1. Introduction
Thermoelectric materials and devices can realize direct conversion between heat and electricity that have attracted great interest owing to their widespread applications, such as solid-state cooling, power generation, and waste heat recovery [
1,
2]. The efficiency for energy conversion is characterized by a dimensionless figure of merit
ZT =
S2σ
T/κ, where
T,
S, σ, and κ are the absolute temperature, Seebeck coefficient, electrical conductivity and thermal conductivity, respectively. An outstanding thermoelectric material requires a higher power factor (
PF =
S2σ) and a lower thermal conductivity.
Until now, alloys still exhibit the best thermoelectric performance, such as Bi
2Te
3, PbTe, SnSe,
etc. [
3,
4,
5], and show good practical prospects. In view of the low cost of raw materials and the high stability, thermoelectric oxides have been considered as promising candidates for high temperature applications. Typical oxides [
1,
6,
7,
8,
9] (such as Ca
3Co
4O
9, CaMnO
3, and ZnO) have been extensively investigated over the past 20 years. However, their
ZT values are still too low to be used in commercial applications due to the mediocre electrical conductivity and high thermal conductivity. Recently, a
p-type oxide material, BiCuSeO, with a low intrinsic thermal conductivity has attracted great attention; its optimized
ZT value can reach 1.4 at 923 K [
10,
11,
12]. Therefore, we consider looking for a low thermal conductivity oxide for
n-type thermoelectric applications.
As reported by
Ruleova et al. [
13], Bi
2O
2Se exhibits a very low thermal conductivity (0.7~0.75 Wm
−1·K
−1 at 800 K), combined with moderate power factor; hence it is expected to be a potential thermoelectric oxide. This compound is formed by partial replacement of selenium atoms with oxygen atoms in Bi
2Se
3, which belongs to so-called Sillen compounds and shows a (Na
0.25Bi
0.75)
2O
2Cl-type structure (D
4h17). Similarly, Bi
2O
2Se exhibits a layered structure that is composed of tetragonal (BiO)
n layers with Se occupying inter-layer positions, which result in the low thermal conductivity. Up to now, the reports about the thermoelectric performance of Bi
2O
2Se are still insufficient [
14,
15,
16,
17]. We have attempted to enhance the thermoelectric properties of Bi
2O
2Se by tetravalence Sn doping, or introducing Bi deficiencies. The Bi deficiencies did not significantly affect the electrical conductivity, but were in favor of orientation alignment of grains. The introduction of Sn brought about a high electrical conductivity and the highest
ZT value can reach 0.20 at 773 K [
18].
However, its electrical conductivity is still not enough (~60 S cm
−1 at 773 K). In general, silver addition is used to enhance electrical conductivity and achieve the favorable results [
19,
20,
21], thus it is considered to optimize the electrical conductivity of Bi
2O
2Se. In this work, we prepared the Bi
2O
2Se/Ag composites by spark plasma sintering (SPS), and evaluated their thermoelectric performances.
