Tuning the Pore Geometry of Ordered Mesoporous Carbons for Enhanced Adsorption of Bisphenol-A
Abstract
:1. Introduction
2. Results and Discussion
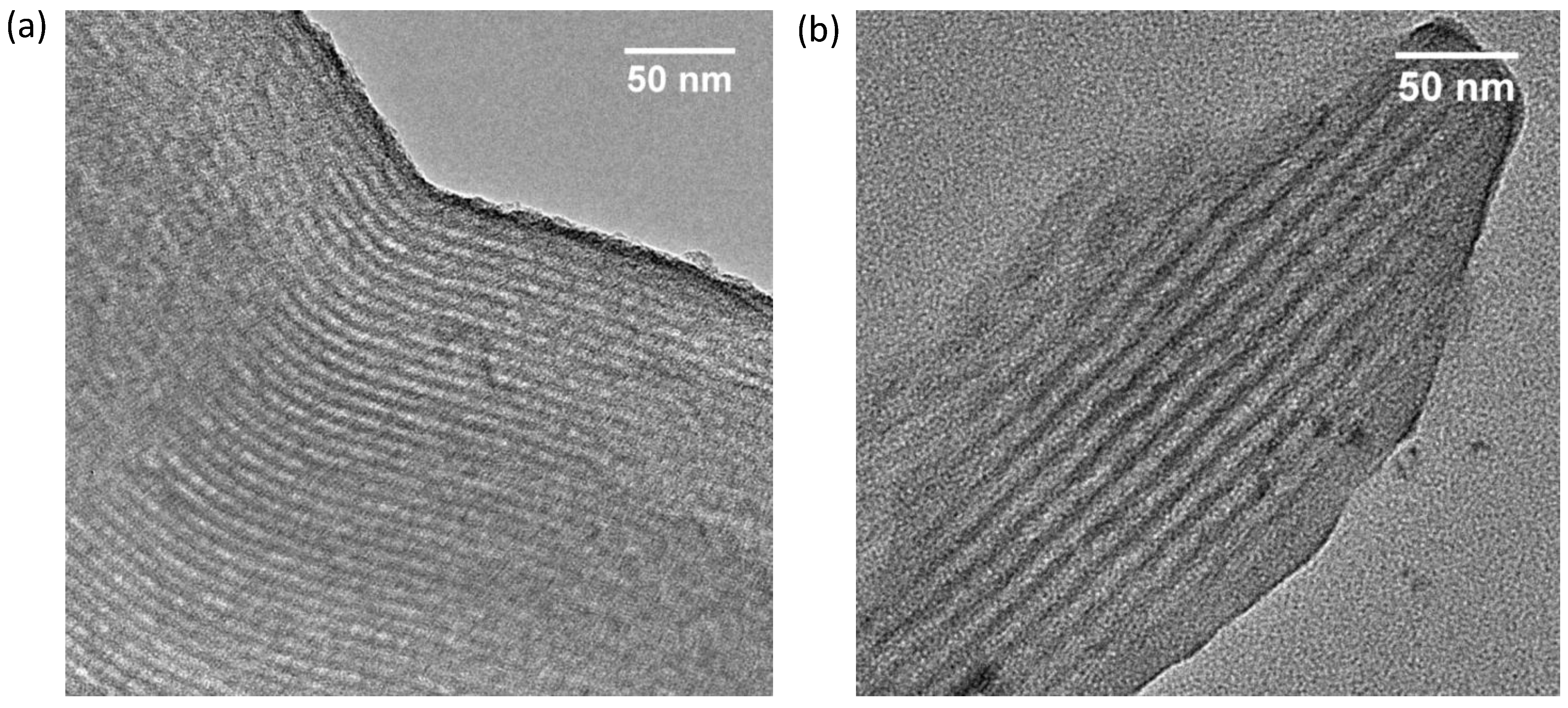
2.1. Characterization of the Adsorbents
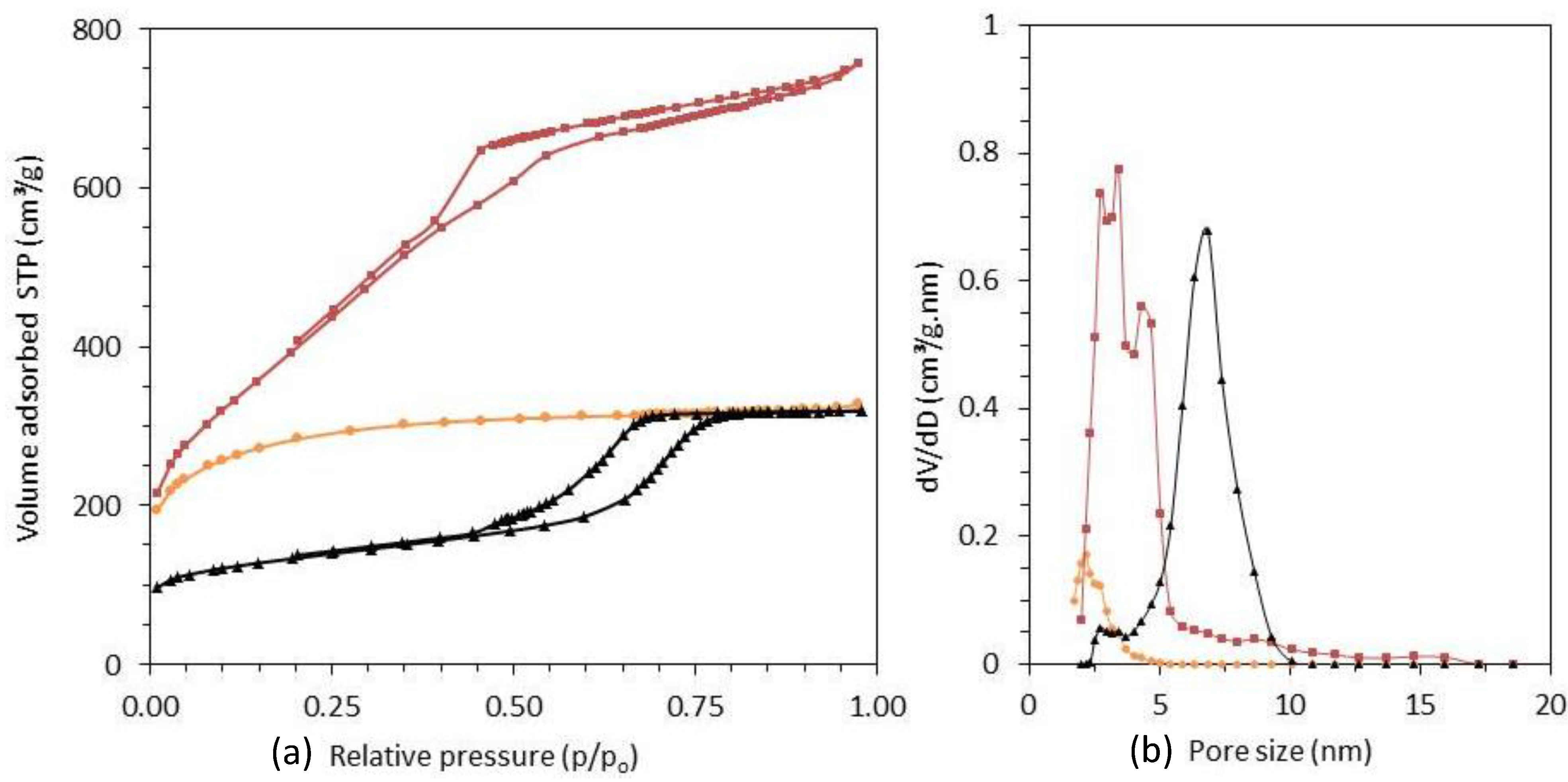
| Material | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore diameter D (nm) | Unit cell parameter a a0 (nm) | Wall thickness a (nm) |
|---|---|---|---|---|---|
| PAC | 1027 | 0.50 | <3 | - | - |
| CMK-3 | 1420 | 1.14 | 4.0 | 9.6 | 5.6 |
| SMC | 476 | 0.49 | 7.0 | 11.6 | 4.6 |

| Material | Elemental analysis [wt %] | |||
|---|---|---|---|---|
| C | H | N | O | |
| PAC | 85.90 | 0.45 | 0.29 | 13.36 |
| CMK-3 | 93.33 | 0.68 | 0.07 | 5.92 |
| SMC | 93.93 | 0.50 | 0.08 | 5.49 |
2.2. Adsorption Isotherms
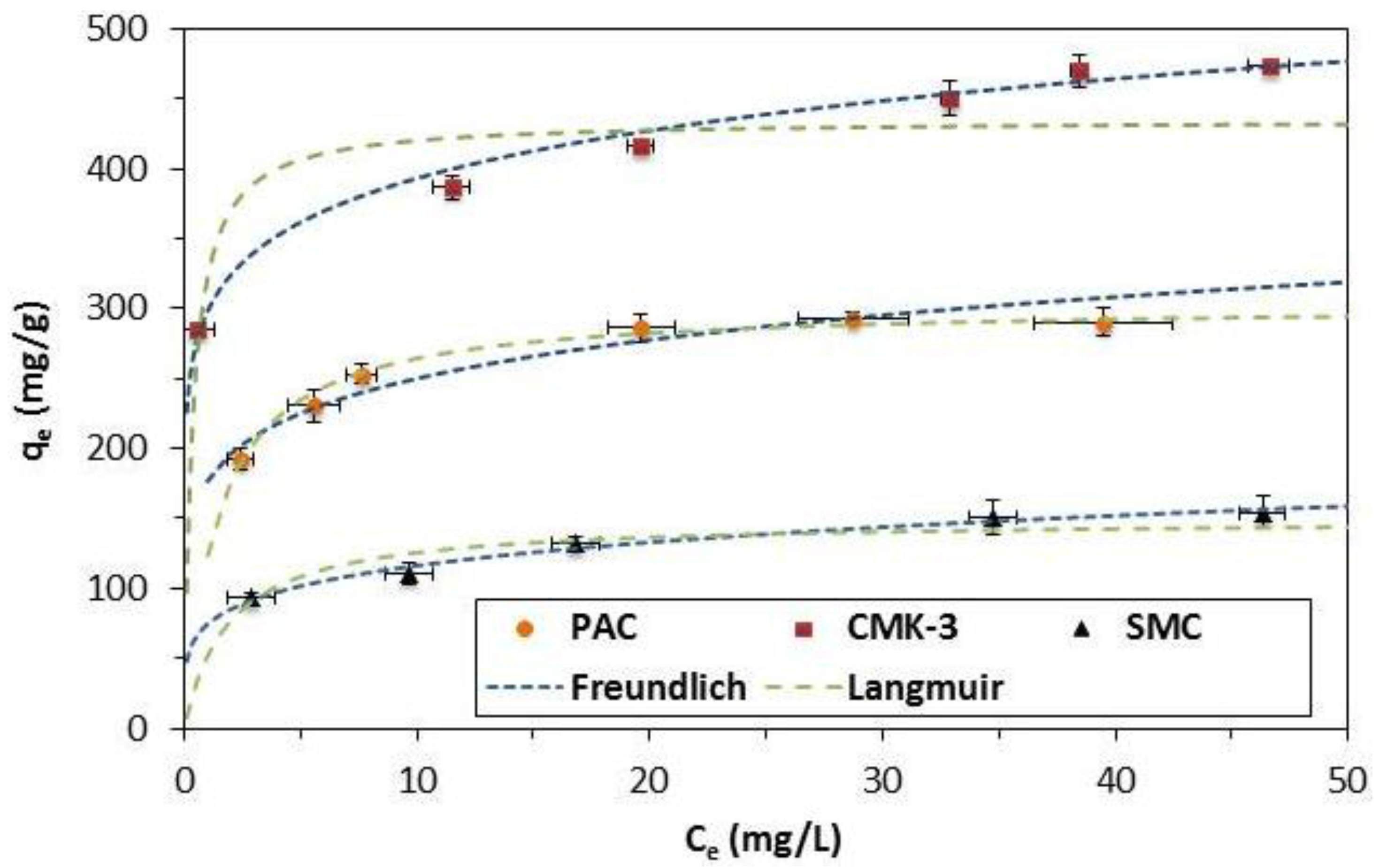
| Material | Experimental | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|---|
| qmax (mg/g) | Adsorbed BPA (molecules/nm2) | qmax (mg/g) | KL (L/mg) | R2 | 1/n | KF (mg/g (L/mg)1/n) | R2 | |
| PAC | 290 | 0.75 | 307 | 0.64 | 0.98 | 0.14 | 181 | 0.93 |
| CMK-3 | 474 | 0.88 | 447 | 2.81 | 0.82 | 0.12 | 296 | 0.99 |
| SMC | 154 | 0.86 | 156 | 0.40 | 0.87 | 0.19 | 74 | 0.98 |
2.3. Adsorption Kinetics
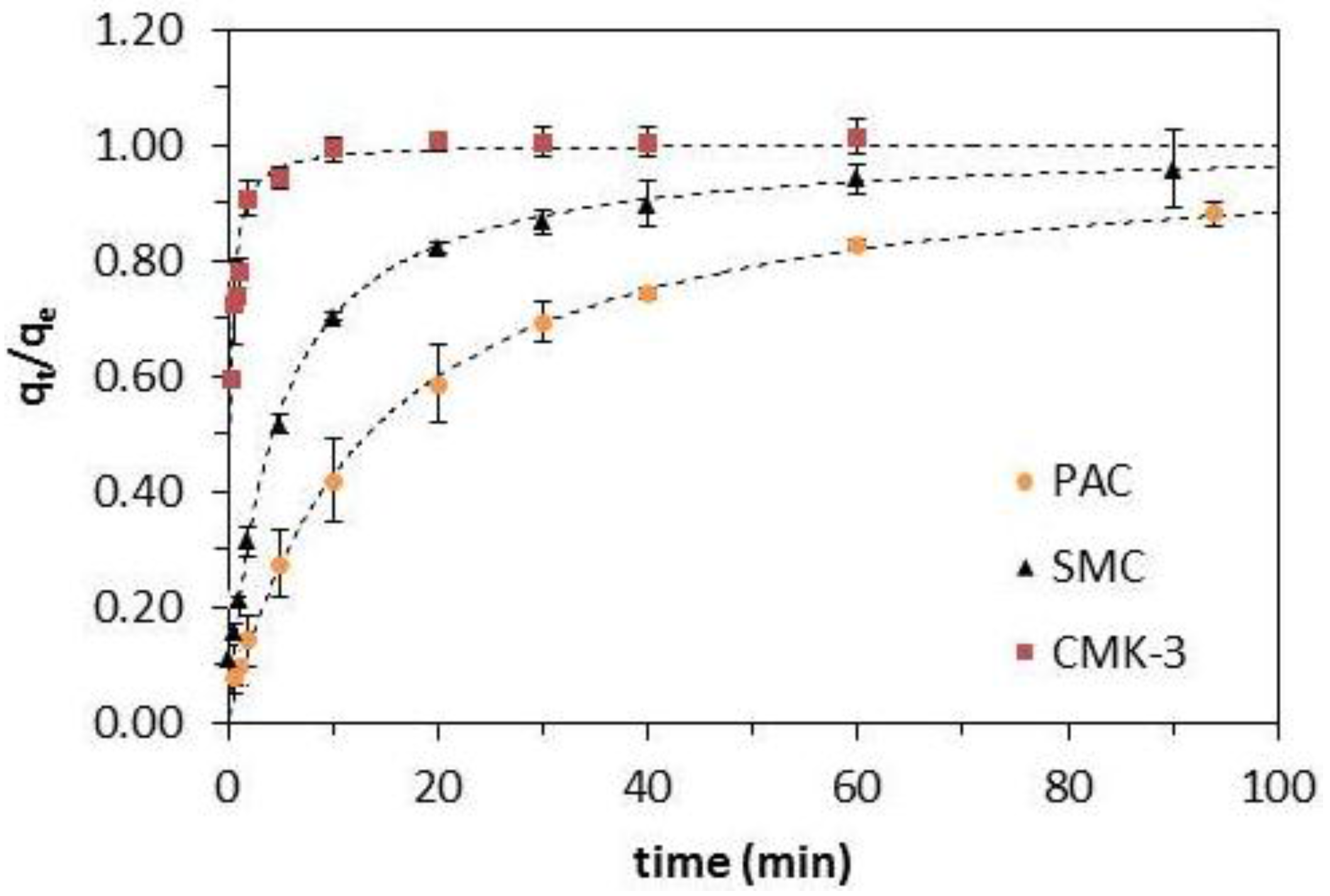
| Material | Pseudo-first-order model | Pseudo-second-order model | Weber-Morris | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qe (exp) (mg/g) | qe (calc) (mg/g) | k1 (1/min) | R2 | qe (calc) (mg/g) | k2 (g/(mg∙min) | t1/2 (min) | R2 | kd (min∙g/(mg)) | C (mg/g) | R2 | |
