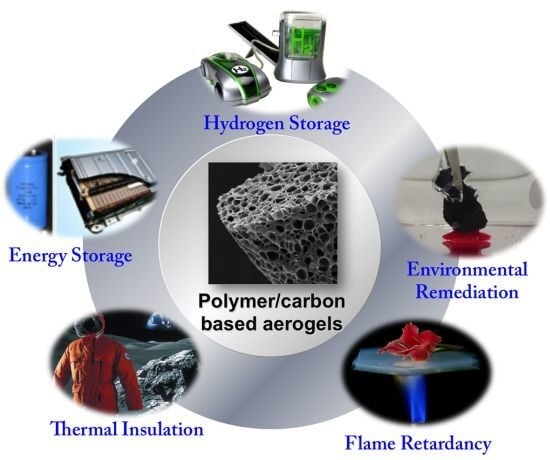
Polymer/Carbon-Based Hybrid Aerogels: Preparation, Properties and Applications
Abstract
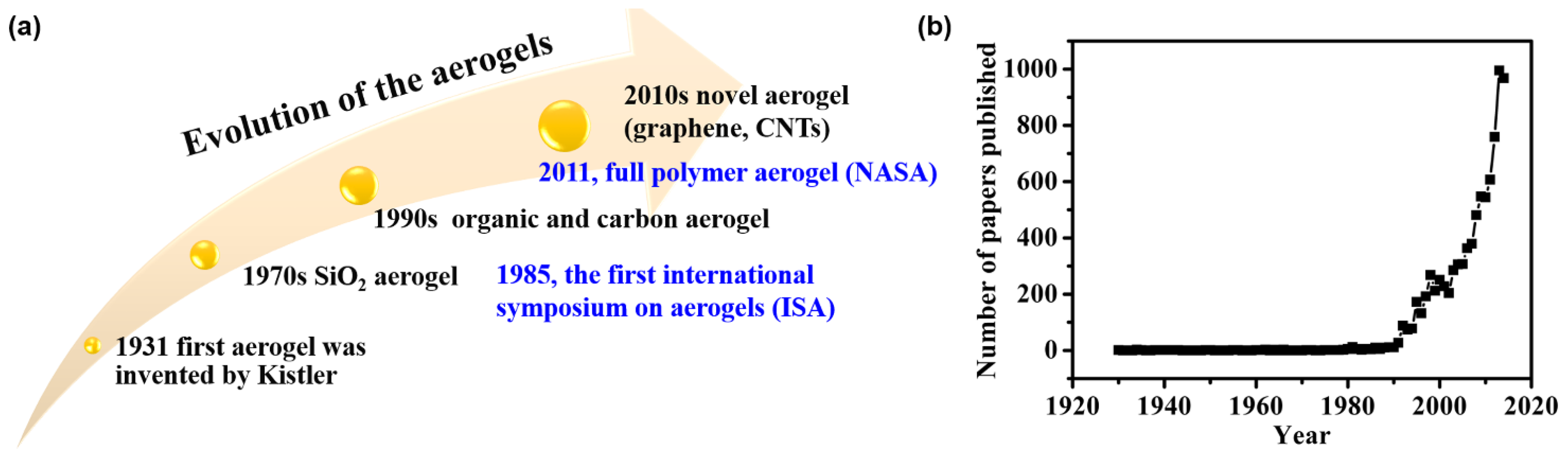
:1. Introduction
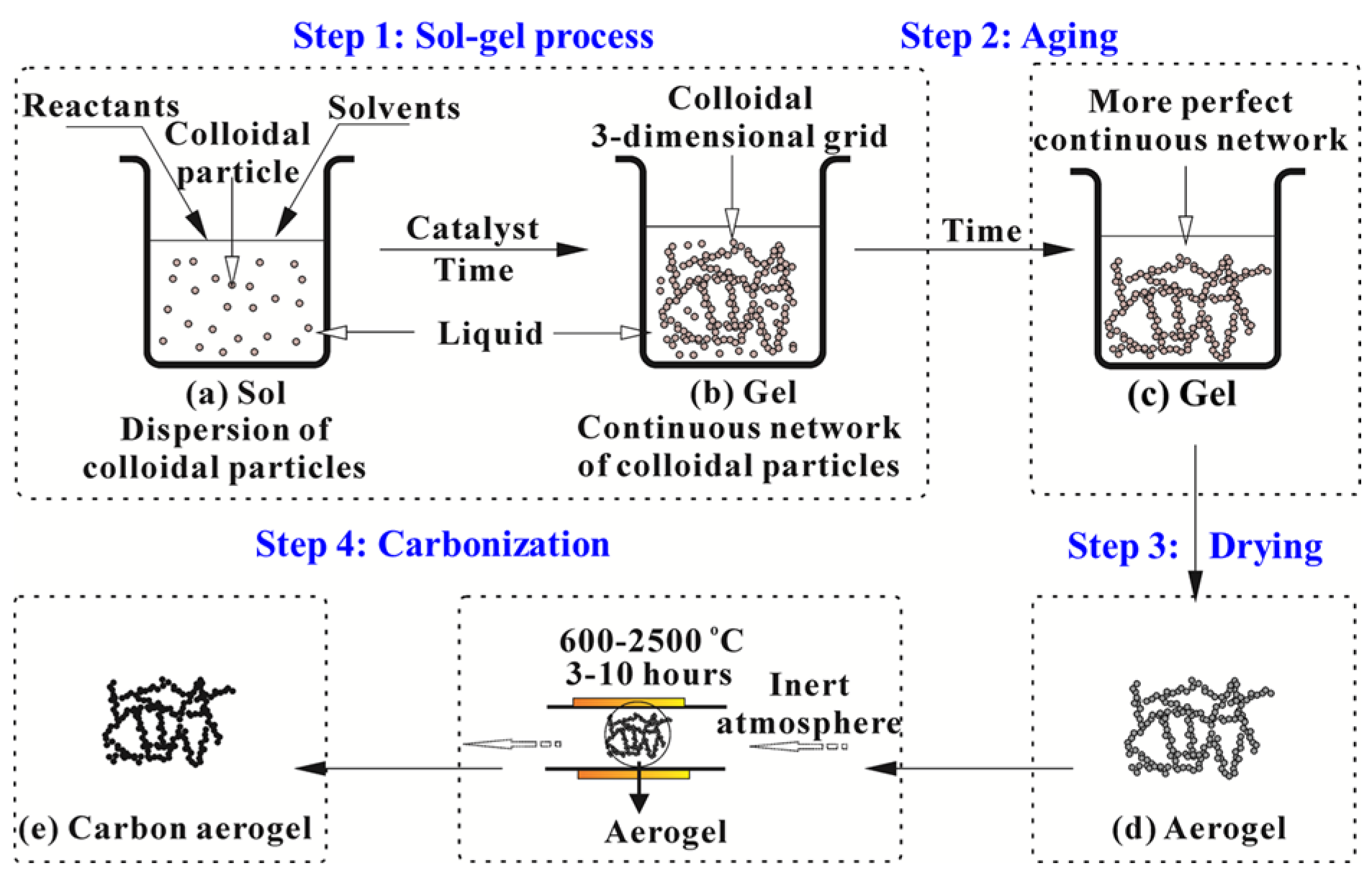
2. Preparation of Polymer/Carbon Aerogels
- (i)
- Sol-gel transition (gelation): Nanoscale sol particles are crosslinked and hierarchically assembled into a wet gel spontaneously or catalyzed by catalysts via hydrolysis or condensation reactions.
- (ii)
- Network perfection (aging): Mechanically reinforce the tenuous solid skeleton generated during the sol-gel process.
- (iii)
- Gel-aerogel transition (drying): The solvent inside the wet gel is replaced by air without serious microstructure damage.
2.1. The Sol-Gel Process
2.2. Aging
2.3. Gel-Aerogel Transition (Drying)
2.3.1. Supercritical Drying
2.3.2. Ambient Pressure Drying
2.3.3. Freeze-Drying
2.3.4. Other Drying Methods
2.4. Carbonization of Organic Aerogels
3. Structures and Properties of Polymer/Carbon-Based Aerogels
3.1. Polymer Aerogels
3.1.1. Cellulose-Based Aerogels
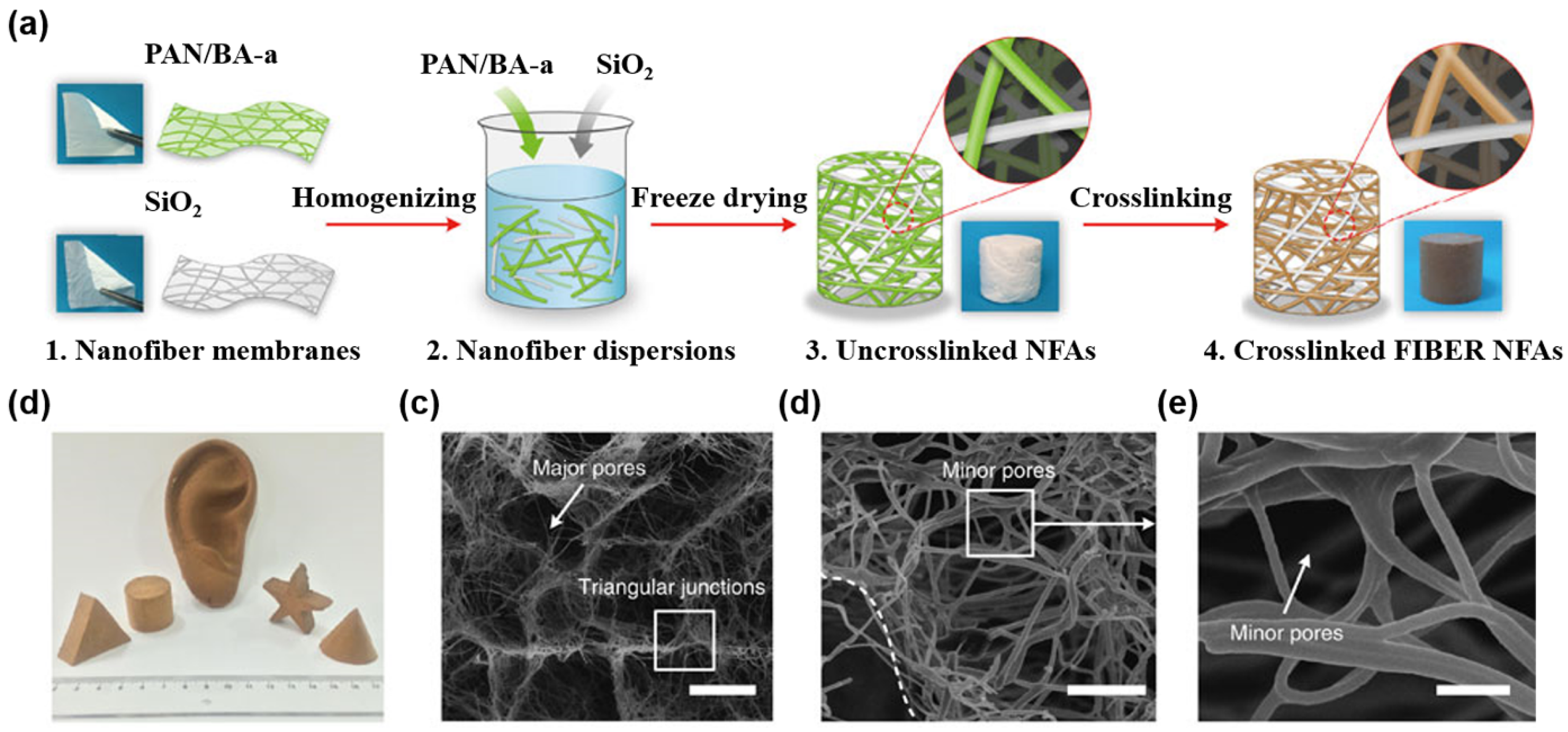
3.1.2. Polysaccharide-Based Aerogels
3.1.3. Resin-Based Aerogels
3.1.4. Polyimide-Based Aerogels
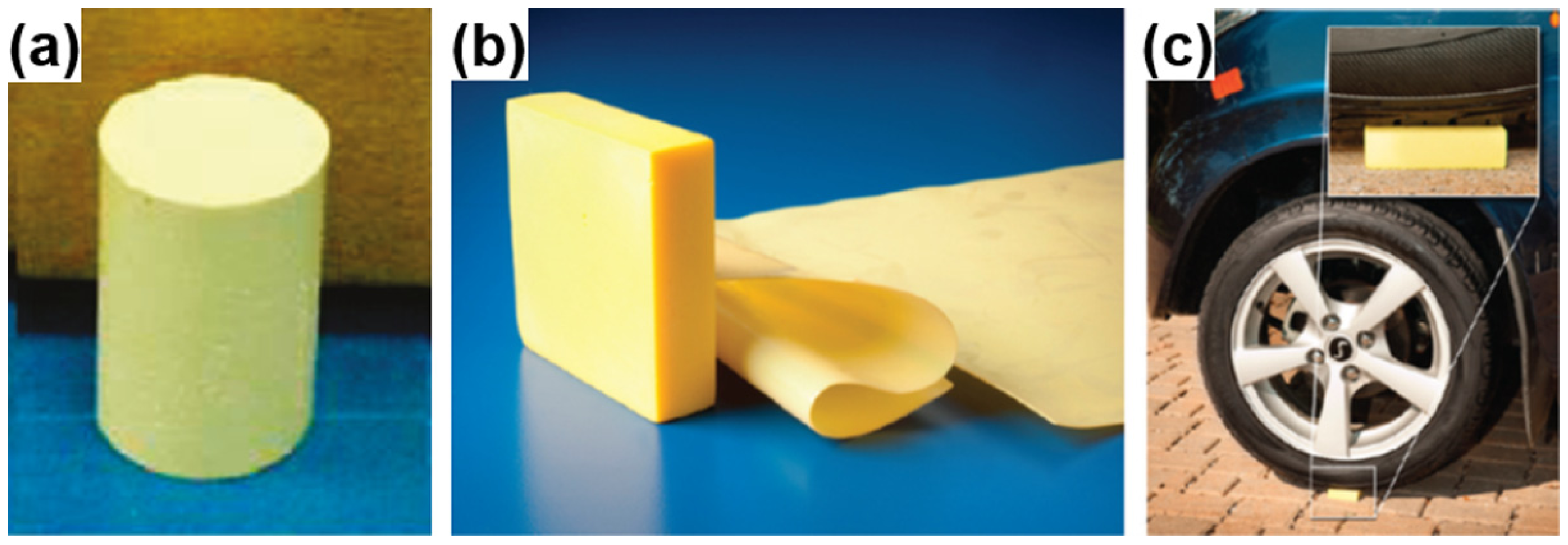

3.1.5. PVA-Based Aerogels
3.1.6. Polymer-Based Hybrid Aerogels
Clay-Polymer Hybrid Aerogels
CNT/CNF-Polymer Hybrid Aerogels
GO-Polymer Hybrid Aerogels
3.2. Carbon Aerogels
3.2.1. Carbon Aerogels from Carbonization
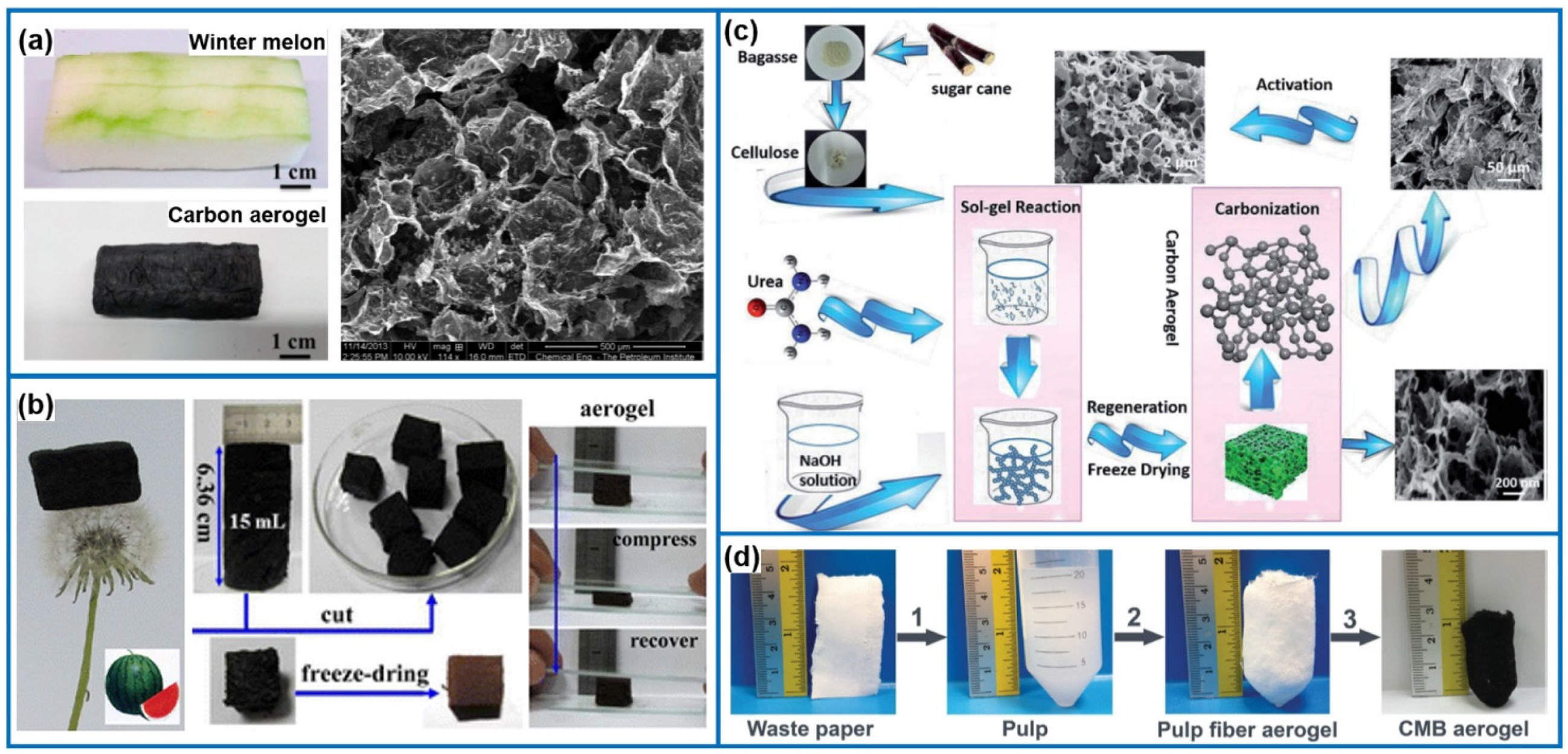
3.2.2. Graphene/CNT Based Carbon Aerogels
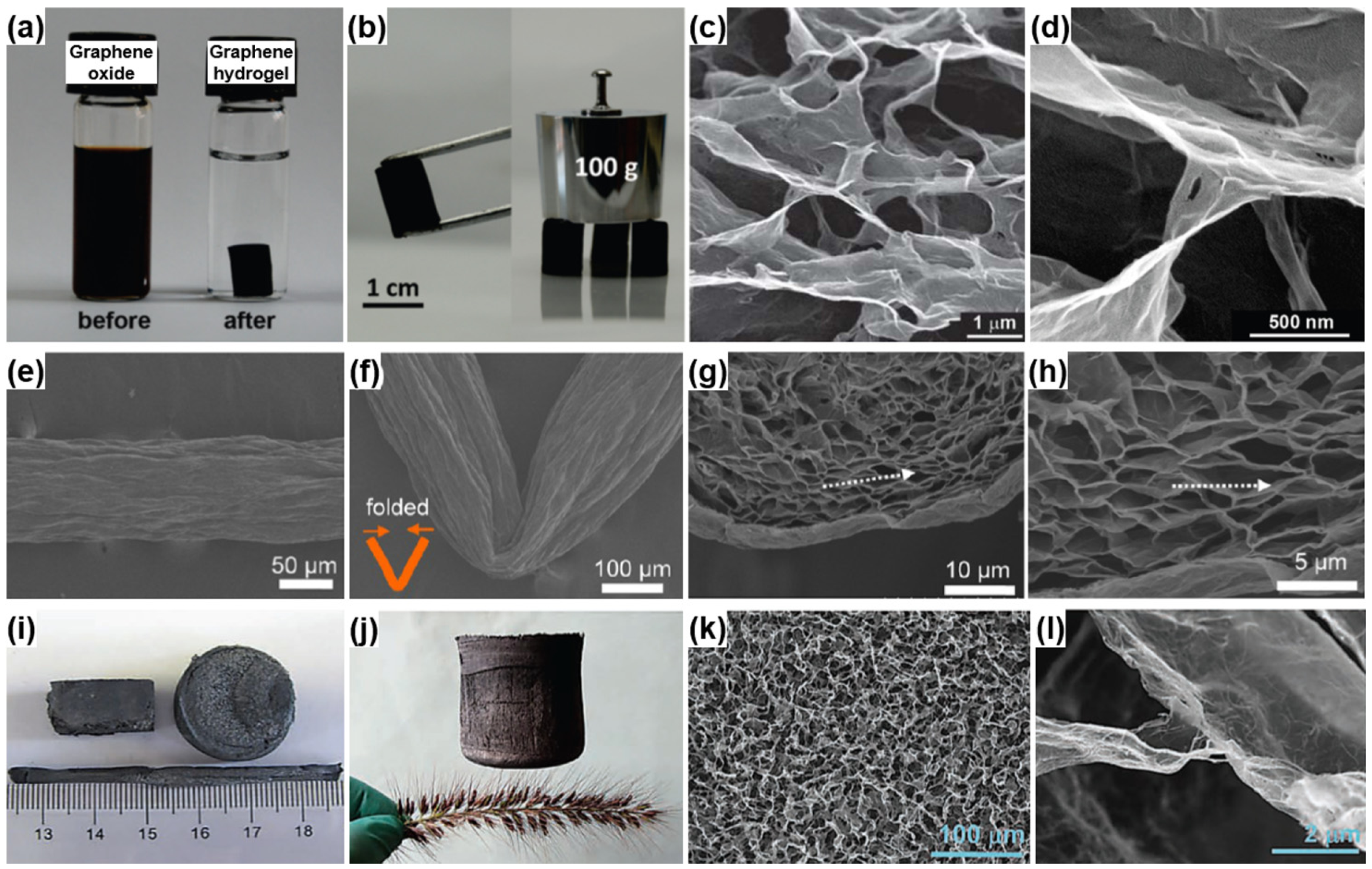
3.2.3. Carbon-Based Hybrid Aerogels
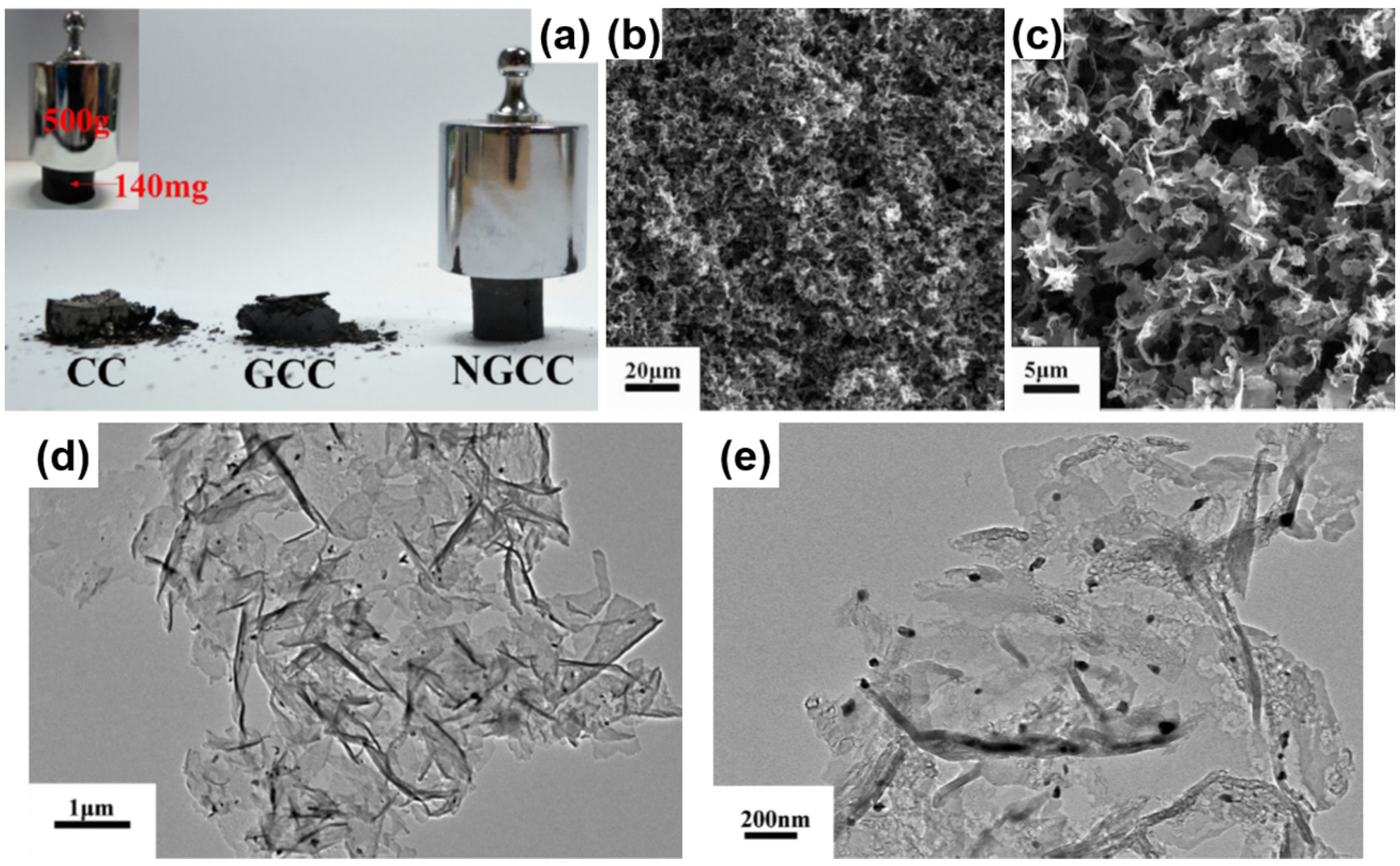
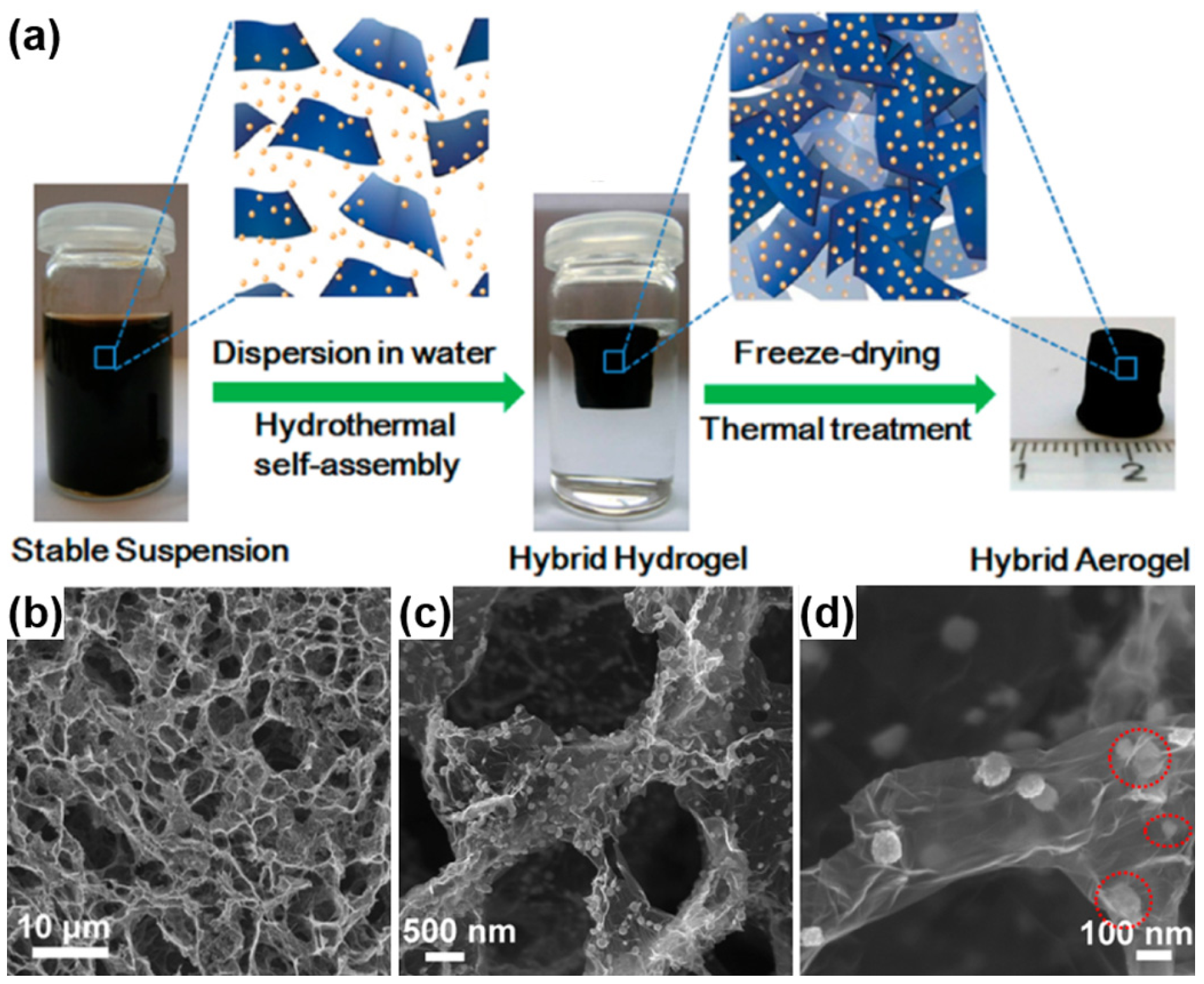
4. Applications of Polymer/Carbon-Based Aerogels
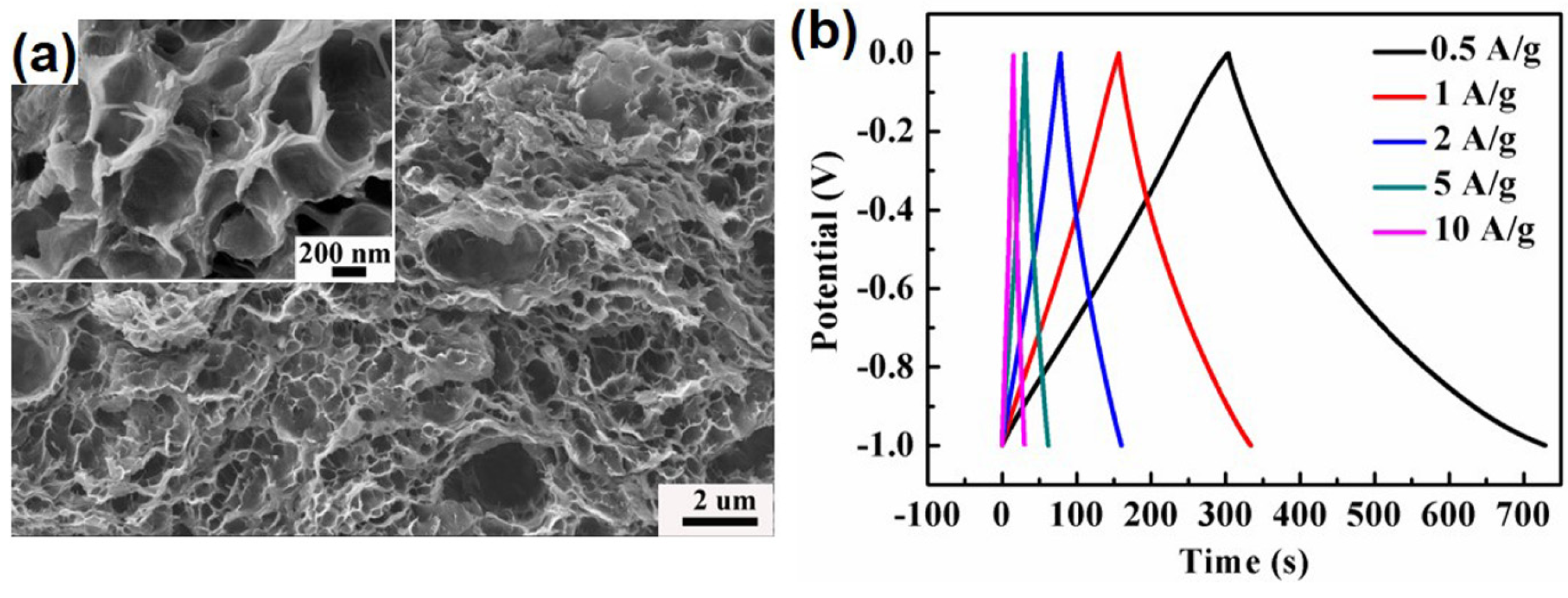
4.1. Energy Storage
4.2. Absorption
| Sorbent Materials | Absorbed Substances | Sorption Capacity (g·g−1) | Ref. |
|---|---|---|---|
| Graphene/PVDF aerogels | Oils and organic solvents | 20–70 | [32] |
| PVA/CNF aerogels | Oils and organic solvents | 44–96 | [33] |
| CNF aerogels | Water, oils and organic solvents | 139–375 | [111] |
| Ultra-flyweight aerogels | Oils and organic solvents | 215–913 | [155] |
| Winter melon carbon aerogels | Oils and organic solvents | 16–50 | [156] |
| Carbon microbelt aerogels | Oils and organic solvents | 50–188 | [160] |
| Ni-doped graphene/carbon cryogels | Oils and organic solvents | 22.2–23.2 | [179] |
| Twisted carbon fiber aerogels | Oils and organic solvents | 50–192 | [186] |
| Vegetable fiber | Crude oil | 1–100 | [188] |
| Nanowire membrane | Oils and some organic solvents | 4–20 | [189] |
| Wool-based nonwoven | Diesel, crude oil, SN 150 | 9–15 | [190] |
| Polymers | Oils and organic solvents | 5–25 | [191] |
| Exfoliated graphite | Heavy oil | 60–90 | [192] |
| Activated carbons | Benzene, toluene | <1 | [193] |
| Carbon nanotube sponges | Oils and organic solvents | 80–180 | [194] |
| Graphene/CNT foam | Compressor oil, organic solvents | 80–140 | [195] |
| CNT sponge doped with boron | Oils and organic solvents | 25–125 | [196] |
| Graphene-based sponges | Oils and organic solvents | 60–160 | [197] |
| Reduced graphite oxide foam | Cyclohexane, chlorobenzene, toluene, petroleum, motor oil | 5–40 | [198] |
| Nitrogen doped graphene foam | Oils and organic solvents | 200–600 | [199] |
| Carbonaceous nanofiber aerogels | Oils and organic solvents | 40–115 | [200] |
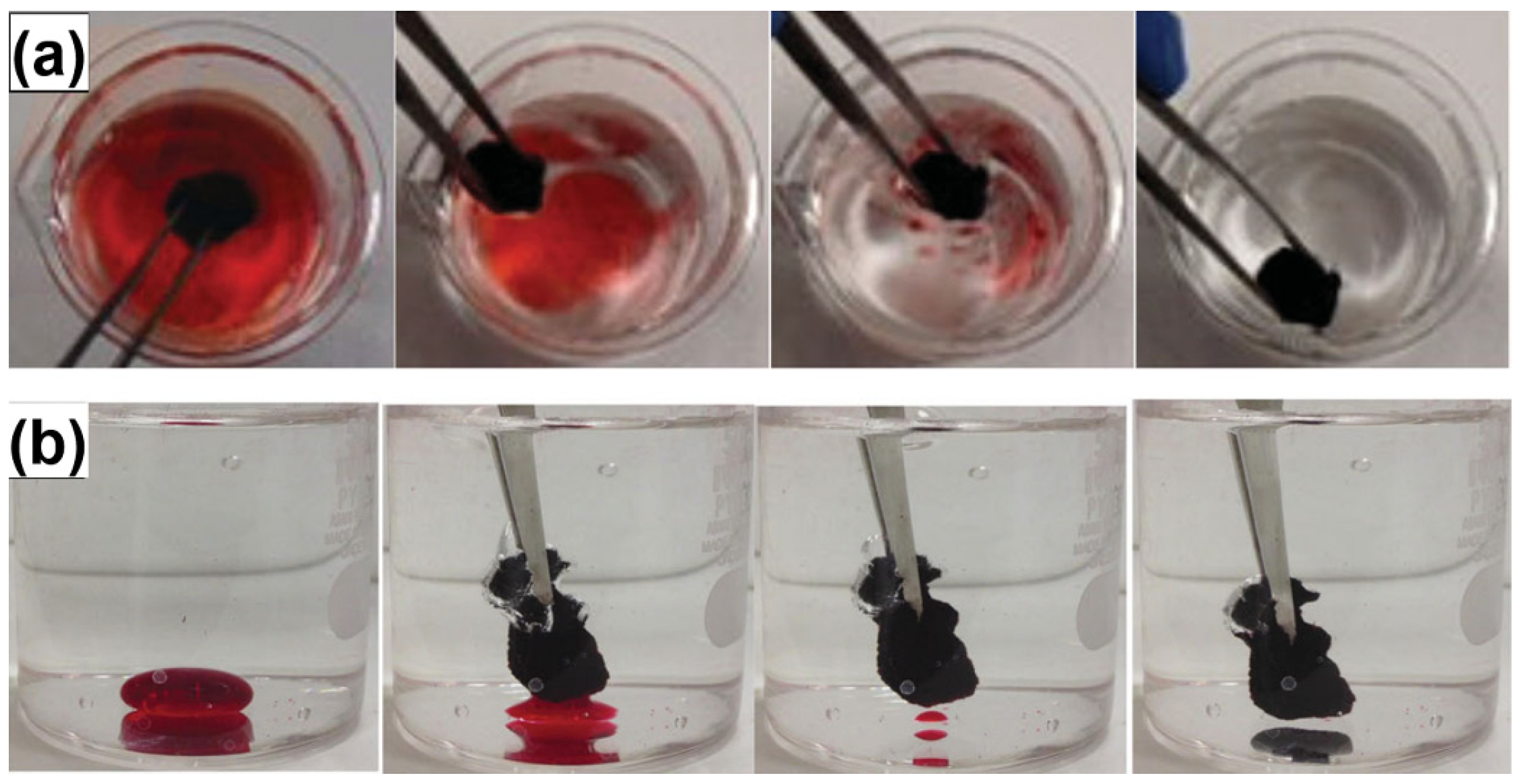
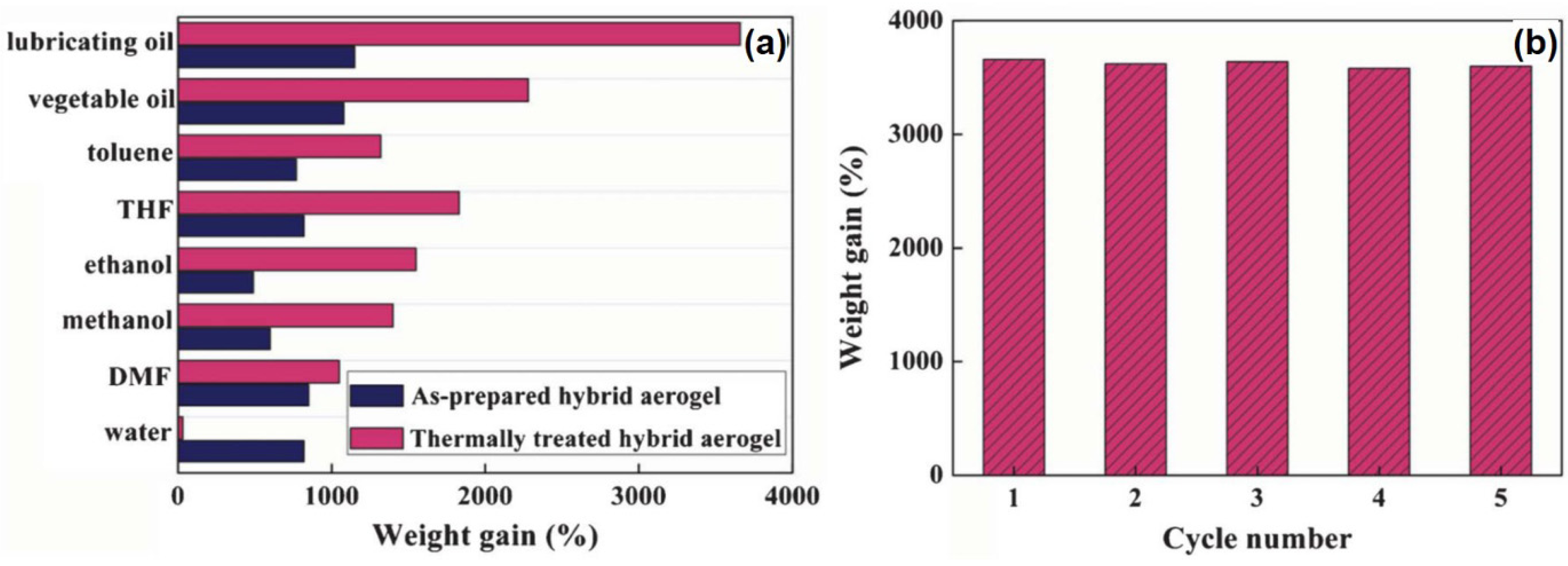
4.3. Adsorption
4.4. Thermal Insulations
4.5. Flame Retardant Materials
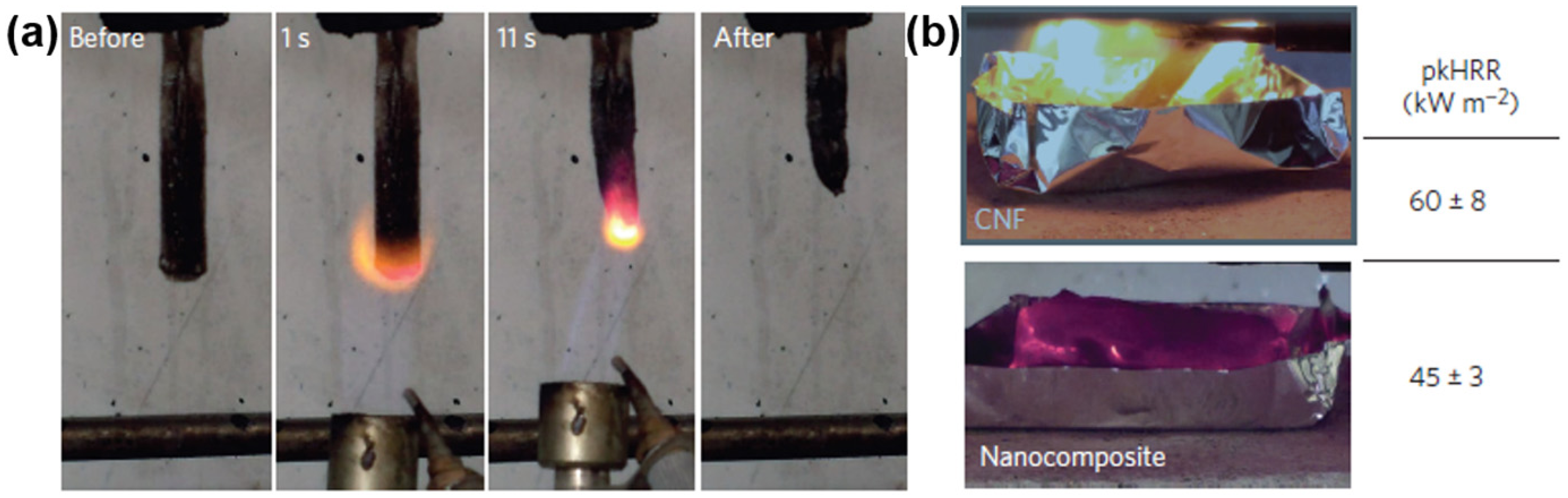
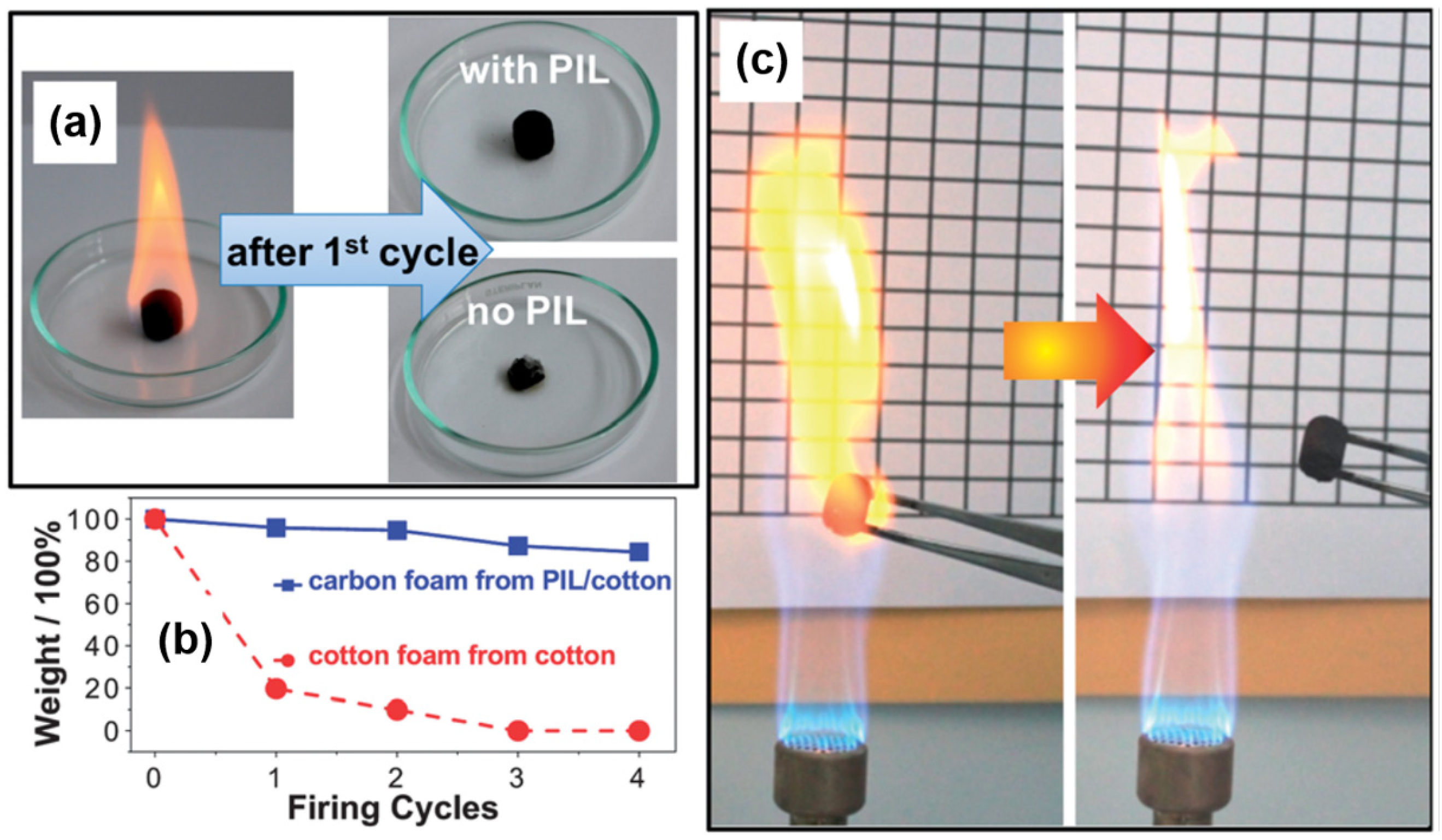
4.6. Catalyst Carriers
4.7. Hydrogen Storage
5. Conclusions and Outlook
5.1. Conclusions
5.2. Challenges and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Du, A.; Zhou, B.; Zhang, Z.H.; Shen, J. A special material or a new state of matter: A review and reconsideration of the aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef]
- Burger, T.; Fricke, J. Aerogels: Production, modification and applications. Phys. Chem. Chem. Phys. 1998, 102, 1523–1528. [Google Scholar] [CrossRef]
- Fricke, J.; Emmerling, A. Aerogels—Preparation, properties, applications. Struct. Bond. 1992, 77, 37–87. [Google Scholar]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4265. [Google Scholar] [CrossRef] [PubMed]
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Toward aerogel based thermal superinsulation in buildings: A comprehensive review. Renew. Sust. Energy Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- Bheekhun, N.; Abu Talib, A.; Hassan, M.R. Aerogels in aerospace: An overview. Adv. Mater. Sci. Eng. 2013, 466, 577–585. [Google Scholar] [CrossRef]
- Koebel, M.; Rigacci, A.; Achard, P. Aerogel-based thermal superinsulation: An overview. J. Sol-Gel Sci. Technol. 2012, 63, 315–339. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel insulation for building applications: A state-of-the-art review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef]
- Hrubesh, L.W. Aerogel applications. J. Non-Cryst. Solids 1998, 225, 335–342. [Google Scholar] [CrossRef]
- Fricke, J.; Emmerling, A. Aerogels—Recent progress in production techniques and novel applications. J. Sol-Gel Sci. Technol. 1998, 13, 299–303. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent expanded aerogels and jellies. Nature 1931, 127, 741–741. [Google Scholar] [CrossRef]
- Nicolaon, G.A.; Teichner, S.J. On a new process of preparation of silica xerogels and aerogels and their textural properties. Bull. Soc. Chim. Fr. 1968, 5, 1900–1906. [Google Scholar]
- Russo, R.E.; Hunt, A.J. Comparison of ethyl versus methyl sol gels for silica aerogels using polar nephelometry. J. Non-Cryst. Solids 1986, 86, 219–230. [Google Scholar] [CrossRef]
- Tewari, P.H.; Hunt, A.J.; Lofftus, K.D. Ambient-temperature supercritical drying of transparent silica aerogels. Mater. Lett. 1985, 3, 363–367. [Google Scholar] [CrossRef]
- Pekala, R.W. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Wright, S.; Sandberg, A.; Nguyen, B.N.; Van Keuls, F.W.; Mueller, C.H.; Rodriguez-Solis, R.; Miranda, F.A.; Aal, N.A. Dielectric and other properties of polyimide aerogels containing fluorinated blocks. ACS Appl. Mater. Interfaces 2014, 6, 6062–6068. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.B.; Hollinger, E.; Wang, Y.Z.; Schiraldi, D.A. Facile fabrication of poly(vinyl alcohol) gels and derivative aerogels. Polymer 2014, 55, 380–384. [Google Scholar] [CrossRef]