2. Results and Discussion
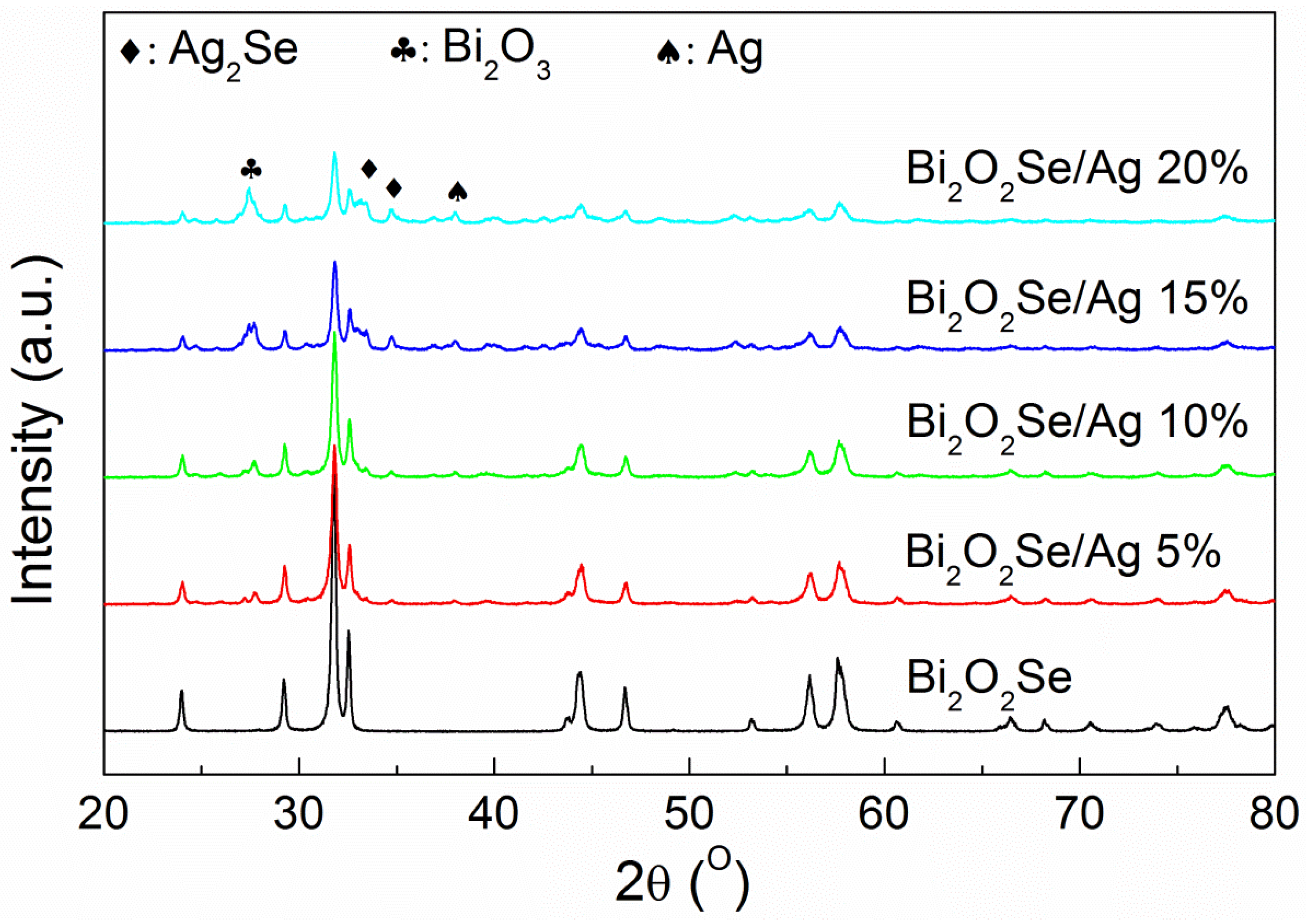
Figure 1 shows the X-ray diffraction (XRD) patterns at room temperature of Bi
2O
2Se/Ag composites. The main phase corresponds to Bi
2O
2Se with a tetragonal structure in
I4/mmm space group. As seen in
Figure 1, the intensity of main phase in composites is decreased, while the second phases can be observed clearly as the Ag additive increasing. After matching the peaks, the second phases are composed of Ag, Ag
2Se and Bi
2O
3. Extra silver is added as an independent additive, therefore the presence of an Ag phase is reasonable. Since Se exists in the form of a single atomic layer in the lattice, the bond between (BiO)
n layers and Se layers should be weak; so it is possible to cause the chemical reaction between the silver and selenium. A reasonable assumption is that:
Obviously, more Ag additive will lead to the stronger second phase as the reaction proceeds. Meanwhile, the strength of the main phase decreases with the reaction of Bi
2O
2Se. This is consistent with the variation of peaks in
Figure 1. That is to say, the introduction of the silver additive will undermine the stability of the Bi
2O
2Se structure, which is not an expected result.
