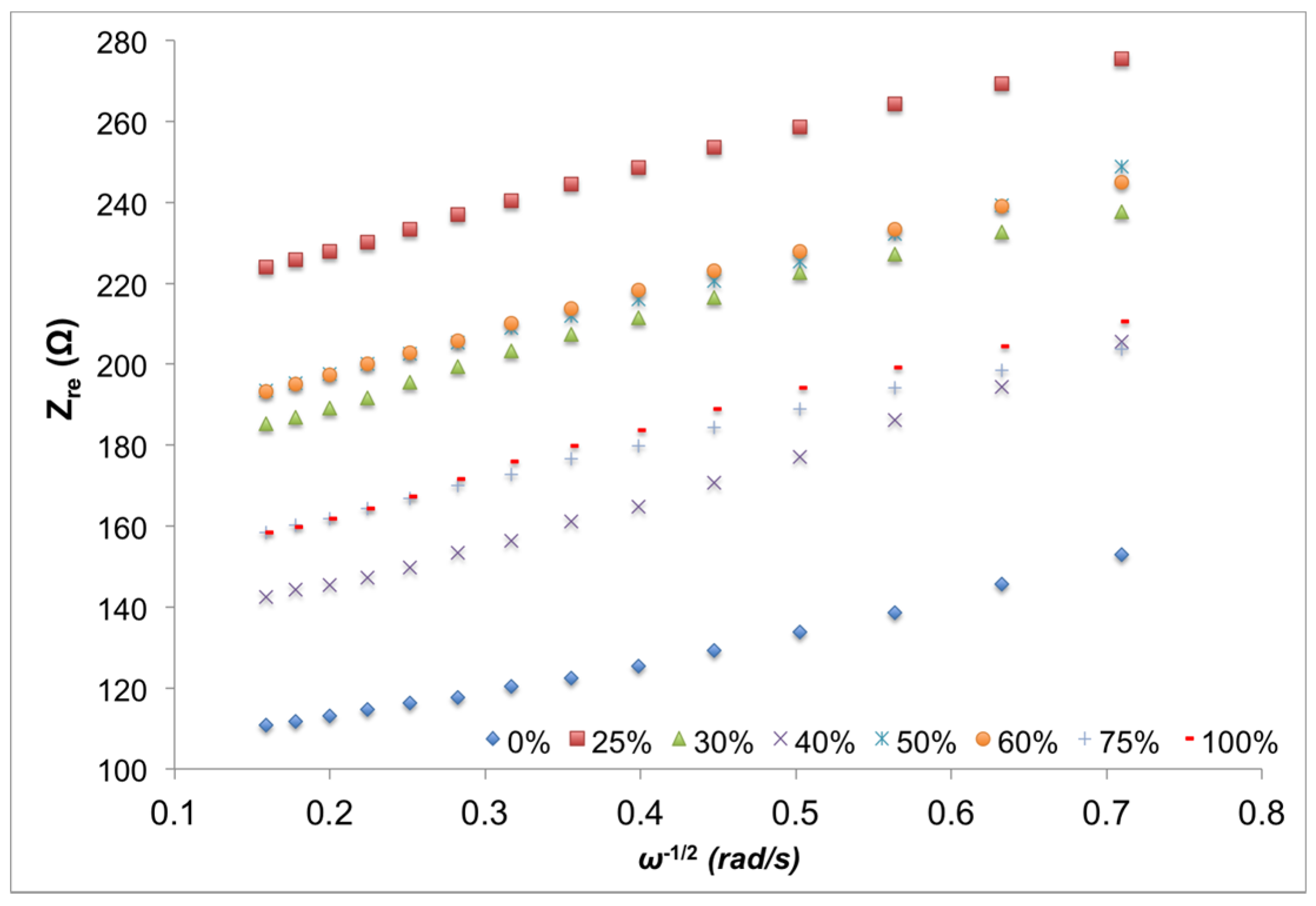
3.3. Battery Performance

| Volume Percent of ILs (vol %) | Avg. Rest Voltage (V) | Avg. Discharge Capacity (mAh/g) |
|---|---|---|
| 0 | 3.342 | 73.04 |
| 25 | 3.800 | 86.74 |
| 30 | 3.853 | 85.47 |
| 40 | 3.807 | 87.61 |
| 50 | 3.942 | 91.79 |
| 60 | 3.630 | 76.03 |
| 75 | 3.202 | 78.48 |
| 100 | 3.073 | 75.71 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, J.Y.; Wang, Y.Y.; Wan, C.C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Sources 1999, 77, 183–197. [Google Scholar] [CrossRef]
- Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.-S.; Kudo, T.; Honma, I. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Wakihara, M. Recent developments in lithium ion batteries. Mater. Sci. Eng. R Rep. 2001, 33, 109–134. [Google Scholar] [CrossRef]
- Patil, A.; Patil, V.; Shin, D.W.; Choi, J.-W.; Paik, D.-S.; Yoon, S.-J. Issue and challenges facing rechargeable thin film lithium batteries. Mater. Res. Bull. 2008, 43, 1913–1942. [Google Scholar] [CrossRef]
- Acar, H.; Çınar, S.; Thunga, M.; Kessler, M.R.; Hashemi, N.; Montazami, R. Study of physically transient insulating materials as a potential platform for transient electronics and bioelectronics. Adv. Funct. Mater. 2014, 24, 4135–4143. [Google Scholar] [CrossRef]
- Meis, C.; Hashemi, N.; Montazami, R. Investigation of spray-coated silver-microparticle electrodes for ionic electroactive polymer actuators. J. Appl. Phys. 2014, 115, 134302. [Google Scholar] [CrossRef]
- Hong, W.; Meis, C.; Heflin, J.R.; Montazami, R. Evidence of counterion migration in ionic polymer actuators via investigation of electromechanical performance. Sens. Actuators B Chem. 2014, 205, 371–376. [Google Scholar] [CrossRef]
- Hong, W.; Almomani, A.; Montazami, R. Influence of ionic liquid concentration on the electromechanical performance of ionic electroactive polymer actuators. Org. Electron. 2014, 15, 2982–2987. [Google Scholar] [CrossRef]
- Amiri Moghadam, A.A.; Hong, W.; Kouzani, A.; Kaynak, A.; Zamani, R.; Montazami, R. Nonlinear dynamic modeling of ionic polymer conductive network composite actuators using rigid finite element method. Sens. Actuators A Phys. 2014, 217, 168–182. [Google Scholar] [CrossRef]
- Alfeeli, B.; Ali, S.; Jain, V.; Montazami, R.; Heflin, J.; Agah, M. MEMS-based gas chromatography columns with nano-structured stationary phases. In Proceedings of the 2008 IEEE on Sensors, Lecce, Italy, 26–29 October 2008; pp. 728–731.