- Yamashita, J.; Ojima, T.; Shioya, M.; Hatori, H.; Yamada, Y. Organic and carbon aerogels derived from poly(vinyl chloride). Carbon 2003, 41, 285–294. [Google Scholar] [CrossRef]
- Banerjee, S.; Das, R.K.; Terech, P.; de Geyer, A.; Aymonier, C.; Loppinet-Serani, A.; Raffy, G.; Maitra, U.; del Guerzo, A.; Desvergne, J. Hybrid organogels and aerogels from co-assembly of structurally different low molecular weight gelators. J. Mater. Chem. C 2013, 1, 3305–3316. [Google Scholar] [CrossRef]
- Terech, P.; Aymonier, C.; Loppinet-Serani, A.; Bhat, S.; Banerjee, S.; Das, R.; Maitra, U.; del Guerzo, A.; Desvergne, J. Structural relationships in 2,3-bis-n-decyloxyanthracene and 12-hydroxystearic acid molecular gels and aerogels processed in supercritical CO2. J. Phys. Chem. 2010, 114, 11409–11419. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Haghgoo, M.; Yousefi, A.A.; Mehr, M.J.Z.; Celzard, A.; Fierro, V.; Leonard, A.; Job, N. Characterization of multi-walled carbon nanotube dispersion in resorcinol-formaldehyde aerogels. Microporous Mesoporous Mater. 2014, 184, 97–104. [Google Scholar] [CrossRef]
- Guo, K.; Song, H.H.; Chen, X.H.; Du, X.; Zhong, L. Graphene oxide as an anti-shrinkage additive for resorcinol-formaldehyde composite aerogels. Phys. Chem. Chem. Phys. 2014, 16, 11603–11608. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.M.; Sharshar, T.; Abd-Elwahed, A.R.; Tawfik, A. Study of transport properties and conduction mechanism of pure and composite resorcinol formaldehyde aerogel doped with Co-ferrite. Mater. Sci. Eng. B 2013, 178, 897–910. [Google Scholar] [CrossRef]
- Irin, F.; Das, S.; Atore, F.O.; Green, M.J. Ultralow percolation threshold in aerogel and cryogel templated composites. Langmuir 2013, 29, 11449–11456. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Bag, S.; Malliakas, C.D.; Kanatzidis, M.G. Selective surfaces: High-surface-area zinc tin sulfide chalcogels. Chem. Mater. 2011, 23, 2447–2456. [Google Scholar] [CrossRef]
- Shafaei-Fallah, M.; He, J.; Rothenberger, A.; Kanatzidis, M.G. Ion-exchangeable cobalt polysulfide chalcogel. J. Am. Chem. Soc. 2011, 133, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Brown, P.; Hope-Weeks, L.J. Gold modified cadmium sulfide aerogels. J. Sol-Gel Sci. Technol. 2011, 57, 68–75. [Google Scholar] [CrossRef]
- Bag, S.; Kanatzidis, M.G. Chalcogels: Porous metal-chalcogenide networks from main-group metal ions. Effect of surface polarizability on selectivity in gas separation. J. Am. Chem. Soc. 2010, 132, 14951–14959. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.T.; Liu, Y.; Brock, S.L. Tuning the optical band gap of quantum dot assemblies by varying network density. ACS Nano 2009, 3, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, C.B.; Li, J.; Xu, L.M.; Xiao, G.Y.; Yan, D.Y. A facile approach to superhydrophobic and superoleophilic graphene/polymer aerogels. J. Mater. Chem. A 2014, 2, 3057–3064. [Google Scholar] [CrossRef]
- Zheng, Q.F.; Cai, Z.Y.; Gong, S.Q. Green synthesis of polyvinyl alcohol (PVA)-cellulose nanofibril (CNF) hybrid aerogels and their use as superabsorbents. J. Mater. Chem. A 2014, 2, 3110–3118. [Google Scholar] [CrossRef]
- Donius, A.E.; Liu, A.; Berglund, L.A.; Wegst, U.G.K. Superior mechanical performance of highly porous, anisotropic nanocellulose-montmorillonite aerogels prepared by freeze casting. J. Mech. Behav. Biomed. Mater. 2014, 37, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Wicklein, B.; Kocjan, A.; Salazar-Alvarez, G.; Carosio, F.; Camino, G.; Antonietti, M.; Bergström, L. Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat. Nanotechnol. 2014, 10, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.B.; Wang, Y.Z.; Schiraldi, D.A. Preparation and flammability of poly(vinyl alcohol) composite aerogels. ACS Appl. Mater. Interfaces 2014, 6, 6790–6796. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.B.; Liu, B.; Huang, W.; Wang, J.S.; Zeng, G.; Wu, W.H.; Schiraldi, D.A. Fabrication and properties of irradiation-cross-linked poly(vinyl alcohol)/clay aerogel composites. ACS Appl. Mater. Interfaces 2014, 6, 16227–16236. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.B.; Chiou, B.S.; Wang, Y.Z.; Schiraldi, D.A. Biodegradable pectin/clay aerogels. ACS Appl. Mater. Interfaces 2013, 5, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.F.; Javadi, A.; Sabo, R.; Cai, Z.Y.; Gong, S.Q. Polyvinyl alcohol (PVA)-cellulose nanofibril (CNF)-multiwalled carbon nanotube (MWCNT) hybrid organic aerogels with superior mechanical properties. RSC Adv. 2013, 3, 20816–20823. [Google Scholar] [CrossRef]
- Wu, W.; Wang, K.; Zhan, M.S. Preparation and performance of polyimide-reinforced clay aerogel composites. Ind. Eng. Chem. Res. 2012, 51, 12821–12826. [Google Scholar] [CrossRef]
- Kabiri, S.; Tran, D.N.H.; Altalhi, T.; Losic, D. Outstanding adsorption performance of graphene-carbon nanotube aerogels for continuous oil removal. Carbon 2014, 80, 523–533. [Google Scholar] [CrossRef]
- Kolla, P.; Lai, C.L.; Mishra, S.; Fong, H.; Rhine, W.; Smirnova, A. CVD grown CNTs within iron modified and graphitized carbon aerogel as durable oxygen reduction catalysts in acidic medium. Carbon 2014, 79, 518–528. [Google Scholar] [CrossRef]
- Aliev, A.E.; Mayo, N.K.; Baughman, R.H.; Avirovik, D.; Priya, S.; Zarnetske, M.R.; Blottman, J.B. Thermal management of thermoacoustic sound projectors using a free-standing carbon nanotube aerogel sheet as a heat source. Nanotechnology 2014, 25, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; He, X.D.; Shang, Y.Y.; Peng, Q.Y.; Qin, Y.Y.; Shi, E.Z.; Yang, Y.B.; Wu, S.T.; Xu, W.J.; Du, S.Y.; et al. Multifunctional graphene sheet-nanoribbon hybrid aerogels. J. Mater. Chem. A 2014, 2, 14994–15000. [Google Scholar] [CrossRef]
- Kang, T.J.; Kim, T.; Jang, E.Y.; Im, H.; Lepro-Chavez, X.; Ovalle-Robles, R.; Oh, J.; Kozlov, M.E.; Baughman, R.H.; Lee, H.H.; et al. Nanotube aerogel sheet flutter for actuation, power generation, and infrasound detection. Sci. Rep. 2014, 4, 6105–6109. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Tng, D.Z.Y.; Lim, C.X.T.; Liu, P.; Nguyen, S.T.; Xiao, P.F.; Marconnet, A.; Lim, C.Y.H.; Duong, H.M. Thermal and electrical properties of graphene/carbon nanotube aerogels. Colloids Surf. A 2014, 445, 48–53. [Google Scholar] [CrossRef]