| PAC | 277 | 264 | 0.0657 | 0.98 | 315 | 0.00024 | 13.4 | 0.99 | 31.2 | 13.2 | 0.94 |
| CMK-3 | 295 | 281 | 2.64 | 0.77 | 291 | 0.01647 | 0.208 | 0.96 | 13.7 | 215 | 0.65 |
| SMC | 147 | 137 | 0.0236 | 0.97 | 149 | 0.00157 | 4.27 | 0.99 | 15.0 | 29.6 | 0.86 |

| PAC | kd (min∙g/mg) | C | R2 | SMC | kd (min∙g/mg) | C | R2 |
|---|---|---|---|---|---|---|---|
| 62–88 µm | 31.2 | 13.2 | 0.94 | 62–88 µm | 15.0 | 29.6 | 0.86 |
| 177–250 µm | 20.9 | −3.93 | 0.99 | 177–250 µm | 13.5 | 6.10 | 0.97 |
| 510–700 µm | 7.62 | −6.68 | 0.98 | 510–700 µm | 8.33 | 2.60 | 0.99 |
3. Experimental Section
3.1. Synthesis of Ordered Mesoporous Carbons
3.2. Characterization
3.3. Adsorption Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, T.-Y.; Liu, L.; Yuan, Z.-Y. Direct synthesis of ordered mesoporous carbons. Chem. Soc. Rev. 2013, 42, 3977–4003. [Google Scholar] [CrossRef] [PubMed]
- Muylaert, I.; Verberckmoes, A.; de Decker, J.; van der Voort, P. Ordered mesoporous phenolic resins: Highly versatile and ultra stable support materials. Adv. Colloid Interface Sci. 2012, 175, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, R.; Joo, S.H.; Kruk, M.; Jaroniec, M. Ordered mesoporous carbons. Adv. Mater. 2001, 13, 677–681. [Google Scholar] [CrossRef]
- Sui, Q.; Huang, J.; Liu, Y.; Chang, X.; Ji, G.; Deng, S.; Xie, T.; Yu, G. Rapid removal of bisphenol A on highly ordered mesoporous carbon. J. Environ. Sci. 2011, 23, 177–182. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, D. Ordered mesoporous materials as adsorbents. Chem. Commun. 2011, 47, 3332–3338. [Google Scholar] [CrossRef]