Figure 1.
Room temperature X-ray diffraction patterns of Bi2O2Se/Ag composites.
Figure 1.
Room temperature X-ray diffraction patterns of Bi2O2Se/Ag composites.
The fractured-surface microstructure in Bi
2O
2Se/Ag composites is presented in
Figure 2. The pure sample shows a typical lamellar structure as seen in
Figure 2a, and the thickness of flaked grains is around 200 nm. It exhibits a loose structure that shows a relative density of 84.5%. The microstructure obviously becomes more compact with the composite of silver, and the obtained experimental densities are 9.264, 9.170, 9.028, and 8.856 g·cm
−3 for Bi
2O
2Se/Ag composites with volume ratios 5%, 10%, 15%, and 20%, respectively. As shown in
Figure 2b, the grain size of sample significantly increases, and the binding between grains becomes closer compared with the pure sample. As we know, Bi
2O
3 is a common sintering aid; therefore it can notably improve the sintering performance of Bi
2O
2Se ceramics by Equation (1). However, the loose nanostructure has been destroyed, which will lead to a deterioration of thermal conductivity. With the increasing content of Ag, a similar change can be found; a small amount of pores were observed, which is consistent with the change of density being caused by the chemical reaction. Further, some nano-particles can be discovered in
Figure 2d; they may originate from the precipitation of second phases, which can act as scattering centers for enhancing phonon scattering.
Figure 2.
Field emission scanning electron microscopy of Bi2O2Se/Ag fracture surfaces, (a) pure sample; (b) 5 vol.% Ag; (c) 10 vol.% Ag; and (d) 20 vol.% Ag.
Figure 2.
Field emission scanning electron microscopy of Bi2O2Se/Ag fracture surfaces, (a) pure sample; (b) 5 vol.% Ag; (c) 10 vol.% Ag; and (d) 20 vol.% Ag.
The temperature dependence of electrical conductivity for Bi
2O
2Se/Ag composites from 300 to 673 K is shown in
Figure 3a. A remarkable enhancement of electrical conductivity can be obtained by Ag addition. Their values at 673 K can reach 1.29, 43.84, 101.55, 243.91, and 691.79 S·cm
−1 for different volume ratio of Ag (0%–20%), respectively. The pure sample exhibits typical semiconductor behavior, in which electrical conductivity increases with the temperature rising. Since the introduction of silver, a similar variation can be found in
Figure 3a. The electrical conductivity increased first and then decreased as the temperature increased. It is well known that Ag is an excellent conductor (~10
5 S·cm
−1), which has a negative temperature coefficient. This does not match with the obtained results, thus the change of electrical conductivity may be mainly controlled by conductive phase Ag
2Se. As reported by Day [
22], that the resistivity of Ag
2Se decreased before 400 K and then increased with temperature after phase transition, the same variation in Bi
2O
2Se/Ag composites can be observed as seen in
Figure 3a. Therefore, we can infer that the abnormal electrical conductivity stems from the effect of Ag
2Se. With the introduction of silver, it is uniformly dispersed in the matrix; however, a small amount of Ag
2Se formed by Equation (1) and was wrapped in large grains as shown in
Figure 2b. Carrier transport between them is not smooth and the electrical conductivity exhibits a limited increase. As the volume ratio increases to 10%, more conductive phase will be formed, the carrier concentration and transport route can be optimized. Further increasing the content of Ag to 20%, excessive second phases precipitated in the form of nanoparticles; the electrical conductivity shows significant enhancement combined with high carrier concentration and good transport properties in composite.
Figure 3.
Temperature dependence of (a) electrical conductivity; and (b) Seebeck coefficient for Bi2O2Se/Ag composites.
Figure 3.
Temperature dependence of (a) electrical conductivity; and (b) Seebeck coefficient for Bi2O2Se/Ag composites.