- Appetecchi, G.B.; Romagnoli, P.; Scrosati, B. Composite gel membranes: A new class of improved polymer electrolytes for lithium batteries. Electrochem. Commun. 2001, 3, 281–284. [Google Scholar] [CrossRef]
- Wachtler, M.; Ostrovskii, D.; Jacobsson, P.; Scrosati, B. A study on PVdF-based SiO2-containing composite gel-type polymer electrolytes for lithium batteries. Electrochim. Acta 2004, 50, 357–361. [Google Scholar] [CrossRef]
- Esterly, D.M. Manufacturing of Poly (vinylidene fluoride) and Evaluation of its Mechanical Properties. Master’s Thesis, Virginia Polytechnic Institute and State University, Blacksburg, Virginia, 9 August 2002. [Google Scholar]
- Gentili, V.; Panero, S.; Reale, P.; Scrosati, B. Composite gel-type polymer electrolytes for advanced, rechargeable lithium batteries. J. Power Sources 2007, 170, 185–190. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. Analysis Method: FTIR studies of β-phase crystal formation in stretched PVDF films. Polym. Test. 2003, 22, 699–704. [Google Scholar] [CrossRef]
- Kim, J.R.; Choi, S.W.; Jo, S.M.; Lee, W.S.; Kim, B.C. Electrospun PVdF-based fibrous polymer electrolytes for lithium ion polymer batteries. Electrochim. Acta 2004, 50, 69–75. [Google Scholar] [CrossRef]
- Zhang, H.P.; Zhang, P.; Li, Z.H.; Sun, M.; Wu, Y.P.; Wu, H.Q. A novel sandwiched membrane as polymer electrolyte for lithium ion battery. Electrochem. Commun. 2007, 9, 1700–1703. [Google Scholar] [CrossRef]
- Ji, G.-L.; Zhu, B.-K.; Cui, Z.-Y.; Zhang, C.-F.; Xu, Y.-Y. PVDF porous matrix with controlled microstructure prepared by TIPS process as polymer electrolyte for lithium ion battery. Polymer 2007, 48, 6415–6425. [Google Scholar] [CrossRef]
- Nakagawa, H.; Izuchi, S.; Kuwana, K.; Nukuda, T.; Aihara, Y. Liquid and polymer gel electrolytes for lithium batteries composed of room-temperature molten salt doped by lithium salt. J. Electrochem. Soc. 2003, 150, A695–A700. [Google Scholar] [CrossRef]
- Wang, Y.; Travas-Sejdic, J.; Steiner, R. Polymer gel electrolyte supported with microporous polyolefin membranes for lithium ion polymer battery. Solid State Ion. 2002, 148, 443–449. [Google Scholar] [CrossRef]
- Boudin, F.; Andrieu, X.; Jehoulet, C.; Olsen, I.I. Microporous PVdF gel for lithium-ion batteries. J. Power Sources 1999, 81–82, 804–807. [Google Scholar] [CrossRef]
- Choi, S.W.; Jo, S.M.; Lee, W.S.; Kim, Y.R. An electrospun poly(vinylidene fluoride) nanofibrous membrane and its battery applications. Adv. Mater. 2003, 15, 2027–2032. [Google Scholar] [CrossRef]