- Liu, L.; Deng, Q.-F.; Liu, Y.-P.; Ren, T.-Z.; Yuan, Z.-Y. HNO3-activated mesoporous carbon catalyst for direct dehydrogenation of propane to propylene. Catal. Commun. 2011, 16, 81–85. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ryoo, R.; Joo, S.H. Characterization of ordered mesoporous carbons synthesized using MCM-48 silicas as templates. J. Phys. Chem. B 2000, 104, 7960–7968. [Google Scholar] [CrossRef]
- Jun, S.; Joo, S.H.; Ryoo, R.; Kruk, M.; Jaroniec, M.; Liu, Z.; Ohsuna, T.; Terasaki, O. Synthesis of new, nanoporous carbon with hexagonally ordered mesostructure. J. Am. Chem. Soc. 2000, 122, 10712–10713. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Jun, S. Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J. Phys. Chem. B 1999, 103, 7743–7746. [Google Scholar] [CrossRef]
- Kim, T.-W.; Park, I.-S.; Ryoo, R. A synthetic route to ordered mesoporous carbon materials with graphitic pore walls. Angew. Chem. Int. Ed. Engl. 2003, 42, 4375–4379. [Google Scholar] [CrossRef] [PubMed]
- Gierszal, K.P.; Jaroniec, M.; Kim, T.-W.; Kim, J.; Ryoo, R. High temperature treatment of ordered mesoporous carbons prepared by using various carbon precursors and ordered mesoporous silica templates. New J. Chem. 2008, 32, 981–993. [Google Scholar] [CrossRef]
- Liang, C.; Hong, K.; Guiochon, G.A.; Mays, J.W.; Dai, S. Synthesis of a large-scale highly ordered porous carbon film by self-assembly of block copolymers. Angew. Chem. 2004, 116, 5909–5913. [Google Scholar] [CrossRef]