Temperature dependence of Seebeck coefficient for Bi
2O
2Se/Ag composites is shown in
Figure 3b. In the whole temperature range from 300 to 673 K, the Seebeck coefficients are all negative, indicating a
n-type electrical conduction behavior. The pure sample shows a high Seebeck coefficient, which ranges from −363.3 to −559.9 μVK
−1. This value can be obtained from the layered structure, which consists of insulating (BiO)
n layers and the conductive Se layers stacked alternately, forming natural superlattices. The two dimensional carriers confinement in the natural superlattices in Bi
2O
2Se is similar to the structure of BiCuSeO system [
23,
24]. With the addition of Ag, the absolute Seebeck coefficients exhibit dramatic reduction, −154.8, −61.7, −46.3, and −29.5 μVK
−1 for Bi
2O
2Se/Ag composites with volume ratios 5%, 10%, 15%, and 20%, respectively. As mentioned before [
22], the Seebeck coefficient of Ag
2Se is from −140 to −20 μVK
−1, which is affected by the process conditions, and it also shows a turning point attributed to the phase transition. A similar phenomenon can be observed in composites by Ag addition. With the formation of Ag
2Se, the carrier concentration increased, therefore a decrease of Seebeck coefficient can be obtained. Moreover, it can be found in
Figure 3b that the variation of value is small when volume ratio of Ag is over 10%, which indicates the electrical conductivity is not only affected by carrier concentration.
Power factor (PF) is calculated from electrical conductivity and Seebeck coefficient as S2σ. The values of PF at 673 K are 0.353, 1.050, 0.387, 0.522, and 0.604 μW·cm−1·K−2 for different volume ratio of Ag (0%–20%), respectively. For high content of Ag, the power factor increases with the electrical conductivity increasing, even with a low Seebeck coefficient. The highest value of power factor can be obtained in a sample of 5 vol.% Ag, which is associated with the optimized electrical conductivity while maintaining a moderate Seebeck coefficient. This shows once again that thermoelectric performance is a comprehensive consideration, with just high electrical conductivity or high Seebeck coefficient, it is difficult to obtain high power factor. We usually need to compromise to get an optimum result.
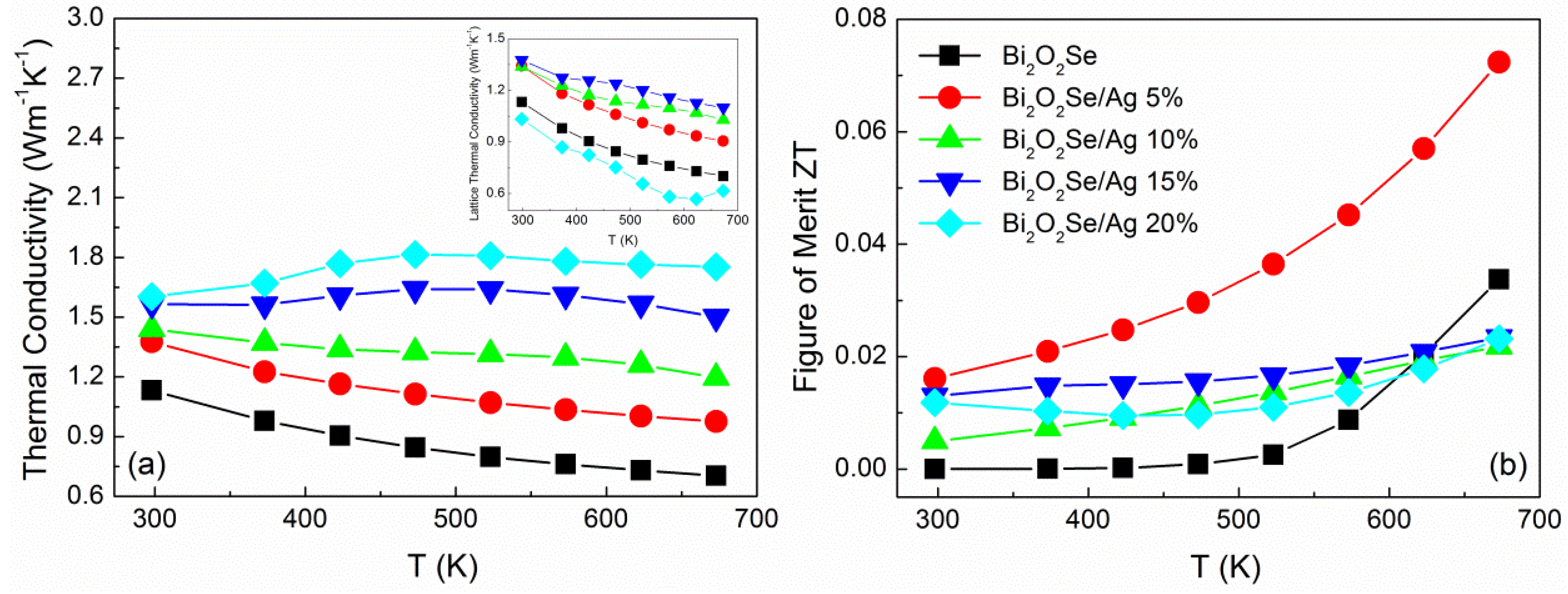
Temperature dependence of thermal conductivity (κ) between 300 and 673 K is shown in
