All-Ceramic Single Crown Restauration of Zirconia Oral Implants and Its Influence on Fracture Resistance: An Investigation in the Artificial Mouth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grouping and Sample Preparation

| Characteristics | Unit | Y-TZP |
|---|---|---|
| Components | ZrO2/Y2O3 | |
| Composition | wt% | 95/5 |
| Density | g/cm3 | >6.0 |
| Grain size | µm | <0.6 |
| Bending strength | MPa | >1200 |
| 48 Zirconia Implants | |||||
|---|---|---|---|---|---|
| Group “C” | Group “N” | ||||
| n = 24 | n = 24 | ||||
| Implants with single crown restauration | Implants without restauration (“as delivered”) | ||||
| C0 | C5 | C10 | N0 | N5 | N10 |
| n = 8 | n = 8 | n = 8 | n = 8 | n = 8 | n = 8 |
| 0 cycles | 5 × 106 cycles | 10 × 106 cycles | 0 cycles | 5 × 106 cycles | 10 × 106 cycles |
 | Dynamic loading | Dynamic loading |  | Dynamic loading | Dynamic loading |
| Static loading test | |||||
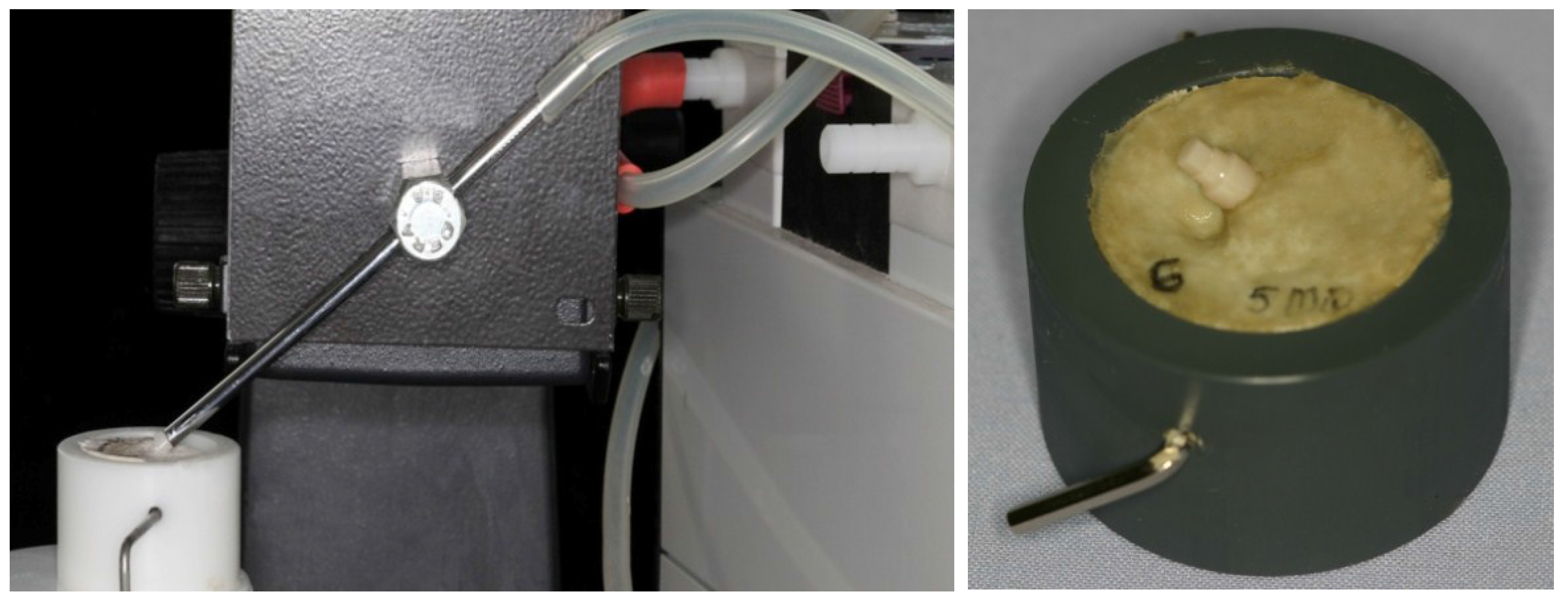
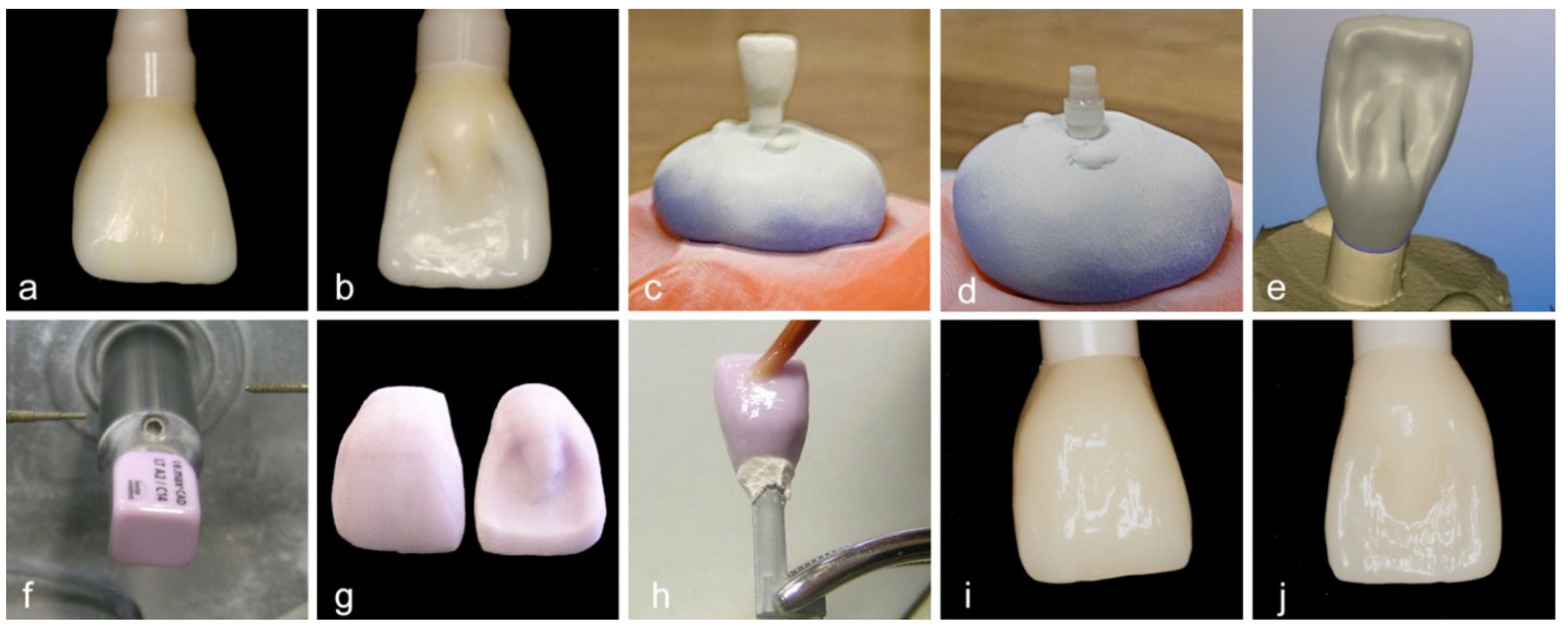
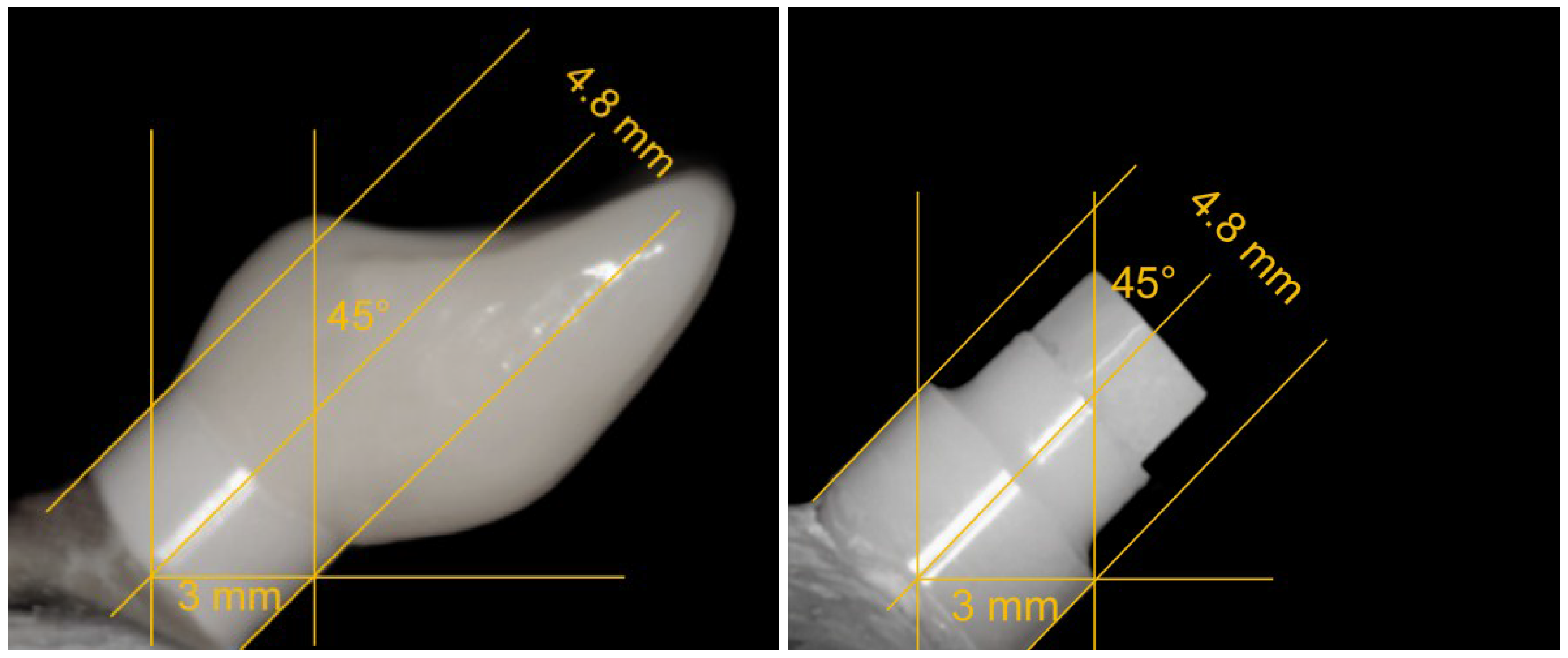
2.2. Dynamic Loading Test
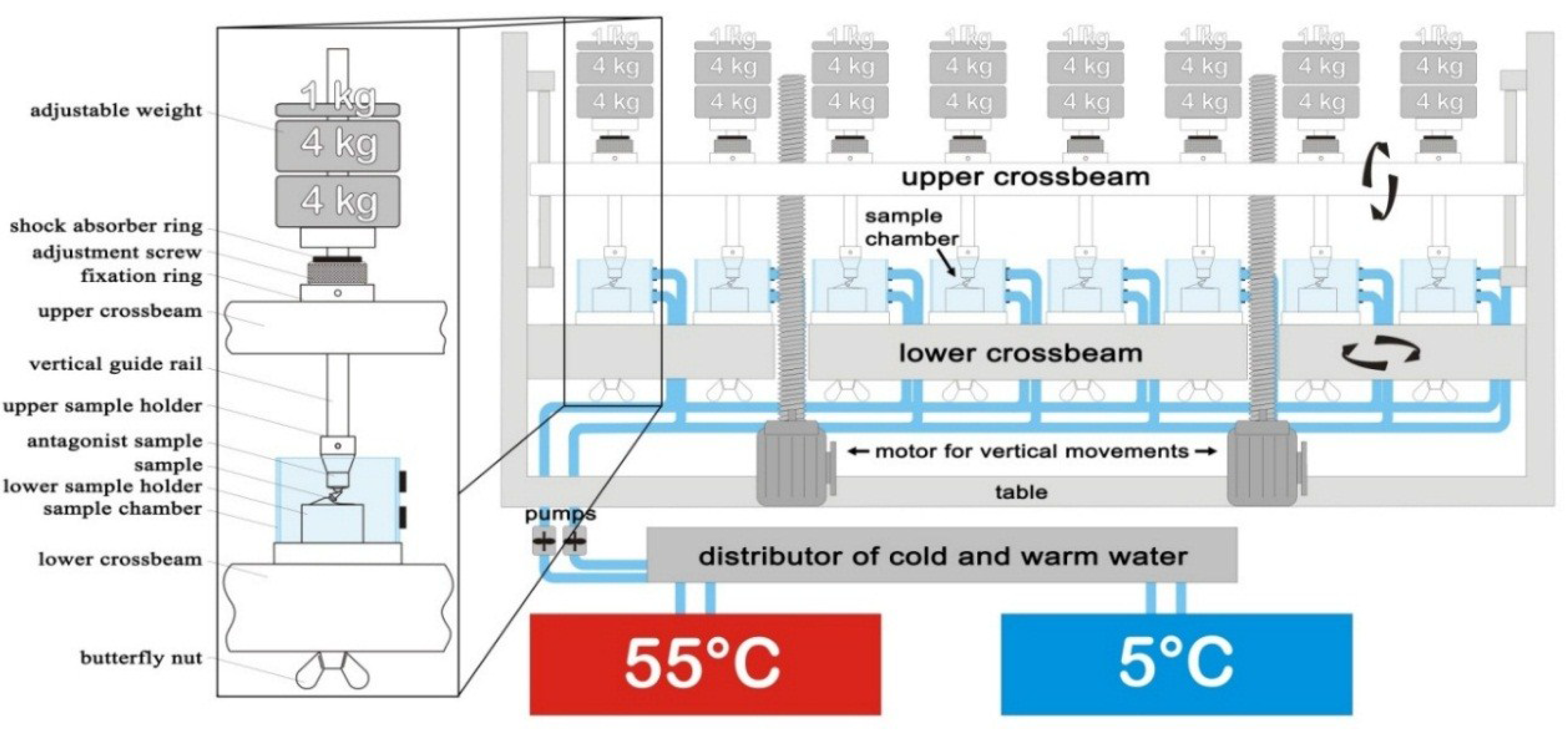
| Chewing Cycles | 5,000,000/10,000,000 |
|---|---|
| Cycle frequency | 1.6 Hz |
| Vertical movement | 6 mm |
| Horizontal movement | 0.5 mm |
| Descending speed | 60 mm/s |
| Rising speed | 55 mm/s |
| Forward speed | 60 mm/s |
| Backward speed | 55 mm/s |
| Applied weight per sample | 10 kg (98 N) |
| Hot dwell time | 60 s |
| Hot bath temperature | 55 °C |
| Cold dwell time | 60 s |
| Cold bath temperature | 5 °C |
| Intermediate pause | 12 s |
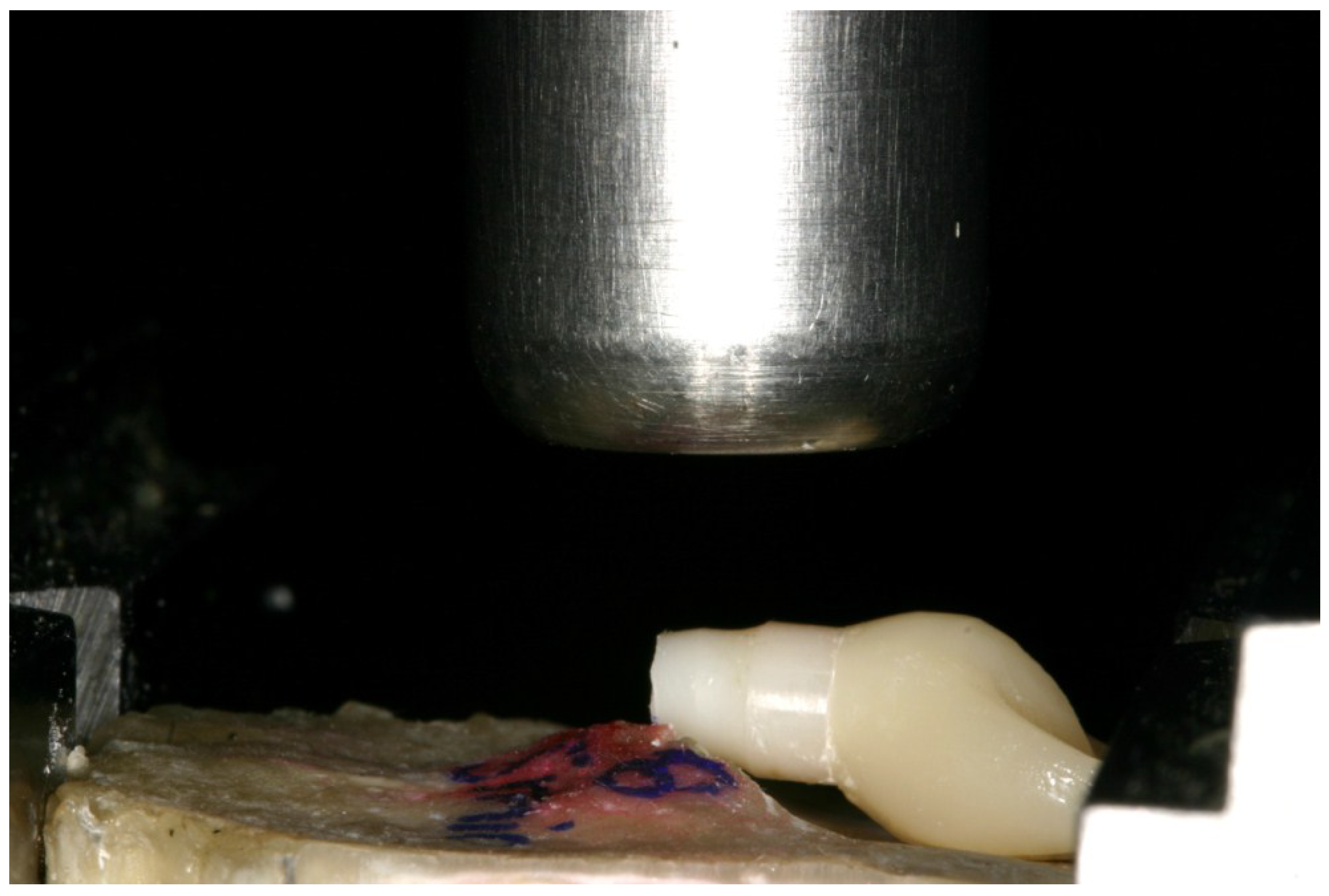
2.3. Static Loading Test