- Guo, K.; Hu, Z.J.; Song, H.H.; Du, X.; Zhong, L.; Chen, X.H. Low-density graphene/carbon composite aerogels prepared at ambient pressure with high mechanical strength and low thermal conductivity. RSC Adv. 2015, 5, 5197–5204. [Google Scholar] [CrossRef]
- Liang, J.F.; Cai, Z.; Li, L.D.; Guo, L.; Geng, J.X. Scalable and facile preparation of graphene aerogel for air purification. RSC Adv. 2014, 4, 4843–4847. [Google Scholar] [CrossRef]
- Chen, L.; Feng, M.; Zhan, H.B. Fundamental electrochemistry of three-dimensional graphene aerogels. RSC Adv. 2014, 4, 30689–30696. [Google Scholar] [CrossRef]
- Xu, Y.X.; Sheng, K.X.; Li, C.; Shi, G.Q. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ellsworth, M.W. Graphene aerogels. ECS Trans. 2009, 5, 241–247. [Google Scholar]
- Chen, K.; Bao, Z.H.; Liu, D.; Zhu, X.R.; Zhang, Z.H.; Zhou, B. Confined synthesis and properties of porous silicon from silica aerogel templates by magnesiothermic reduction. Acta Phys. Chim. Sin. 2011, 27, 2719–2725. [Google Scholar]
- Leventis, N.; Sadekar, A.; Chandrasekaran, N.; Sotiriou-Leventis, C. Click synthesis of monolithic silicon carbide aerogels from polyacrylonitrile-coated 3D silica networks. Chem. Mater. 2010, 22, 2790–2803. [Google Scholar] [CrossRef]
- Worsley, M.A.; Kuntz, J.D.; Cervantes, O.; Han, T.; Gash, A.E.; Satcher, J.H.; Baumann, T.F. Route to high surface area TiO2/C and TiCN/C composites. J. Mater. Chem. 2009, 19, 7146–7150. [Google Scholar] [CrossRef]
- Worsley, M.A.; Kuntz, J.D.; Pauzauskie, P.J.; Cervantes, O.; Zaug, J.M.; Gash, A.E.; Satcher, J.H.; Baumann, T.F. High surface area carbon nanotube-supported titanium carbonitride aerogels. J. Mater. Chem. 2009, 19, 5503–5506. [Google Scholar] [CrossRef]
- Davis, M.; Zhang, K.; Wang, S.R.; Hope-Weeks, L.J. Enhanced electrical conductivity in mesoporous 3D indium-tin oxide materials. J. Mater. Chem. 2012, 22, 20163–20165. [Google Scholar] [CrossRef]
- Chervin, C.N.; Clapsaddle, B.J.; Chiu, H.W.; Gash, A.E., Jr.; Satcher, J.H.; Kauzlarich, S.M. Role of cyclic ether and solvent in a non-alkoxide sol-gel synthesis of yttria-stabilized zirconia nanoparticles. Chem. Mater. 2006, 18, 4865–4874. [Google Scholar] [CrossRef]
- Chervin, C.N.; Clapsaddle, B.J.; Chiu, H.W.; Gash, A.E.; Satcher, J.H.; Kauzlarich, S.M. Aerogel synthesis of yttria-stabilized zirconia by a non-alkoxide sol-gel route. Chem. Mater. 2005, 17, 3345–3351. [Google Scholar] [CrossRef]
- Colvin, J.D.; Fournier, K.B.; May, M.J.; Scott, H.A. A computational study of X-ray emission from laser-irradiated Ge-doped foams. Phys. Plasmas 2010, 17, 131–144. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, C.C.; Cheng, W.Y.; Lu, S.Y. Dispersing WO3 in carbon aerogel makes an outstanding supercapacitor electrode material. Carbon 2014, 69, 287–293. [Google Scholar] [CrossRef]
- Yin, L.W.; Zhang, Z.W.; Li, Z.Q.; Hao, F.B.; Li, Q.; Wang, C.X.; Fan, R.H.; Qi, Y.X. Spinel ZnMn2O4 nanocrystal-anchored 3D hierarchical carbon aerogel hybrids as anode materials for lithium ion batteries. Adv. Funct. Mater. 2014, 24, 4176–4185. [Google Scholar] [CrossRef]
- Wu, Z.S.; Yang, S.B.; Sun, Y.; Parvez, K.; Feng, X.L.; Muellen, K. 3D nitrogen-doped graphene aerogel-supported Fe3O4 nanoparticles as efficient eletrocatalysts for the oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085. [Google Scholar] [CrossRef] [PubMed]
- Rhine, W.; Wang, J.; Begag, R. Polyimide Aerogels, Carbon Aerogels, and Metal Carbide Aerogels and Methods of Making Same. US Pantent 20040132845, 8 July 2004. [Google Scholar]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Leventis, N.; Koebel, M.M. Aerogels Handbook, 1st ed.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Einarsrud, M.A.; Nilsen, E.; Rigacci, A.; Pajonk, G.M.; Buathier, S.; Valette, D.; Durant, M.; Chevalier, B.; Nitz, P.; Ehrburger-Dolle, F. Strengthening of silica gels and aerogels by washing and aging processes. J. Non-Cryst. Solids 2001, 285, 1–7. [Google Scholar] [CrossRef]
- Carrott, P.J.M.; Marques, L.M.; Carrott, M. Core-shell polymer aerogels prepared by co-polymerisation of 2,4-dihydroxybenzoic acid, resorcinol and formaldehyde. Microporous Mesoporous Mater. 2012, 158, 170–174. [Google Scholar] [CrossRef]
- Wu, D.C.; Fu, R.W.; Sun, Z.Q.; Yu, Z.Q. Low-density organic and carbon aerogels from the sol-gel polymerization of phenol with formaldehyde. J. Non-Cryst. Solids 2005, 351, 915–921. [Google Scholar] [CrossRef]
- Qin, G.T.; Wei, W.; Guo, S.C. Semi-continuous drying of RF gels with supercritical acetone. Carbon 2003, 41, 851–853. [Google Scholar] [CrossRef]
- Li, W.C.; Lu, A.H.; Guo, S.C. Characterization of the microstructures of organic and carbon aerogels based upon mixed cresol-formaldehyde. Carbon 2001, 39, 1989–1994. [Google Scholar] [CrossRef]
- Liang, C.H.; Sha, G.Y.; Guo, S.C. Resorcinol-formaldehyde aerogels prepared by supercritical acetone drying. J. Non-Cryst. Solids 2000, 271, 167–170. [Google Scholar] [CrossRef]
- Tamon, H.; Ishizaka, H.; Araki, T.; Okazaki, M. Control of mesoporous structure of organic and carbon aerogels. Carbon 1998, 36, 1257–1262. [Google Scholar] [CrossRef]
- Bock, V.; Emmerling, A.; Fricke, J. Influence of monomer and catalyst concentration on RF and carbon aerogel structure. J. Non-Cryst. Solids 1998, 225, 69–73. [Google Scholar] [CrossRef]
- Bock, V.; Emmerling, A.; Saliger, R.; Fricke, J. Structural investigation of resorcinol formaldehyde and carbon aerogels using SAXS and BET. J. Porous Mater. 1997, 4, 287–294. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Meng, Q.H. The preparation and characterization graphene-cross-linked phenol-formaldehyde hybrid carbon xerogels. J. Sol-Gel Sci. Technol. 2013, 67, 304–311. [Google Scholar] [CrossRef]
- Reuss, M.; Ratke, L. RF-aerogels catalysed by ammonium carbonate. J. Sol-Gel Sci. Technol. 2010, 53, 85–92. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Hu, H.Q.; Li, W.C.; Zhang, X.Y. Cresol-formaldehyde based carbon aerogel as electrode material for electrochemical capacitor. J. Power Sources 2006, 162, 738–742. [Google Scholar] [CrossRef]
- Bruck, S.; Ratke, L. RF-aerogels: A new binding material for foundry application. J. Sol-Gel Sci. Technol. 2003, 26, 663–666. [Google Scholar] [CrossRef]
- Brandt, R.; Petricevic, R.; Probstle, H.; Fricke, J. Acetic acid catalyzed carbon aerogels. J. Porous Mater. 2003, 10, 171–178. [Google Scholar] [CrossRef]