Figure 4a. The thermal conductivity at 673 K is 0.704, 0.976, 1.196, 1.501, 1.752 W·m
−1·K
−1 for Bi
2O
2Se/Ag composites with volume ratios 5%, 10%, 15%, and 20%, respectively. The huge increase in thermal conductivity may come from the presence of conductive phase Ag
2Se, as in the reported result [
22] of about 1.5~4 W·m
−1·K
−1. Especially in higher Ag content, the composites exhibit a similar feature to Ag
2Se that thermal conductivity increases with temperature and then levels off after phase transition. As we know, thermal conductivity (κ) can be expressed by the sum of the lattice thermal conductivity (κ
l) and electronic thermal conductivity (κ
e) as κ = κ
l + κ
e. Usually, the electronic thermal conductivity can be calculated by Wiedemann-Franz’s law as below:
where
L is the Lorenz constant, σ is the electrical conductivity, and
T is the absolute temperature. The calculated κ
e in the entire temperature range is similar to the change of electrical conductivity, which shows a significant rise from 0.002 to 1.136 W·m
−1·K
−1. Based on the electronic thermal conductivity, the lattice thermal conductivity can be calculated. At lower content of silver, κ
l increased with more Ag
2Se formation and ceramics density improvement. When the vol.% is increased to 20%, the lattice thermal conductivity suddenly reduced to 0.616 W·m
−1·K
−1, which may derive from the precipitation of nanoparticles that can obviously improve on the scattering of phonons.
Figure 4.
Temperature dependence of (a) thermal conductivity; and (b) ZT value for Bi2O2Se/Ag composites.
Figure 4.
Temperature dependence of (a) thermal conductivity; and (b) ZT value for Bi2O2Se/Ag composites.
Based upon the electrical and thermal transport properties, the dimensionless figure of merit (
ZT) is calculated as shown in
Figure 4b. The highest
ZT value reaches 0.072 at 673 K for Bi
2O
2Se/5 vol.% Ag, which demonstrates 115% improvement compared with pristine Bi
2O
2Se. With more Ag additive, the
ZT values are lower than the pure sample at high temperature. This indicates the great improvement of electrical conductivity is insufficient to compensate for the loss of Seebeck coefficient and thermal conductivity. Meanwhile, the Ag addition will affect the stability of Bi
2O
2Se, therefore it may not be a good choice for the enhancement of thermoelectric performance of Bi
2O
2Se.