2.4. Statistical Analysis
3. Results
3.1. Dynamic Loading Test
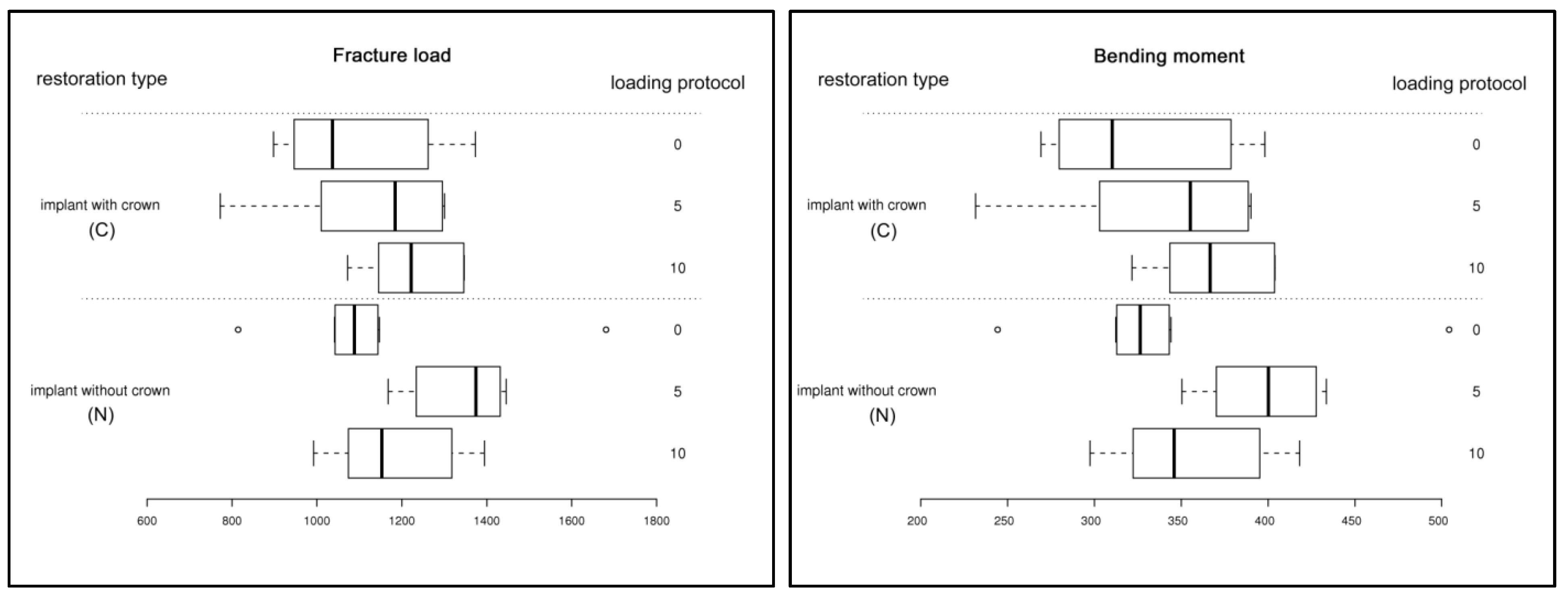
3.2. Static Loading Test
| Subgroup | n | Fracture load [N] F | Bending moment [Ncm] M | ||
|---|---|---|---|---|---|
| mean | SD | mean | SD | ||
| C0 | 8 | 1095.2 | 183.4 | 325.6 | 53.3 |
| C5 | 8 | 1131.5 | 189.1 | 339.5 | 56.7 |
| C10 | 8 | 1230.6 | 109.7 | 369.2 | 32.9 |
| N0 | 8 | 1130.5 | 245.6 | 339.2 | 73.7 |
| N5 | 8 | 1336.6 | 110.4 | 397.5 | 32.1 |
| N10 | 8 | 1184.4 | 147.0 | 355.3 | 44.1 |
3.3. Statistical Analysis
| Comparison | Bending moment | Fracture load | ||
|---|---|---|---|---|
| adj. p-value | Significance | adj. p-value | Significance | |
| C0 : C5 | 0.994 | not significant | 0.9981 | not significant |
| C0 : C10 | 0.5322 | not significant | 0.6137 | not significant |
| C0 : N0 | 0.9945 | not significant | 0.9983 | not significant |
| C0 : N5 | 0.0728 | not significant | 0.0732 | not significant |
| C0 : N10 | 0.8497 | not significant | 0.9001 | not significant |
| C5 : C10 | 0.8493 | not significant | 0.8537 | not significant |
| C5 : N0 | 1.0 | not significant | 1.0 | not significant |
| C5 : N5 | 0.2239 | not significant | 0.1802 | not significant |
| C5 : N10 | 0.9886 | not significant | 0.989 | not significant |
| C10 : N0 | 0.8439 | not significant | 0.8485 | not significant |
| C10 : N5 | 0.8727 | not significant | 0.8146 | not significant |
| C10 : N10 | 0.9939 | not significant | 0.9942 | not significant |
| N0 : N5 | 0.2192 | not significant | 0.1761 | not significant |
| N0 : N10 | 0.9876 | not significant | 0.9881 | not significant |
| N5 : N10 | 0.5658 | not significant | 0.4894 | not significant |
