Green Perylene Bisimide Dyes: Synthesis, Photophysical and Electrochemical Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. General
2.2. Synthesis
2.2.1. Perylene Bisimide (4)
2.2.2. Synthesis of 1-Nitroperylene Bisimide (3)
2.2.3. Synthesis of 1-Aminoperylene Bisimide (2)
2.2.4. General Procedure for Alkylation (1a–1c)
3. Results and Discussion
3.1. Synthesis
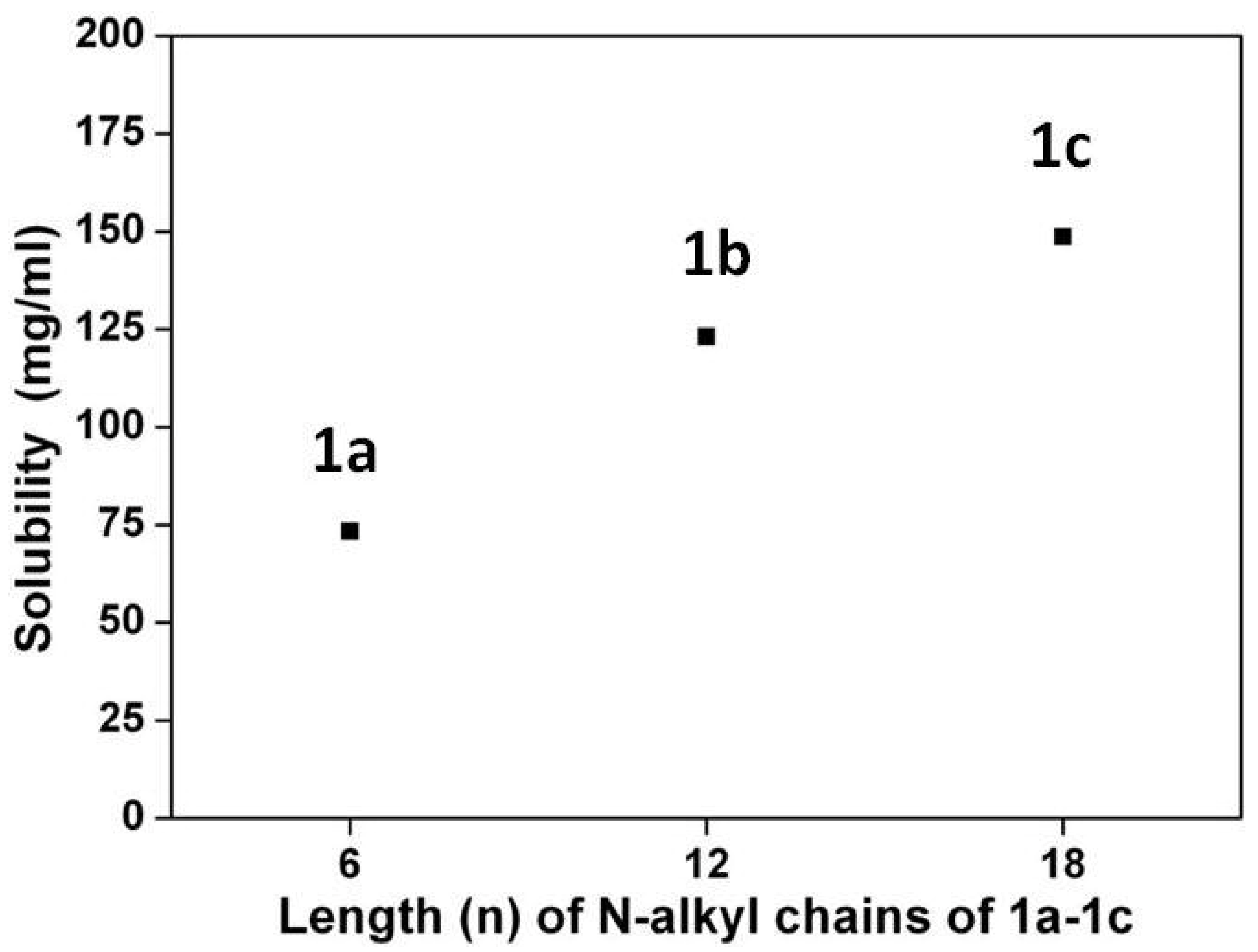

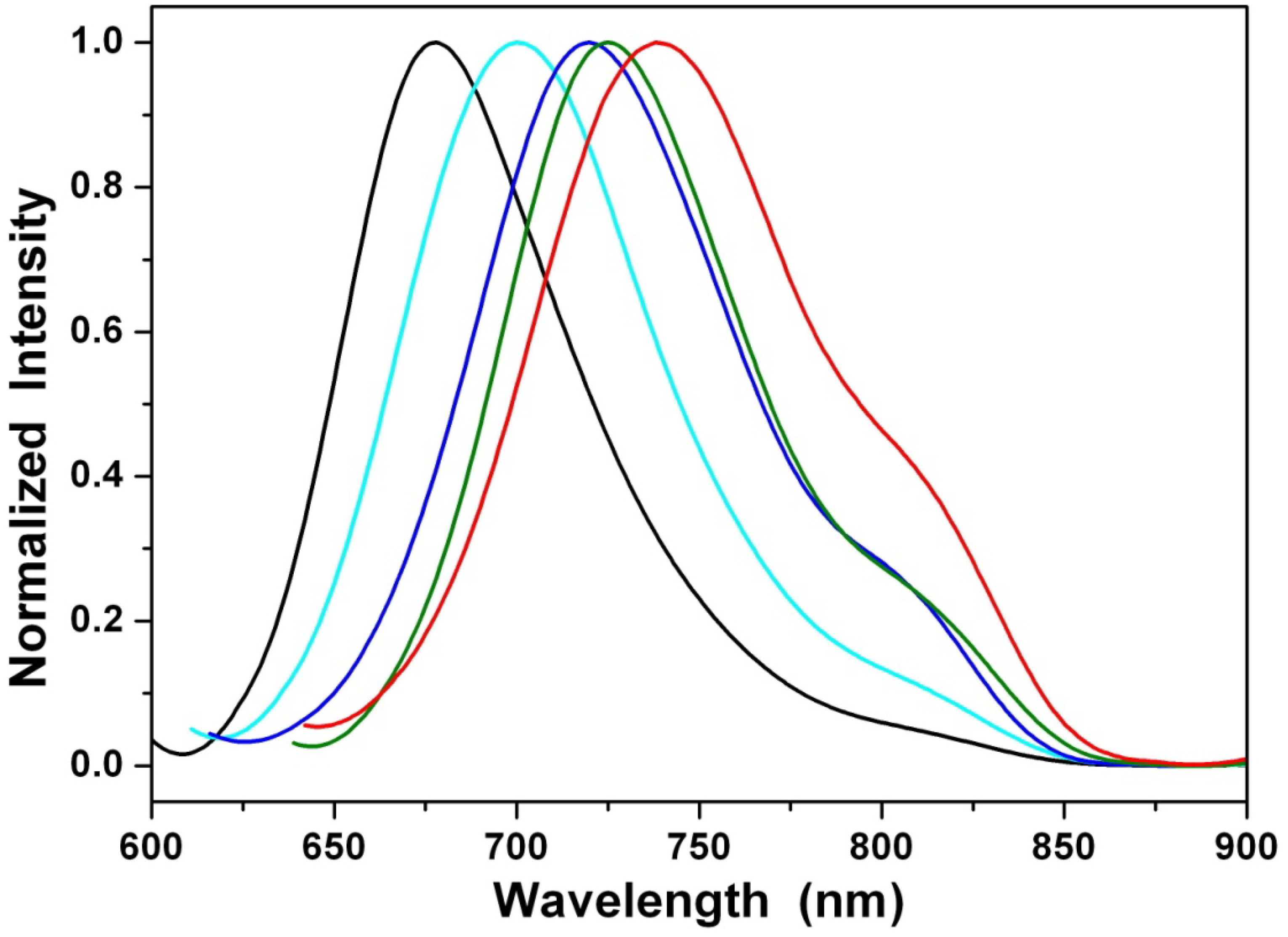
3.2. Optical Properties
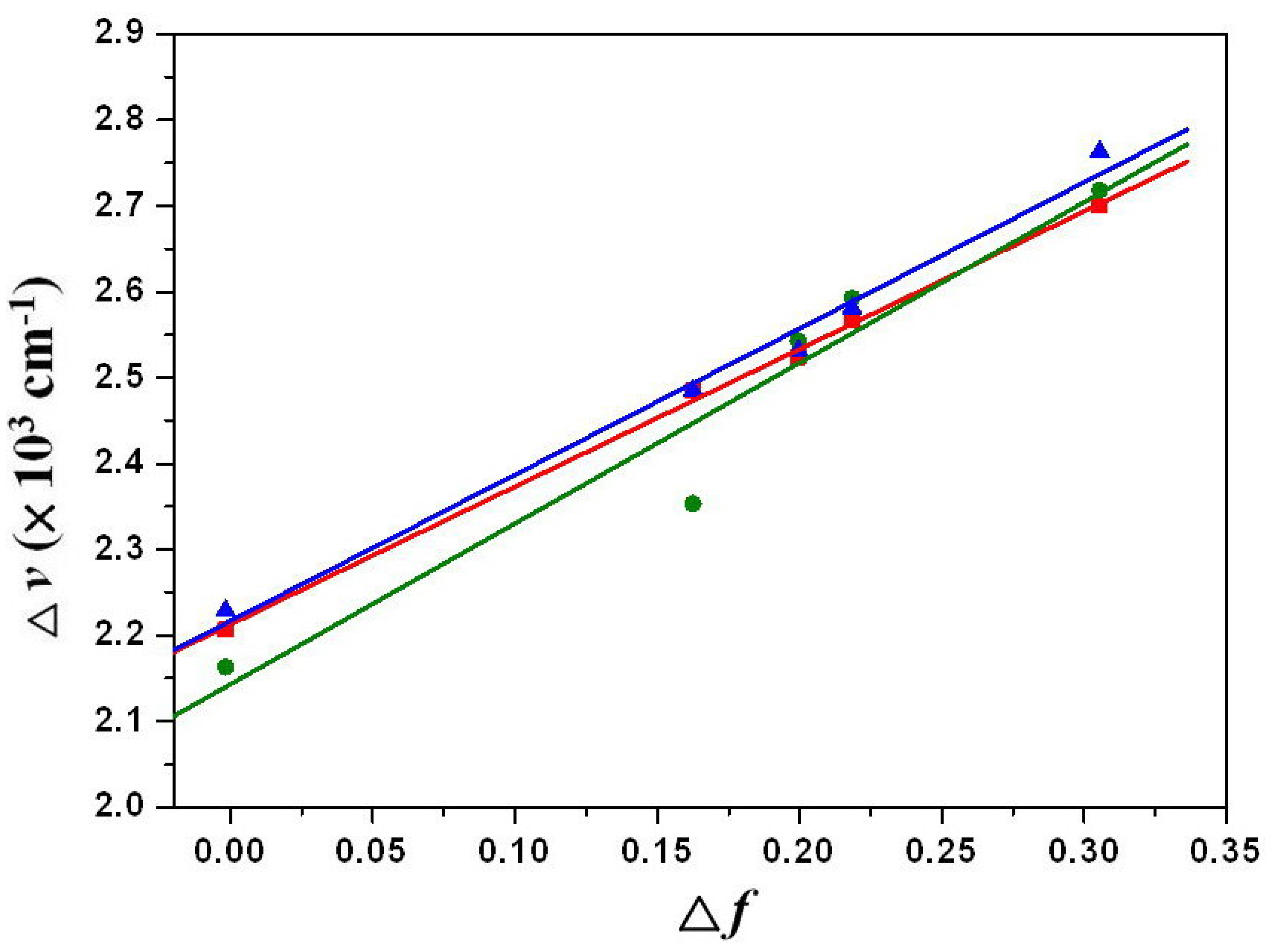
 , can be estimated by the Lippert-Mataga equation and expressed as:
, can be estimated by the Lippert-Mataga equation and expressed as:


 values can be calculated as 9.0 D, 11.7 D and 12.8 D for 1a–1c. These values indicate that the alkylamino-substituted PBIs (1a–1c) have larger dipole moment changes than that (7.4 D) of the amino-substituted compound (2).