- Liang, C.; Dai, S. Synthesis of mesoporous carbon materials via enhanced hydrogen-bonding interaction. J. Am. Chem. Soc. 2006, 128, 5316–5317. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Synthesis of ordered mesoporous carbons with channel structure from an organic-organic nanocomposite. Chem. Commun. 2005, 16, 2125–2127. [Google Scholar] [CrossRef]
- Zhang, F.; Meng, Y.; Gu, D.; Yan, Y.; Yu, C.; Tu, B.; Zhao, D. A facile aqueous route to synthesize highly ordered mesoporous polymers and carbon frameworks with Ia 3̅ d bicontinuous cubic structure. J. Am. Chem. Soc. 2005, 127, 13508–13509. [Google Scholar] [CrossRef] [PubMed]
- Schlienger, S.; Graff, A.-L.; Celzard, A.; Parmentier, J. Direct synthesis of ordered mesoporous polymer and carbon materials by a biosourced precursor. Green Chem. 2012, 14, 313–316. [Google Scholar] [CrossRef]
- Muylaert, I.; Borgers, M.; Bruneel, E.; Schaubroeck, J.; Verpoort, F.; van der Voort, P. Ultra stable ordered mesoporous phenol/formaldehyde polymers as a heterogeneous support for vanadium oxide. Chem. Commun. 2008, 37, 4475–4477. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.; Shi, Y.; Cheng, L.; Feng, D.; Wu, Z.; Chen, Z.; Wan, Y.; Stein, A.; et al. A family of highly ordered mesoporous polymer resin and carbon structures from organic–organic self-assembly. Chem. Mater. 2006, 18, 4447–4464. [Google Scholar] [CrossRef]