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jung, R.E.; Zembic, A.; Pjetursson, B.E.; Zwahlen, M.; Thoma, D.S. Systematic review of the survival rate and the incidence of biological, technical, and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin. Oral Implants Res. 2012, 23 (Suppl. 6), 2–21. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (fdps) after a mean observation period of at least 5 years. Clin. Oral Implants Res. 2012, 23 (Suppl. 6), 22–38. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloy. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Parr, G.R.; Gardner, L.K.; Toth, R.W. Titanium: The mystery metal of implant dentistry. Dental materials aspects. J. Prosthet. Dent. 1985, 54, 410–414. [Google Scholar] [CrossRef]
- Chaturvedi, T.P. An overview of the corrosion aspect of dental implants (titanium and its alloys). Indian J. Dent. Res. 2009, 20, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Flatebø, R.S.; Johannessen, A.C.; Grønningsaeter, A.G.; Bøe, O.E.; Gjerdet, N.R.; Grung, B.; Leknes, K.N. Host response to titanium dental implant placement evaluated in a human oral model. J. Periodontol. 2006, 77, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Al-Hezaimi, K.; Almas, K.; Romanos, G.E. Is titanium sensitivity associated with allergic reactions in patients with dental implants? A systematic review. Clin. Implant Dent. Relat. Res. 2013, 15, 47–52. [Google Scholar] [CrossRef]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin. Oral Implants Res. 2008, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Payne, A.G.; De Silva, R.K.; Duncan, W.J. Titanium allergy: Could it affect dental implant integration? Clin. Or. Implant. Res. 2011, 22, 673–680. [Google Scholar] [CrossRef]