- Tonanon, N.; Wareenin, Y.; Siyasukh, A.; Tanthapanichakoon, W.; Nishihara, H.; Mukai, S.R.; Tamon, H. Preparation of resorcinol formaldehyde (RF) carbon gels: Use of ultrasonic irradiation followed by microwave drying. J. Non-Cryst. Solids 2006, 352, 5683–5686. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.K.; Yan, L.L.; Liu, J.X.; Wang, F.C. Compressive behaviors and morphological changes of resorcinol-formaldehyde aerogel at high strain rates. Microporous Mesoporous Mater. 2010, 133, 134–140. [Google Scholar] [CrossRef]
- Schwan, M.; Ratke, L. Flexibilisation of resorcinol-formaldehyde aerogels. J. Mater. Chem. A 2013, 1, 13462–13468. [Google Scholar] [CrossRef]
- Kawagishi, K.; Saito, H.; Furukawa, H. Superior nanoporous polyimides via supercritical CO2 drying of jungle-gym-type polyimide gels. Macromol. Papid Commun. 2007, 28, 96–100. [Google Scholar] [CrossRef]
- Matson, D.W.; Smith, R.D. Supercritical fluid technologies for ceramic-processing applications. J. Am. Ceram. Soc. 1989, 72, 871–881. [Google Scholar] [CrossRef]
- Shen, D.X.; Liu, F.G.; Yang, H.X.; Yang, S.Y. Intrinsically highly hydrophobic semi-alicyclic fluorinated polyimide aerogel with ultra low dielectric constants. Chem. Lett. 2013, 42, 1230–1232. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Saito, T.; Isogai, A. Aerogels with 3D ordered nanofiber skeletons of liquid-crystalline nanocellulose derivatives as tough and transparent insulators. Angew. Chem. Int. Ed. 2014, 53, 10394–10397. [Google Scholar] [CrossRef] [PubMed]
- Rudaz, C.; Courson, R.; Bonnet, L.; Calas-Etienne, S.; Sallee, H.; Budtova, T. Aeropectin: Fully biomass-based mechanically strong and thermal superinsulating aerogel. Biomacromolecules 2014, 15, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Pajonk, G.M.; Repellinlacroix, M.; Abouarnadasse, S.; Chaouki, J.; Klvana, D. From sol-gel to aerogels and cryogels. J. Non-Cryst. Solids 1990, 121, 66–67. [Google Scholar] [CrossRef]
- Kalinin, S.V.; Kheifets, L.I.; Mamchik, A.I.; Knot’Ko, A.G.; Vertegel, A.A. Influence of the drying technique on the structure of silica gels. J. Sol-Gel Sci. Technol. 1999, 15, 31–35. [Google Scholar] [CrossRef]
- Jin, H.; Nishiyama, Y.; Wada, M.; Kuga, S. Nanofibrillar cellulose aerogels. Colloids Surf. A 2004, 240, 63–67. [Google Scholar] [CrossRef]
- Li, G.Y.; Wang, Z.G. Microporous polyimides with uniform pores for adsorption and separation of CO2 gas and organic vapors. Macromolecules 2013, 46, 3058–3066. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, G.; Dong, Q.; Qian, B.Q.; Zhang, M.D.; Li, C.P.; Qiu, J.S. An ionic liquid template approach to graphene-carbon xerogel composites for supercapacitors with enhanced performance. J. Mater. Chem. A 2014, 2, 14329–14333. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A.; Ritter, J.A. Preparation and properties of resorcinol-formaldehyde organic and carbon gels. Adv. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Maldonado-Hodar, F.J. Carbon aerogels for catalysis applications: An overview. Carbon 2005, 43, 455–465. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Wang, J.; Shen, J.; Deng, Z.S.; Lai, Z.Q.; Zhou, B.; Attia, S.M.; Chen, L.Y. The investigation of the adsorption character of carbon aerogels. Nanostruct. Mater. 1999, 11, 375–381. [Google Scholar] [CrossRef]
- Pekala, R.W.; Farmer, J.C.; Alviso, C.T.; Tran, T.D.; Mayer, S.T.; Miller, J.M.; Dunn, B. Carbon aerogels for electrochemical applications. J. Non-Cryst. Solids 1998, 225, 74–80. [Google Scholar] [CrossRef]
- Lin, C.; Ritter, J.A. Carbonization and activation of sol-gel derived carbon xerogels. Carbon 2000, 38, 849–861. [Google Scholar] [CrossRef]
- Lin, C.; Ritter, J.A. Effect of synthesis pH on the structure of carbon xerogels. Carbon 1997, 35, 1271–1278. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Malow, E.J.; Silva, R.; Wright, S.; Quade, D.; Vivod, S.L.; Guo, H.Q.; Guo, J.; Cakmak, M. Mechanically strong, flexible polyimide aerogels cross-linked with aromatic triamine. ACS Appl. Mater. Interfaces 2012, 4, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Kistler, S.S. Coherent expanded aerogels. J. Phys. Chem. 1932, 36, 52–64. [Google Scholar] [CrossRef]
- Cai, J.; Kimura, S.; Wada, M.; Kuga, S.; Zhang, L. Cellulose aerogels from aqueous alkali hydroxide-urea solution. ChemSusChem 2008, 1, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Zhang, X.X.; Lu, C.H.; Lan, L.D.; Yuan, G.P. Polyaniline-decorated cellulose aerogel nanocomposite with strong interfacial adhesion and enhanced photocatalytic activity. RSC Adv. 2014, 4, 8966–8972. [Google Scholar] [CrossRef]
- Deng, M.L.; Zhou, Q.; Du, A.K.; van Kasteren, J.; Wang, Y.Z. Preparation of nanoporous cellulose foams from cellulose-ionic liquid solutions. Mater. Lett. 2009, 63, 1851–1854. [Google Scholar] [CrossRef]
- Hoepfner, S.; Ratke, L.; Milow, B. Synthesis and characterisation of nanofibrillar cellulose aerogels. Cellulose 2008, 15, 121–129. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Yang, D.; Sun, Q.; Liu, Y.; Zhao, H. Lignocellulose aerogel from wood-ionic liquid solution (1-allyl-3-methylimidazolium chloride) under freezing and thawing conditions. Biomacromolecules 2011, 12, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Sescousse, R.; Gavillon, R.; Budtova, T. Aerocellulose from cellulose-ionic liquid solutions: Preparation, properties and comparison with cellulose-NaOH and cellulose-NMMO routes. Carbohydr. Polym. 2011, 83, 1766–1774. [Google Scholar] [CrossRef]
- Gavillon, R.; Budtova, T. Aerocellulose: New highly porous cellulose prepared from cellulose-NaOH aqueous solutions. Biomacromolecules 2008, 9, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.L.; Sharma, S.; Liu, W.Y.; Mu, W.; Liu, W.; Zhang, X.D.; Deng, Y.L. Aerogel microspheres from natural cellulose nanofibrils and their application as cell culture scaffold. Biomacromolecules 2014, 15, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.T.; Feng, J.D.; Ng, S.K.; Wong, J.P.W.; Tan, V.B.C.; Duong, H.M. Advanced thermal insulation and absorption properties of recycled cellulose aerogels. Colloids Surf. A 2014, 445, 128–134. [Google Scholar] [CrossRef]
- Kow, K.W.; Yusoff, R.; Aziz, A.R.A.; Abdullah, E.C. From bamboo leaf to aerogel: Preparation of water glass as a precursor. J. Non-Cryst. Solids 2014, 386, 76–84. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.L. Amphiphilic superabsorbent cellulose nanofibril aerogels. J. Mater. Chem. A 2014, 2, 6337–6342. [Google Scholar] [CrossRef]