- Chen, M.; Ike, M.; Fujita, M. Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ. Toxicol. 2002, 17, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, D.; Yanagiba, Y.; Duan, Z.; Ito, Y.; Okamura, A.; Asaeda, N.; Tagawa, Y.; Li, C.; Taya, K.; Zhang, S.Y.; et al. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol. Lett. 2010, 194, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Belfroid, A.; van Velzen, M.; van der Horst, B.; Vethaak, D. Occurrence of bisphenol A in surface water and uptake in fish: evaluation of field measurements. Chemosphere 2002, 49, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Cousins, I.T.; Staples, C.A.; Klecka, G.M.; Mackay, D. A multimedia assessment of the environmental fate of bisphenol A. Hum. Ecol. Risk Assess. 2002, 8, 1107–1135. [Google Scholar] [CrossRef]
- Liu, G.; Ma, J.; Li, X.; Qin, Q. Adsorption of bisphenol A from aqueous solution onto activated carbons with different modification treatments. J. Hazard. Mater. 2009, 164, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-T.; Lai, C.-W.; Su, T.-Y. Adsorption of bisphenol-A from aqueous solution onto minerals and carbon adsorbents. J. Hazard. Mater. 2006, 134, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, A.; Tamai, M.; Kawasaki, N.; Nakamura, T.; Tanada, S. Adsorption characteristics of bisphenol A onto carbonaceous materials produced from wood chips as organic waste. J. Colloid Interface Sci. 2002, 252, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-Y. Comparison with as-grown and microwave modified carbon nanotubes to removal aqueous bisphenol A. Desalination 2009, 249, 976–982. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Zhu, Y. Decontamination of bisphenol A from aqueous solution by graphene adsorption. Langmuir 2012, 28, 8418–8425. [Google Scholar] [CrossRef] [PubMed]
- Libbrecht, W.; Deruyck, F.; Poelman, H.; Verberckmoes, A.; Thybaut, J.; de Clercq, J.; van der Voort, P. Optimization of soft templated mesoporous carbon synthesis using Definitive Screening Design. Chem. Eng. J. 2015, 259, 126–134. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Z.; Wan, H.; Wan, Y.; Zheng, S.; Zhu, D. Enhanced adsorption of humic acids on ordered mesoporous carbon compared with microporous activated carbon. Environ. Toxicol. Chem. 2011, 30, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Agenson, K. Retention of a wide variety of organic pollutants by different nanofiltration/reverse osmosis membranes: Controlling parameters of process. J. Memb. Sci. 2003, 225, 91–103. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Vogel, D.; Khan, S. Characterising humic acid fouling of nanofiltration membranes using bisphenol A as a molecular indicator. Water Res. 2008, 42, 4049–4058. [Google Scholar] [CrossRef] [PubMed]
- Soni, H.; Padmaja, P. Palm shell based activated carbon for removal of bisphenol A: An equilibrium, kinetic and thermodynamic study. J. Porous Mater. 2014, 21, 275–284. [Google Scholar] [CrossRef]