- Li, J.; Hastings, G. Oxide bioceramics: Inert ceramic materials in medicine and dentistry. In Handbook of Biomaterial Properties, 1st ed.; Black, J., Hastings, G., Eds.; Springer: London, UK, 1998; pp. 340–354. [Google Scholar]
- Kim, D.J.; Lee, M.H.; Lee, D.Y.; Han, J.S. Mechanical properties, phase stability, and biocompatibility of (y, nb)-tzp/al(2)o(3) composite abutments for dental implant. J. Biomed. Mater. Res. 2000, 53, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Aldini, N.N.; Fini, M.; Giavaresi, G.; Martini, L.; Dubini, B.; Ponzi Bossi, M.G.; Rustichelli, F.; Krajewski, A.; Ravaglioli, A.; Mazzocchi, M.; et al. Osteointegration of bioactive glass-coated and uncoated zirconia in osteopenic bone: An in vivo experimental study. J. Biomed. Mater. Res. 2004, 68A, 264–272. [Google Scholar] [CrossRef]
- Covacci, V.; Bruzzese, N.; Maccauro, G.; Andreassi, C.; Ricci, G.A.; Piconi, C.; Marmo, E.; Burger, W.; Cittadini, A. In vitro evaluation of the mutagenic and carcinogenic power of high purity zirconia ceramic. Biomaterials 1999, 20, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Gahlert, M.; Gudehus, T.; Eichhorn, S.; Steinhauser, E.; Kniha, H.; Erhardt, W. Biomechanical and histomorphometric comparison between zirconia implants with varying surface textures and a titanium implant in the maxilla of miniature pigs. Clin. Oral Implants Res. 2007, 18, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Gahlert, M.; Roehling, S.; Sprecher, C.M.; Kniha, H.; Milz, S.; Bormann, K. In vivo