- Lin, J.Y.; Yu, L.B.; Tian, F.; Zhao, N.; Li, X.H.; Bian, F.G.; Wang, J. Cellulose nanofibrils aerogels generated from jute fibers. Carbohydr. Polym. 2014, 109, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Yu, J.Y.; Tang, X.M.; Ge, J.L.; Ding, B. Ultralight nanofibre-assembled cellular aerogels with superelasticity and multifunctionality. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Quignard, F.; Valentin, R.; di Renzo, F. Aerogel materials from marine polysaccharides. New J. Chem. 2008, 32, 1300–1310. [Google Scholar] [CrossRef]
- Wierik, G.; Bergsma, J.; ArendsScholte, A.W.; Boersma, T.; Eissens, A.C.; Lerk, C.F. A new generation of starch products as excipient in pharmaceutical tablets. 1. Preparation and binding properties of high surface area potato starch products. Int. J. Pharm. 1996, 134, 27–36. [Google Scholar]
- Glenn, G.M.; Irving, D.W. Sstarch-based microcellular foams. Cereal Chem. 1995, 72, 155–161. [Google Scholar]
- Budarin, V.; Clark, J.H.; Deswarte, F.; Hardy, J.; Hunt, A.J.; Kerton, F.M. Delicious not siliceous: Expanded carbohydrates as renewable separation media for column chromatography. Chem. Commun. 2005, 23, 2903–2905. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Budarin, V.L.; Clark, J.H. Tuneable mesoporous materials from alpha-d-polysaccharides. ChemSusChem 2008, 1, 408–411. [Google Scholar] [CrossRef] [PubMed]
- El Kadib, A.; Bousmina, M. Chitosan bio-based organic-inorganic hybrid aerogel microspheres. Chem. Eur. J. 2012, 18, 8264–8277. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Bernardi, L.; Gioia, C.; Vierucci, S.; Robitzer, M.; Quignard, F. Chitosan aerogel: A recyclable, heterogeneous organocatalyst for the asymmetric direct aldol reaction in water. Chem. Commun. 2010, 46, 2903–2905. [Google Scholar] [CrossRef] [PubMed]
- Lamarque, G.; Viton, C.; Domard, A. Comparative study of the first heterogeneous deacetylation of alpha- and beta-chitins in a multistep process. Biomacromolecules 2004, 5, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Bao, Z.H.; Du, A.; Zhu, X.R.; Shen, J.; Wu, G.M.; Zhang, Z.H.; Zhou, B. One-pot synthesis, characterization and properties of acid-catalyzed resorcinol/formaldehyde cross-linked silica aerogels and their conversion to hierarchical porous carbon monoliths. J. Sol-Gel Sci. Technol. 2012, 62, 294–303. [Google Scholar] [CrossRef]
- Tian, H.Y.; Buckley, C.E.; Paskevicius, M.; Wang, S.B. Carbon aerogels from acetic acid catalysed resorcinol-furfural using supercritical drying for hydrogen storage. J. Supercrit. Fluids 2011, 55, 1115–1117. [Google Scholar] [CrossRef]
- Tian, H.Y.; Buckley, C.E.; Paskeuicius, M.; Sheppard, D.A. Acetic acid catalysed carbon xerogels derived from resorcinol-furfural for hydrogen storage. Int. J. Hydrog. Energy 2011, 36, 671–679. [Google Scholar] [CrossRef]
- Mayes, R.T.; Tsouris, C.; Kiggans, J.O.; Mahurin, S.M.; DePaoli, D.W.; Dai, S. Hierarchical ordered mesoporous carbon from phloroglucinol-glyoxal and its application in capacitive deionization of brackish water. J. Mater. Chem. 2010, 20, 8674–8678. [Google Scholar] [CrossRef]
- Conceicao, F.L.; Carrott, P.J.M.; Carrott, M. New carbon materials with high porosity in the 1–7 nm range obtained by chemical activation with phosphoric acid of resorcinol-formaldehyde aerogels. Carbon 2009, 47, 1874–1877. [Google Scholar] [CrossRef]
- Reuss, M.; Ratke, L. Subcritically dried RF-aerogels catalysed by hydrochloric acid. J. Sol-Gel Sci. Technol. 2008, 47, 74–80. [Google Scholar] [CrossRef]
- Mulik, S.; Sotiriou-Leventis, C.; Leventis, N. Time-efficient acid-catalyzed synthesis of resorcinol-formaldehyde aerogels. Chem. Mater. 2007, 19, 6138–6144. [Google Scholar] [CrossRef]
- Fairen-Jimenez, D.; Carrasco-Marin, F.; Moreno-Castilla, C. Porosity and surface area of monolithic carbon aerogels prepared using alkaline carbonates and organic acids as polymerization catalysts. Carbon 2006, 44, 2301–2307. [Google Scholar] [CrossRef]
- Wu, D.C.; Fu, R.W. Fabrication and physical properties of organic and carbon aerogel derived from phenol and furfural. J. Porous Mat. 2005, 12, 311–316. [Google Scholar] [CrossRef]
- Barbieri, O.; Ehrburger-Dolle, F.; Rieker, T.P.; Pajonk, G.M.; Pinto, N.; Rao, A.V. Small-angle X-ray scattering of a new series of organic aerogels. J. Non-Cryst. Solids 2001, 285, 109–115. [Google Scholar] [CrossRef]
- Wu, D.C.; Fu, R.M. Synthesis of organic and carbon aerogels from phenol-furfural by two-step polymerization. Microporous Mesoporous Mater. 2006, 96, 115–120. [Google Scholar] [CrossRef]
- Lorjai, P.; Wongkasemjit, S.; Chaisuwan, T.; Jamieson, A.M. Significant enhancement of thermal stability in the non-oxidative thermal degradation of bisphenol-A/aniline based polybenzoxazine aerogel. Polym. Degrad. Stabil. 2011, 96, 708–718. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Agag, T.; Ishida, H.; Qutubuddin, S. Biobased chitosan/polybenzoxazine cross-linked films: Preparation in aqueous media and synergistic improvements in thermal and mechanical properties. Biomacromolecules 2013, 14, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Chaisuwan, T.; Komalwanich, T.; Luangsukrerk, S.; Wongkasemjit, S. Removal of heavy metals from model wastewater by using polybenzoxazine aerogel. Desalination 2010, 256, 108–114. [Google Scholar] [CrossRef]
- Thubsuang, U.; Ishida, H.; Wongkasemjit, S.; Chaisuwan, T. Self-formation of 3D interconnected macroporous carbon xerogels derived from polybenzoxazine by selective solvent during the sol-gel process. J. Mater. Sci. 2014, 49, 4946–4961. [Google Scholar] [CrossRef]
- Lorjai, P.; Chaisuwan, T.; Wongkasemjit, S. Porous structure of polybenzoxazine-based organic aerogel prepared by sol-gel process and their carbon aerogels. J. Sol-Gel Sci. Technol. 2009, 52, 56–64. [Google Scholar] [CrossRef]
- Mahadik-Khanolkar, S.; Donthula, S.; Sotiriou-Leventis, C.; Leventis, N. Polybenzoxazine aerogels. 1. High-yield room-temperature acid-catalyzed synthesis of robust monoliths, oxidative aromatization, and conversion to microporous carbons. Chem. Mater. 2014, 26, 1303–1317. [Google Scholar] [CrossRef]
- Gu, S.; Li, Z.; Miyoshi, T.; Jana, S.C. Polybenzoxazine aerogels with controllable pore structures. RSC Adv. 2015, 5, 26801–26805. [Google Scholar] [CrossRef]