- Konicki, W.; Cendrowski, K.; Chen, X.; Mijowska, E. Application of hollow mesoporous carbon nanospheres as an high effective adsorbent for the fast removal of acid dyes from aqueous solutions. Chem. Eng. J. 2013, 228, 824–833. [Google Scholar] [CrossRef]
- Wang, X.; Liang, C.; Dai, S. Facile synthesis of ordered mesoporous carbons with high thermal stability by self-assembly of resorcinol-formaldehyde and block copolymers under highly acidic conditions. Langmuir 2008, 24, 7500–7505. [Google Scholar] [PubMed]
- Leyva-ramos, R. Role of pore volume and surface diffusion in the adsorption of aromatic compounds on activated carbon. Adsorption 2013, 19, 945–957. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Libbrecht, W.; Vandaele, K.; De Buysser, K.; Verberckmoes, A.; Thybaut, J.W.; Poelman, H.; De Clercq, J.; Van Der Voort, P. Tuning the Pore Geometry of Ordered Mesoporous Carbons for Enhanced Adsorption of Bisphenol-A. Materials 2015, 8, 1652-1665. https://doi.org/10.3390/ma8041652
Libbrecht W, Vandaele K, De Buysser K, Verberckmoes A, Thybaut JW, Poelman H, De Clercq J, Van Der Voort P. Tuning the Pore Geometry of Ordered Mesoporous Carbons for Enhanced Adsorption of Bisphenol-A. Materials. 2015; 8(4):1652-1665. https://doi.org/10.3390/ma8041652
Chicago/Turabian StyleLibbrecht, Wannes, Koen Vandaele, Klaartje De Buysser, An Verberckmoes, Joris W. Thybaut, Hilde Poelman, Jeriffa De Clercq, and Pascal Van Der Voort. 2015. "Tuning the Pore Geometry of Ordered Mesoporous Carbons for Enhanced Adsorption of Bisphenol-A" Materials 8, no. 4: 1652-1665. https://doi.org/10.3390/ma8041652