values can be calculated as 9.0 D, 11.7 D and 12.8 D for 1a–1c. These values indicate that the alkylamino-substituted PBIs (1a–1c) have larger dipole moment changes than that (7.4 D) of the amino-substituted compound (2).
| 1a/1b/1c | λabs (nm) a | λem (nm) a | Stokes shift (nm) | Φ b × 102 |
|---|---|---|---|---|
| cyclohexane | 589/589/589 | 678/675/677 | 89/86/88 | 1.88/2.70/2.57 |
| diethyl ether | 597/601/597 | 700/700/701 | 103/99/104 | 0.47/0.58/0.45 |
| ethyl acetate | 608/610/610 | 720/722/721 | 112/112/111 | 0.24/0.31/0.29 |
| dichloromethane | 610/611/612 | 724/726/726 | 114/115/114 | 0.20/0.29/0.26 |
| acetonitrile | 613/614/614 | 738/737/736 | 125/123/122 | 0.12/0.19/0.17 |
3.3. Quantum Chemistry Computation
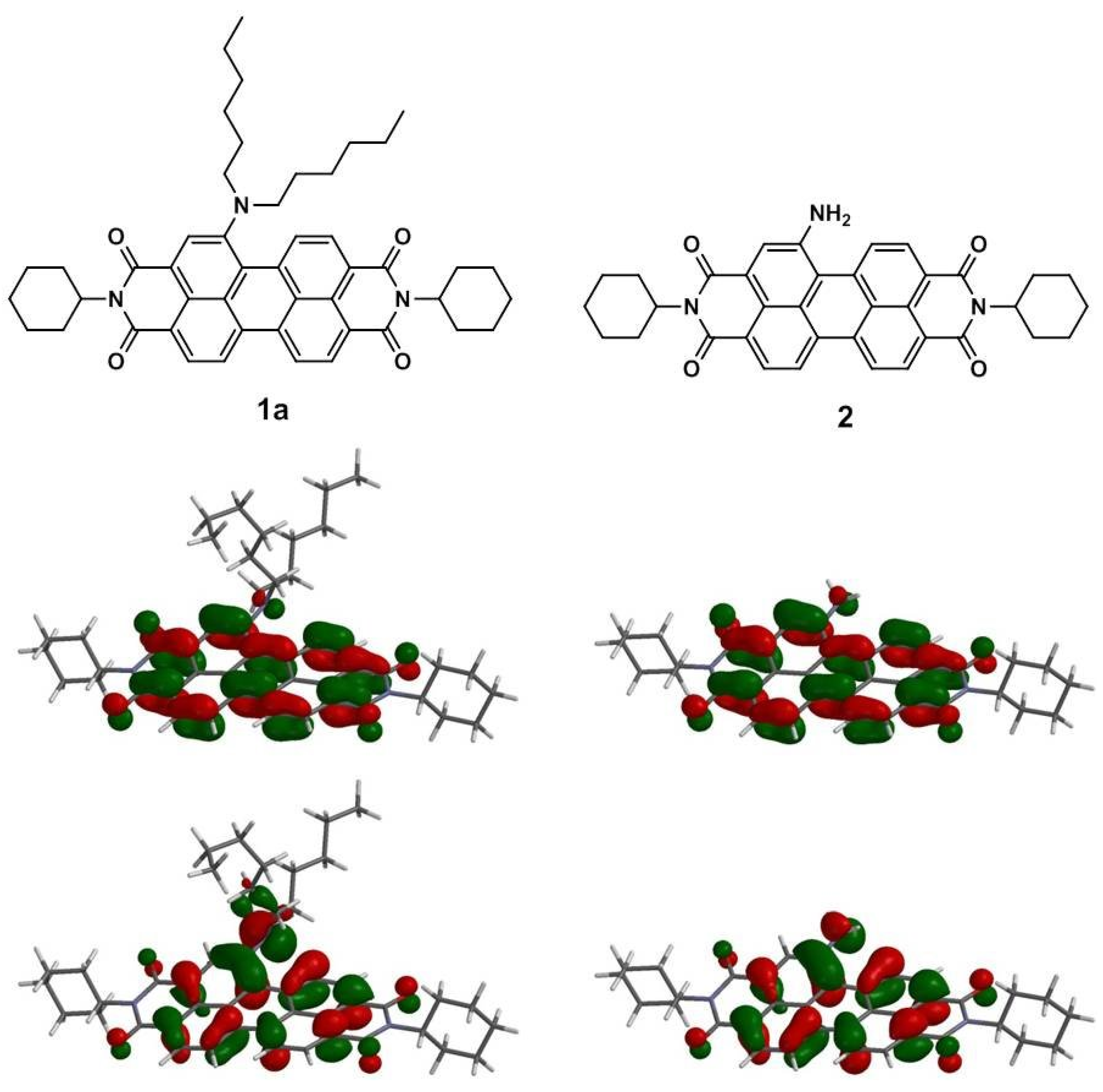

| Compound | HOMO a | LUMO a | Eg a | Eg b | μg c | μe d | Twisting angle (°) |
|---|---|---|---|---|---|---|---|
| 1a | −5.48 | −3.19 | 2.29 | 2.11 | 3.5 | 12.5 | 9.40, 13.43 |