- Montazami, R.; Liu, S.; Liu, Y.; Wang, D.; Zhang, Q.; Heflin, J.R. Thickness dependence of curvature, strain, and response time in ionic electroactive polymer actuators fabricated via layer-by-layer assembly. J. Appl. Phys. 2011, 109, 104301. [Google Scholar] [CrossRef]
- Montazami, R.; Wang, D.; Heflin, J.R. Influence of conductive network composite structure on the electromechanical performance of ionic electroactive polymer actuators. Int. J. Smart Nano Mater. 2012, 3, 204–213. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, R.; Ghaffari, M.; Lin, J.; Liu, S.; Cebeci, H.; de Villoria, R.G.; Montazami, R.; Wang, D.; Wardle, B.L.; et al. Equivalent circuit modeling of ionomer and ionic polymer conductive network composite actuators containing ionic liquids. Sens. Actuators A Phys. 2012, 181, 70–76. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Lin, J.; Wang, D.; Jain, V.; Montazami, R.; Heflin, J.R.; Li, J.; Madsen, L.; Zhang, Q.M. Ion transport and storage of ionic liquids in ionic polymer conductor network composites. Appl. Phys. Lett. 2010, 96, 223503. [Google Scholar] [CrossRef]
- Liu, S.; Montazami, R.; Liu, Y.; Jain, V.; Lin, M.; Zhou, X.; Heflin, J.R.; Zhang, Q.M. Influence of the conductor network composites on the electromechanical performance of ionic polymer conductor network composite actuators. Sens. Actuators A Phys. 2010, 157, 267–275. [Google Scholar] [CrossRef]
- Manuel Stephan, A. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Scrosati, B. Recent advances in lithium ion battery materials. Electrochim. Acta 2000, 45, 2461–2466. [Google Scholar] [CrossRef]
- Scrosati, B.; Croce, F.; Panero, S. Progress in lithium polymer battery R&D. J. Power Sources 2001, 100, 93–100. [Google Scholar] [CrossRef]
- Fernicola, A.; Scrosati, B.; Ohno, H. Potentialities of ionic liquids as new electrolyte media in advanced electrochemical devices. Ionics 2006, 12, 95–102. [Google Scholar] [CrossRef]
- Egashira, M.; Todo, H.; Yoshimoto, N.; Morita, M. Lithium ion conduction in ionic liquid-based gel polymer electrolyte. J. Power Sources 2008, 178, 729–735. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Huang, J.; Xu, J.J.; Khalfan, A.; Greenbaum, S.G. Li ion conducting polymer gel electrolytes based on ionic liquid/PVDF-HFP blends. J. Electrochem. Soc. 2007, 154, A1048–A1057. [Google Scholar] [CrossRef] [PubMed]
- Sirisopanaporn, C.; Fernicola, A.; Scrosati, B. New, ionic liquid-based membranes for lithium battery application. J. Power Sources 2009, 186, 490–495. [Google Scholar] [CrossRef]
- Sato, T.; Marukane, S.; Narutomi, T.; Akao, T. High rate performance of a lithium polymer battery using a novel ionic liquid polymer composite. J. Power Sources 2007, 164, 390–396. [Google Scholar] [CrossRef]