performance of zirconia and titanium implants: A histomorphometric study in mini pig maxillae. Clin. Oral Implants Res. 2012, 23, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Gahlert, M.; Röhling, S.; Wieland, M.; Sprecher, C.M.; Kniha, H.; Milz, S. Osseointegration of zirconia and titanium dental implants: A histological and histomorphometrical study in the maxilla of pigs. Clin. Oral Implants Res. 2009, 20, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Wolkewitz, M.; Hinze, M.; Han, J.S.; Bachle, M.; Butz, F. Biomechanical and histological behavior of zirconia implants: An experiment in the rat. Clin. Oral Implants Res. 2009, 20, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Schliephake, H.; Hefti, T.; Schlottig, F.; Gedet, P.; Staedt, H. Mechanical anchorage and peri-implant bone formation of surface-modified zirconia in minipigs. J. Clin. Periodontol. 2010, 37, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Manicone, P.F.; Rossi Iommetti, P.; Raffaelli, L. An overview of zirconia ceramics: Basic properties and clinical applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Christel, P.; Meunier, A.; Heller, M.; Torre, J.P.; Peille, C.N. Mechanical properties and short-term in vivo evaluation of yttrium-oxide-partially-stabilized zirconia. J. Biomed. Mater. Res. 1989, 23, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Swain, M.V. Impact of oral fluids on dental ceramics: What is the clinical relevance? Dent. Mater. 