- Kim, S.Y.; Noh, Y.J.; Lim, J.; You, N. Silica aerogel/polyimide composites with preserved aerogel pores using multi-step curing. Macromol. Res. 2014, 22, 108–111. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Wright, S.; Sandberg, A.; Nguyen, B.N.; van Keuls, F.W.; Mueller, C.H.; Rodriguez-Solis, R.; Miranda, F.A. Low dielectric polyimide aerogels as substrates for lightweight patch antennas. ACS Appl. Mater. Interfaces 2012, 4, 6346–6353. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.Q.; Meador, M.A.B.; McCorkle, L.; Quade, D.J.; Guo, J.A.; Hamilton, B.; Cakmak, M.; Sprowl, G. Polyimide aerogels cross-linked through amine functionalized polyoligomeric silsesquioxane. ACS Appl. Mater. Interfaces 2011, 3, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Chidambareswarapattar, C.; Larimore, Z.; Sotiriou-Leventis, C.; Mang, J.T.; Leventis, N. One-step room-temperature synthesis of fibrous polyimide aerogels from anhydrides and isocyanates and conversion to isomorphic carbons. J. Mater. Chem. 2010, 20, 9666–9678. [Google Scholar] [CrossRef]
- Simpson, J.O.; St Clair, A.K. Fundamental insight on developing low dielectric constant polyimides. Thin Solid Films 1997, 308, 480–485. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Mohite, D.P.; Larimore, Z.J.; Mang, J.T.; Churu, G.; Lu, H.B. Polyimide aerogels by ring-opening metathesis polymerization (ROMP). Chem. Mater. 2011, 23, 2250–2261. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Bai, H.; Li, C.; Wang, X.L.; Shi, G.Q. A pH-sensitive graphene oxide composite hydrogel. Chem. Commun. 2010, 46, 2376–2378. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, H.S. Oscillatory shear induced gelation of graphene-poly(vinyl alcohol) composite hydrogels and rheological premonitor of ultra-light aerogels. Polymer 2014, 55, 287–294. [Google Scholar] [CrossRef]
- Wang, P.P.; Zhao, J.; Xuan, R.F.; Wang, Y.; Zou, C.; Zhang, Z.Q.; Wan, Y.Z.; Xu, Y. Flexible and monolithic zinc oxide bionanocomposite foams by a bacterial cellulose mediated approach for antibacterial applications. Dalton Trans. 2014, 43, 6762–6768. [Google Scholar] [CrossRef] [PubMed]
- Biener, J.; Stadermann, M.; Suss, M.; Worsley, M.A.; Biener, M.M.; Rose, K.A.; Baumann, T.F. Advanced carbon aerogels for energy applications. Energy Environ. Sci. 2011, 4, 656–667. [Google Scholar] [CrossRef]
- White, R.J.; Brun, N.; Budarin, V.L.; Clark, J.H.; Titirici, M. Always look on the ”light” side of life: Sustainable carbon aerogels. ChemSusChem 2014, 7, 670–689. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Xu, Z.; Gao, C. Multifunctional, ultra-flyweight, synergistically assembled carbon aerogels. Adv. Mater. 2013, 25, 2554–2560. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. Carbon aerogel from winter melon for highly efficient and recyclable oils and organic solvents absorption. ACS Sustain. Chem. Eng. 2014, 2, 1492–1497. [Google Scholar] [CrossRef]
- Ren, Y.M.; Zhang, J.M.; Xu, Q.; Chen, Z.M.; Yang, D.Y.; Wang, B.; Jiang, Z. Biomass-derived three-dimensional porous N-doped carbonaceous aerogel for efficient supercapacitor electrodes. RSC Adv. 2014, 4, 23412–23419. [Google Scholar] [CrossRef]
- Wu, X.L.; Wen, T.; Guo, H.L.; Yang, S.B.; Wang, X.K.; Xu, A.W. Biomass-derived sponge-like carbonaceous hydrogels and aerogels for supercapacitors. ACS Nano 2013, 7, 3589–3597. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Zhao, Z.H.; Tian, J.; Li, H.D.; Sang, Y.H.; Yu, G.W.; Cai, H.Q.; Liu, H.; Wong, C.P.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.C.; Huang, X.; Wu, X.; Cao, X.H.; Tan, C.L.; Yin, Z.Y.; Lu, X.H.; Sun, L.T.; Zhang, H. Carbon microbelt aerogel prepared by waste paper: An efficient and recyclable sorbent for oils and organic solvents. Small 2014, 10, 3544–3550. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Li, C.; Liang, H.W.; Chen, J.F.; Yu, S.H. Ultralight, flexible, and fire-resistant carbon nanofiber aerogels from bacterial cellulose. Angew. Chem. Int. Ed. 2013, 52, 2925–2929. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Huang, Z.H.; Liang, H.W.; Yao, W.T.; Yu, Z.Y.; Yu, S.H. Flexible all-solid-state high-power supercapacitor fabricated with nitrogen-doped carbon nanofiber electrode material derived from bacterial cellulose. Energy Environ. Sci. 2013, 6, 3331–3338. [Google Scholar] [CrossRef]
- Chen, L.F.; Huang, Z.H.; Liang, H.W.; Gao, H.L.; Yu, S.H. Three-dimensional heteroatom-doped carbon nanofiber networks derived from bacterial cellulose for supercapacitors. Adv. Funct. Mater. 2014, 24, 5104–5111. [Google Scholar] [CrossRef]
- Liang, H.W.; Wu, Z.Y.; Chen, L.F.; Li, C.; Yu, S.H. Bacterial cellulose derived nitrogen-doped carbon nanofiber aerogel: An efficient metal-free oxygen reduction electrocatalyst for zinc-air battery. Nano Energy 2015, 11, 366–376. [Google Scholar] [CrossRef]
- Long, C.L.; Qi, D.P.; Wei, T.; Yan, J.; Jiang, L.L.; Fan, Z.J. Nitrogen-doped carbon networks for high energy density supercapacitors derived from polyaniline coated bacterial cellulose. Adv. Funct. Mater. 2014, 24, 3953–3961. [Google Scholar] [CrossRef]
- Chen, L.F.; Huang, Z.H.; Liang, H.W.; Guan, Q.F.; Yu, S.H. Bacterial-cellulose-derived carbon nanofiber@MnO2 and nitrogen-doped carbon nanofiber electrode materials: An asymmetric supercapacitor with high energy and power density. Adv. Mater. 2013, 25, 4746–4752. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Xu, M.W.; Bao, S.J.; Yang, F.; Chai, H. Design and synthesis of carbonized polypyrrole-coated graphene aerogel acting as an efficient metal-free catalyst for oxygen reduction. RSC Adv. 2014, 4, 16979–16984. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wu, P.Y. A facile one-pot route towards three-dimensional graphene-based microporous N-doped carbon composites. RSC Adv. 2014, 4, 45619–45624. [Google Scholar] [CrossRef]
- Pham, V.H.; Nguyen-Phan, T.D.; Jang, J.; Vu, T.; Lee, Y.J.; Song, I.K.; Shin, E.W.; Chung, J.S. Nitrogen-doped mesoporous reduced graphene oxide for high-performance supercapacitors. RSC Adv. 2014, 4, 22455–22462. [Google Scholar] [CrossRef]