- Hayamizu, K.; Aihara, Y.; Nakagawa, H.; Nukuda, T.; Price, W.S. Ionic conduction and ion diffusion in binary room-temperature ionic liquids composed of [emim][BF4] and LiBF4. J. Phys. Chem. B 2004, 108, 19527–19532. [Google Scholar] [CrossRef]
- Li, J.; Wilmsmeyer, K.; Hou, J.; Madsen, L. The role of water in transport of ionic liquids in polymeric artificial muscle actuators. Soft Matter 2009, 5, 2596–2602. [Google Scholar]
- Hou, J.; Zhang, Z.; Madsen, L.A. Cation/anion associations in ionic liquids modulated by hydration and ionic medium. J. Phys. Chem. B 2011, 15, 4576–4582. [Google Scholar] [CrossRef]
- Li, T.; Balbuena, P.B. Theoretical studies of lithium perchlorate in ethylene carbonate, propylene carbonate, and their mixtures. J. Electrochem. Soc. 1999, 146, 3613–3622. [Google Scholar] [CrossRef]
- Pandey, G.; Hashmi, S. Experimental investigations of an ionic-liquid-based, magnesium ion conducting, polymer gel electrolyte. J. Power Sources 2009, 187, 627–634. [Google Scholar] [CrossRef]
- Rodrigues, S.; Munichandraiah, N.; Shukla, A. A review of state-of-charge indication of batteries by means of a.c. impedance measurements. J. Power Sources 2000, 87, 12–20. [Google Scholar] [CrossRef]
- Wang, X.; Hao, H.; Liu, J.; Huang, T.; Yu, A. A novel method for preparation of macroposous lithium nickel manganese oxygen as cathode material for lithium ion batteries. Electrochim. Acta 2011, 56, 4065–4069. [Google Scholar] [CrossRef]
- Appetecchi, G.; Croce, F.; de Paolis, A.; Scrosati, B. A poly (vinylidene fluoride)-based gel electrolyte membrane for lithium batteries. J. Electroanal. Chem. 1999, 463, 248–252. [Google Scholar] [CrossRef]
- Reale, P.; Panero, S.; Scrosati, B. Sustainable high-voltage lithium ion polymer batteries. J. Electrochem. Soc. 2005, 152, A1949–A1954. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Chen, Y.; Montazami, R. Ionic Liquid-Doped Gel Polymer Electrolyte for Flexible Lithium-Ion Polymer Batteries. Materials 2015, 8, 2735-2748. https://doi.org/10.3390/ma8052735
Zhang R, Chen Y, Montazami R. Ionic Liquid-Doped Gel Polymer Electrolyte for Flexible Lithium-Ion Polymer Batteries. Materials. 2015; 8(5):2735-2748. https://doi.org/10.3390/ma8052735
Chicago/Turabian StyleZhang, Ruisi, Yuanfen Chen, and Reza Montazami. 2015. "Ionic Liquid-Doped Gel Polymer Electrolyte for Flexible Lithium-Ion Polymer Batteries" Materials 8, no. 5: 2735-2748. https://doi.org/10.3390/ma8052735