2014, 30, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Andreiotelli, M.; Kohal, R.J. Fracture strength of zirconia implants after artificial aging. Clin. Implant Dent. Relat. Res. 2009, 11, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Wolkewitz, M.; Tsakona, A. The effects of cyclic loading and preparation on the fracture strength of zirconium-dioxide implants: An in vitro investigation. Clin. Oral Implants Res. 2011, 22, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.R.; Coelho, P.G.; Fernandes, C.A.; Navarro, J.M.; Dias, R.A.; Thompson, V.P. Reliability of one-piece ceramic implant. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Finke, H.C.; Klaus, G. Stability of prototype two-piece zirconia and titanium implants after artificial aging: An in vitro pilot study. Clin. Implant Dent. Rel. Res. 2009, 11, 323–329. [Google Scholar] [CrossRef]
- Kohal, R.J.; Klaus, G.; Strub, J.R. Zirconia-implant-supported all-ceramic crowns withstand long-term load: A pilot investigation. Clin. Oral Implants Res. 2006, 17, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Rakosi, T.; Jonas, I.; Graber, T. Orthodontic diagnosis. In Cephalometric Analysis, 1st ed.; Rateitschak, K.H., Ed.; Georg Thieme Stuttgart: New York, NY, USA, 1993; p. 198. [Google Scholar]
- Cannizzaro, G.; Torchio, C.; Felice, P.; Leone, M.; Esposito, M. Immediate occlusal versus non-occlusal loading of single zirconia implants. A multicentre pragmatic randomised clinical trial. Eur. J. Or. Implantol. 2010, 3, 111–120. [Google Scholar]
- Payer, M.; Arnetzl, V.; Kirmeier, R.; Koller, M.; Arnetzl, G.; Jakse, N. Immediate provisional restoration of single-piece zirconia implants: A prospective case series - results after 24 months of clinical function. Clin. Oral Implants Res. 2013, 24, 569–575. [Google Scholar] [CrossRef]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 1993, 26, 111–119. [Google Scholar] [CrossRef] [PubMed]