- Aliev, A.E.; Oh, J.Y.; Kozlov, M.E.; Kuznetsov, A.A.; Fang, S.L.; Fonseca, A.F.; Ovalle, R.; Lima, M.D.; Haque, M.H.; Gartstein, Y.N.; et al. Giant-stroke, superelastic carbon nanotube aerogel muscles. Science 2009, 323, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.H.; Liu, J.H.; Karakoti, A.S.; Kumar, A.; Joung, D.; Li, Q.A.; Khondaker, S.I.; Seal, S.; Zhai, L. Ultralight multiwalled carbon nanotube aerogel. ACS Nano 2010, 4, 7293–7302. [Google Scholar] [CrossRef] [PubMed]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H.; Baumann, T.F. Synthesis of graphene aerogel with high electrical conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.P.; Ren, X.C.; Wang, P.; Yu, S.H. Macroscopic multifunctional graphene-based hydrogels and aerogels by a metal ion induced self-assembly process. ACS Nano 2012, 6, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Wu, Q.; Sun, Y.Q.; Bai, H.; Shi, G.Q. Three-dimensional self-assembly of graphene oxide and DNA into multifunctional hydrogels. ACS Nano 2010, 4, 7358–7362. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Bai, H.; Li, C.; Shi, G.Q. A graphene oxide/hemoglobin composite hydrogel for enzymatic catalysis in organic solvents. Chem. Commun. 2011, 47, 4962–4964. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Li, C.; Wang, X.L.; Shi, G.Q. On the gelation of graphene oxide. J. Phys. Chem. C 2011, 115, 5545–5551. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Li, P.G.; Gao, C. Strong, conductive, lightweight, neat graphene aerogel fibers with aligned pores. ACS Nano 2012, 6, 7103–7113. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Abraham, D.; Dobos, G.; Madarasz, J.; Onyestyak, G.; Safran, G.; Geissler, E.; Laszlo, K. Molybdenum doped carbon aerogels with catalytic potential. Carbon 2014, 66, 210–218. [Google Scholar] [CrossRef]
- Gao, W.; Miao, Y.E.; Zhang, C.; Yang, Z.; Liu, Z.Y.; Tjiu, W.W.; Liu, T.X. Ni-doped graphene/carbon cryogels and their applications as versatile sorbents for water purification. ACS Appl. Mater. Interfaces 2013, 5, 7584–7591. [Google Scholar]
- Sillars, F.B.; Fletcher, S.I.; Mirzaeian, M.; Hall, P.J. Effect of activated carbon xerogel pore size on the capacitance performance of ionic liquid electrolytes. Energy Environ. Sci. 2011, 4, 695–706. [Google Scholar] [CrossRef]
- Ren, Y.M.; Xu, Q.; Zhang, J.M.; Yang, H.X.; Wang, B.; Yang, D.Y.; Hu, J.H.; Liu, Z.M. Functionalization of biomass carbonaceous aerogels: Selective preparation of MnO2@CA composites for supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 9689–9697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, D.; Qian, X.Z.; Zhao, C.J.; Xu, Y.L. A novel nano structured LiFePO4/C composite as cathode for Li-ion batteries. J. Power Sources 2014, 249, 431–434. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, G.Q. Preparation of highly conductive graphene hydrogels for fabricating supercapacitors with high rate capability. J. Phys. Chem. C 2011, 115, 17206–17212. [Google Scholar] [CrossRef]
- Worsley, M.A.; Olson, T.Y.; Lee, J.R.I.; Willey, T.M.; Nielsen, M.H.; Roberts, S.K.; Pauzauskie, P.J.; Biener, J., Jr.; Satcher, J.H.; Baumann, T.F. High surface area, sp2-cross-linked three-dimensional graphene monoliths. J. Phys. Chem. Lett. 2011, 2, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Fan, W.; Huang, Y.P.; Zhang, C.; Liu, T.X. Graphene/carbon aerogels derived from graphene crosslinked polyimide as electrode materials for supercapacitors. RSC Adv. 2015, 5, 1301–1308. [Google Scholar] [CrossRef]
- Bi, H.C.; Yin, Z.Y.; Cao, X.H.; Xie, X.; Tan, C.L.; Huang, X.; Chen, B.; Chen, F.T.; Yang, Q.L.; Bu, X.Y.; et al. Carbon fiber aerogel made from raw cotton: A novel, efficient and recyclable sorbent for oils and organic solvents. Adv. Mater. 2013, 25, 5916–5921. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.K.; Tian, H.Y.; Wang, S.B.; Rufford, T.; Zhu, Z.H.; Buckley, C.E. KOH catalysed preparation of activated carbon aerogels for dye adsorption. J. Colloid Interface Sci. 2011, 357, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Annunciado, T.R.; Sydenstricker, T.; Amico, S.C. Experimental investigation of various vegetable fibers as sorbent materials for oil spills. Mar. Pollut. Bull. 2005, 50, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.K.; Liu, X.G.; Akbulut, O.; Hu, J.Q.; Suib, S.L.; Kong, J.; Stellacci, F. Superwetting nanowire membranes for selective absorption. Nat. Nanotechnol. 2008, 3, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Radetic, M.M.; Jocic, D.M.; Jovancic, P.M.; Petrovic, Z.L.; Thomas, H.F. Recycled wool-based nonwoven material as an oil sorbent. Environ. Sci. Technol. 2003, 37, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Sun, H.X.; Tan, D.Z.; Fan, W.J.; Wen, S.H.; Qing, X.J.; Li, G.X.; Li, S.Y.; Deng, W.Q. Superhydrophobic conjugated microporous polymers for separation and adsorption. Energy Environ. Sci. 2011, 4, 2062–2065. [Google Scholar] [CrossRef]
- Toyoda, M.; Inagaki, M. Heavy oil sorption using exfoliated graphite—New application of exfoliated graphite to protect heavy oil pollution. Carbon 2000, 38, 199–210. [Google Scholar] [CrossRef]
- Lillo-Rodenas, M.A.; Cazorla-Amoros, D.; Linares-Solano, A.; Beguin, F.; Clinard, C.; Rouzaud, J.N. HRTEM study of activated carbons prepared by alkali hydroxide activation of anthracite. Carbon 2004, 42, 1305–1310. [Google Scholar] [CrossRef]
- Gui, X.C.; Wei, J.Q.; Wang, K.L.; Cao, A.Y.; Zhu, H.W.; Jia, Y.; Shu, Q.K.; Wu, D.H. Carbon nanotube sponges. Adv. Mater. 2010, 22, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.C.; Chen, J.; Ma, Y.W.; Wang, J.; Chan-Park, M.B.; Liu, X.M.; Wang, L.H.; Huang, W.; Chen, P. Superhydrophobic and superoleophilic hybrid foam of graphene and carbon nanotube for selective removal of oils or organic solvents from the surface of water. Chem. Commun. 2012, 48, 10660–10662. [Google Scholar] [CrossRef] [PubMed]
- Hashim, D.P.; Narayanan, N.T.; Romo-Herrera, J.M.; Cullen, D.A.; Hahm, M.G.; Lezzi, P.; Suttle, J.R.; Kelkhoff, D.; Munoz-Sandoval, E.; Ganguli, S.; et al. Covalently bonded three-dimensional carbon nanotube solids via boron induced nanojunctions. Sci. Rep. 2012, 2, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Duc Dung, N.; Tai, N.H.; Lee, S.B.; Kuo, W.S. Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ. Sci. 2012, 5, 7908–7912. [Google Scholar]
- Niu, Z.Q.; Chen, J.; Hng, H.H.; Ma, J.; Chen, X.D. A leavening strategy to prepare reduced graphene oxide foams. Adv. Mater. 2012, 24, 4144–4150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, C.G.; Hu, Y.; Cheng, H.H.; Shi, G.Q.; Qu, L.T. A versatile, ultralight, nitrogen-doped graphene framework. Angew. Chem. Int. Ed. 2012, 51, 11371–11375. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.W.; Guan, Q.F.; Chen, L.F.; Zhu, Z.; Zhang, W.J.; Yu, S.H. Macroscopic-scale template synthesis of robust carbonaceous nanofiber hydrogels and aerogels and their applications. Angew. Chem. Int. Ed. 2012, 51, 5101–5105. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tong, Z.; Ngai, T.; Wang, C.Y. Nitrogen-rich and fire-resistant carbon aerogels for the removal of oil contaminants from water. ACS Appl. Mater. Interfaces 2014, 6, 6351–6360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tjiu, W.W.; Liu, T.X. One-pot hydrothermal synthesis and reusable oil-adsorbing properties of porous carbonaceous monoliths using multi-walled carbon nanotubes as templates. RSC Adv. 2013, 3, 14938–14941. [Google Scholar] [CrossRef]