- DeLong, R.; Sakaguchi, R.L.; Douglas, W.H.; Pintado, M.R. The wear of dental amalgam in an artificial mouth: A clinical correlation. Dent. Mater. 1985, 1, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Wassell, R.W.; McCabe, J.F.; Walls, A.W. A two-body frictional wear test. J. Dent. Res. 1994, 73, 1546–1553. [Google Scholar] [PubMed]
- Fontijn-Tekamp, F.A.; Slagter, A.P.; Van Der Bilt, A.; Van, T.H.M.A.; Witter, D.J.; Kalk, W.; Jansen, J.A. Biting and chewing in overdentures, full dentures, and natural dentitions. J. Dent. Res. 2000, 79, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Schindler, H.J.; Stengel, E.; Spiess, W.E. Feedback control during mastication of solid food textures—a clinical-experimental study. J. Prosthet. Dent. 1998, 80, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Sanon, C.; Chevalier, J.; Douillard, T.; Cattani-Lorente, M.; Scherrer, S.S.; Gremillard, L. A new testing protocol for zirconia dental implants. Dent. Mater. 2015, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Morneburg, T.R.; Pröschel, P.A. Measurement of masticatory forces and implant loads: A methodologic clinical study. Int. J. Prosthodont. 2002, 15, 20–27. [Google Scholar] [PubMed]
- Duyck, J.; van Oosterwyck, H.; Vander Sloten, J.; de Cooman, M.; Puers, R.; Naert, I. Magnitude and distribution of occlusal forces on oral implants supporting fixed prostheses: An in vivo study. Clin. Oral Implants Res. 2000, 11, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Morneburg, T.R.; Pröschel, P.A. In vivo forces on implants influenced by occlusal scheme and food consistency. Int. J. Prosthodont. 2003, 16, 481–486. [Google Scholar] [PubMed]