- Meena, A.K.; Mishra, G.K.; Rai, P.K.; Rajagopal, C.; Nagar, P.N. Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J. Hazard. Mater. 2005, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.Y.; Cui, Y.; Zhu, J.H.; Han, B.H. Preparation of three-dimensional graphene oxide-polyethylenimine porous materials as dye and gas adsorbents. ACS Appl. Mater. Interfaces 2013, 5, 9172–9179. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Jiang, G.; Fan, M.; Shen, X.; Cui, S.; Russell, A.G. A new aerogel based CO2 adsorbent developed using a simple sol-gel method along with supercritical drying. Chem. Commun. 2014, 50, 12158–12161. [Google Scholar] [CrossRef] [PubMed]
- Masika, E.; Mokaya, R. High surface area metal salt templated carbon aerogels via a simple subcritical drying route: Preparation and CO2 uptake properties. RSC Adv. 2013, 3, 17677–17681. [Google Scholar] [CrossRef]
- Robertson, C.; Mokaya, R. Microporous activated carbon aerogels via a simple subcritical drying route for CO2 capture and hydrogen storage. Microporous Mesoporous Mat. 2013, 179, 151–156. [Google Scholar] [CrossRef]
- Sui, Z.; Meng, Q.; Li, J.; Zhu, J.; Cui, Y.; Han, B. High surface area porous carbons produced by steam activation of graphene aerogels. J. Mater. Chem. A 2014, 2, 9891–9898. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Agag, T.; Ishida, H.; Qutubuddin, S. Biobased chitosan hybrid aerogels with superior adsorption: Role of graphene oxide in CO2 capture. RSC Adv. 2013, 3, 16011–16020. [Google Scholar] [CrossRef]
- Jelle, B.P. Traditional, state-of-the-art and future thermal building insulation materials and solutions—Properties, requirements and possibilities. Energy Build. 2011, 43, 2549–2563. [Google Scholar] [CrossRef]
- Fernandez, J.E. Materials for aesthetic, energy-efficient, and self-diagnostic buildings. Science 2007, 315, 1807–1810. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Li, Q.; Wang, Y.C.; Yi, X.; Zeng, J.; Yu, H.P.; Liu, Y.X.; Li, J. Comparative study of aerogels obtained from differently prepared nanocellulose fibers. ChemSusChem 2014, 7, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Bendahou, D.; Bendahou, A.; Seantier, B.; Grohens, Y.; Kaddami, H. Nano-fibrillated cellulose-zeolites based new hybrid composites aerogels with super thermal insulating properties. Ind. Crop. Prod. 2015, 65, 374–382. [Google Scholar] [CrossRef]
- Leventis, N.; Chidambareswarapattar, C.; Mohite, D.P.; Larimore, Z.J.; Lu, H.B.; Sotiriou-Leventis, C. Multifunctional porous aramids (aerogels) by efficient reaction of carboxylic acids and isocyanates. J. Mater. Chem. 2011, 21, 11981–11986. [Google Scholar] [CrossRef]
- Chen, H.B.; Wang, Y.Z.; Sanchez-Soto, M.; Schiraldi, D.A. Low flammability, foam-like materials based on ammonium alginate and sodium montmorillonite clay. Polymer 2012, 53, 5825–5831. [Google Scholar] [CrossRef]
- Wang, Y.T.; Liao, S.F.; Shang, K.; Chen, M.J.; Huang, J.Q.; Wang, Y.Z.; Schiraldi, D.A. Efficient approach to improving the flame retardancy of poly(vinyl alcohol)/clay aerogels: Incorporating piperazine-modified ammonium polyphosphate. ACS Appl. Mater. Interfaces 2015, 7, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.J.; Siebenburger, M.; Qiu, X.L.; Antonietti, M.; Yuan, J.Y. Low fractions of ionic liquid or poly(ionic liquid) can activate polysaccharide biomass into shaped, flexible and fire-retardant porous carbons. J. Mater. Chem. A 2013, 1, 11887–11893. [Google Scholar] [CrossRef]
- Lin, Y.F.; Chang, C.Y. Magnetic mesoporous iron oxide/carbon aerogel photocatalysts with adsorption ability for organic dye removal. RSC Adv. 2014, 4, 28628–28631. [Google Scholar] [CrossRef]
- Thomas, K.M. Hydrogen adsorption and storage on porous materials. Catal. Today 2007, 120, 389–398. [Google Scholar] [CrossRef]
- Gosalawit-Utke, R.; Niesen, T.K.; Pranzas, K.; Saldan, I.; Pistidda, C.; Karimi, F.; Laipple, D.; Skibsted, J.; Jensen, T.R.; Klassen, T.; et al. 2LiBH4-MgH2 in a resorcinol-furfural carbon aerogel scaffold for reversible hydrogen storage. J. Phys. Chem. C 2012, 116, 1526–1534. [Google Scholar] [CrossRef]
- Gosalawit-Utke, R.; Milanese, C.; Javadian, P.; Girella, A.; Laipple, D.; Puszkiel, J.; Cattaneo, A.S.; Ferrara, C.; Wittayakhun, J.; Skibsted, J.; et al. 2LiBH4–MgH2–0.13TiCl4 confined in nanoporous structure of carbon aerogel scaffold for reversible hydrogen storage. J. Alloy. Compd. 2014, 599, 78–86. [Google Scholar] [CrossRef]
- Gosalawit-Utke, R.; Meethom, S.; Pistidda, C.; Milanese, C.; Laipple, D.; Saisopa, T.; Marini, A.; Klassen, T.; Dornheim, M. Destabilization of LiBH4 by nanoconfinement in PMMA-co-BM polymer matrix for reversible hydrogen storage. Int. J. Hydrog. Energy 2014, 39, 5019–5029. [Google Scholar] [CrossRef]
- Javadian, P.; Jensen, T.R. Enhanced hydrogen reversibility of nanoconfined LiBH4-Mg(BH4)2. Int. J. Hydrog. Energy 2014, 39, 9871–9876. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Javadian, P.; Polanski, M.; Besenbacher, F.; Bystrzycki, J.; Skibsted, J.; Jensen, T.R. Nanoconfined NaAlH4: Prolific effects from increased surface area and pore volume. Nanoscale 2014, 6, 599–607. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC‐BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, L.; Zhang, Y.; Zhang, L.; Miao, Y.-E.; Fan, W.; Liu, T. Polymer/Carbon-Based Hybrid Aerogels: Preparation, Properties and Applications. Materials 2015, 8, 6806-6848. https://doi.org/10.3390/ma8105343
Zuo L, Zhang Y, Zhang L, Miao Y-E, Fan W, Liu T. Polymer/Carbon-Based Hybrid Aerogels: Preparation, Properties and Applications. Materials. 2015; 8(10):6806-6848. https://doi.org/10.3390/ma8105343
Chicago/Turabian StyleZuo, Lizeng, Youfang Zhang, Longsheng Zhang, Yue-E Miao, Wei Fan, and Tianxi Liu. 2015. "Polymer/Carbon-Based Hybrid Aerogels: Preparation, Properties and Applications" Materials 8, no. 10: 6806-6848. https://doi.org/10.3390/ma8105343