- Richter, E.J. In vivo horizontal bending moments on implants. Int. J. Oral Maxillofac. Implants 1998, 13, 232–244. [Google Scholar] [PubMed]
- Kohal, R.J.; Wolkewitz, M.; Mueller, C. Alumina-reinforced zirconia implants: Survival rate and fracture strength in a masticatory simulation trial. Clin. Oral Implants Res. 2010, 21, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Spies, B.C.; Sauter, C.; Wolkewitz, M.; Kohal, R.J. Alumina reinforced zirconia implants: Effects of cyclic loading and abutment modification on fracture resistance. Dent. Mater. 2015, 31, 262–272. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohal, R.-J.; Kilian, J.B.; Stampf, S.; Spies, B.C. All-Ceramic Single Crown Restauration of Zirconia Oral Implants and Its Influence on Fracture Resistance: An Investigation in the Artificial Mouth. Materials 2015, 8, 1577-1589. https://doi.org/10.3390/ma8041577
Kohal R-J, Kilian JB, Stampf S, Spies BC. All-Ceramic Single Crown Restauration of Zirconia Oral Implants and Its Influence on Fracture Resistance: An Investigation in the Artificial Mouth. Materials. 2015; 8(4):1577-1589. https://doi.org/10.3390/ma8041577
Chicago/Turabian StyleKohal, Ralf-Joachim, Jolanta Bernadette Kilian, Susanne Stampf, and Benedikt Christopher Spies. 2015. "All-Ceramic Single Crown Restauration of Zirconia Oral Implants and Its Influence on Fracture Resistance: An Investigation in the Artificial Mouth" Materials 8, no. 4: 1577-1589. https://doi.org/10.3390/ma8041577