| 1b | −5.48 | −3.19 | 2.29 | 2.11 | 3.6 | 15.3 | 9.42, 13.45 |
| 1c | −5.47 | −3.19 | 2.28 | 2.11 | 3.8 | 16.6 | 9.45, 13.49 |
| 2 | −5.62 | −3.21 | 2.41 | 2.24 | 2.7 | 10.1 | 9.23, 17.49 |
| 3 | −6.25 | −3.84 | 2.41 | 2.39 | - | - | 7.89, 15.87 |
| 4 | −5.94 | −3.46 | 2.48 | 2.38 | - | - | 0.00, 0.00 |
3.4. Electrochemical Properties

| Compound | E+1/2 a | E2+1/2 a | E−1/2 a | E2−1/2 a | HOMO b | LUMO b |
|---|---|---|---|---|---|---|
| 1a | 0.85 | 1.32 | −1.02 | −1.17 | −5.47 | −3.36 |
| 1b | 0.86 | 1.34 | −1.05 | −1.21 | −5.48 | −3.37 |
| 1c | 0.84 | 1.33 | −1.01 | −1.20 | −5.46 | −3.35 |
| 2 | 0.97 | 1.36 | −0.97 | −1.09 | −5.57 | −3.33 |
| 3 c | - | - | −0.19 | −0.51 | −6.64 | −4.25 |
| 4 c | - | - | −0.46 | −0.76 | −6.36 | −3.98 |
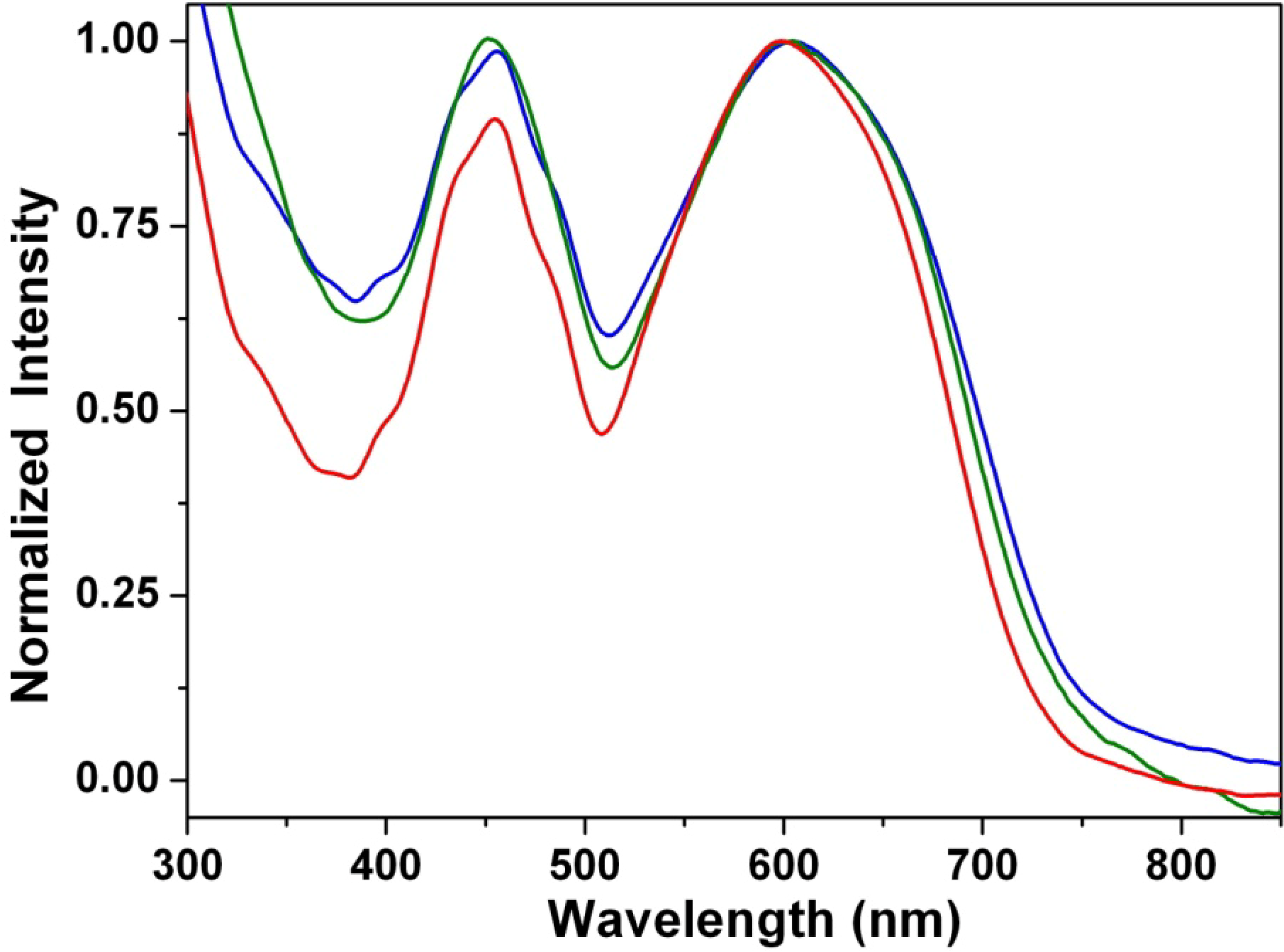
3.5. Stacking Behaviors of Dyes in Solution and Solid State
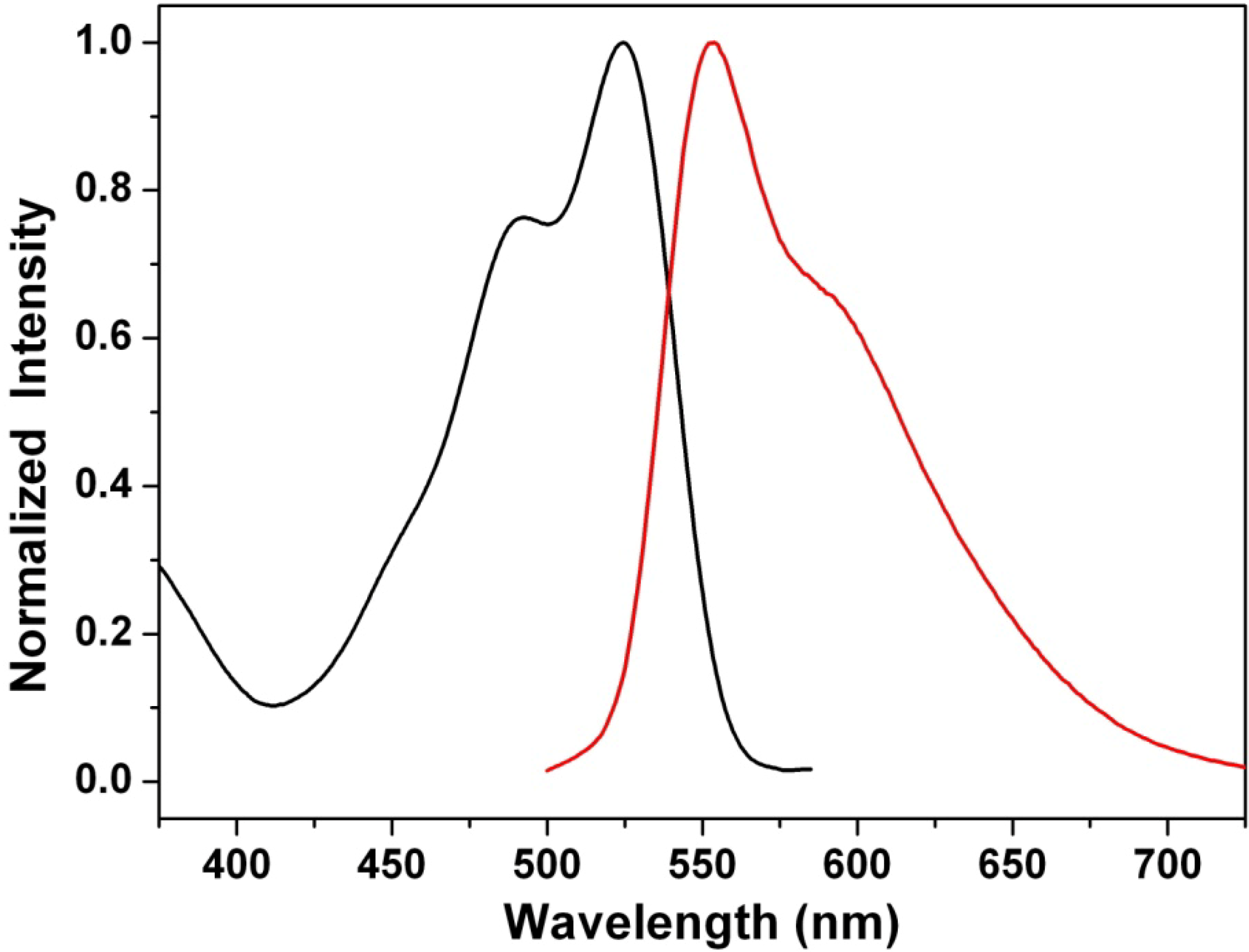
3.6. Influence of the Acidic Condition on the Optical Properties of Dyes
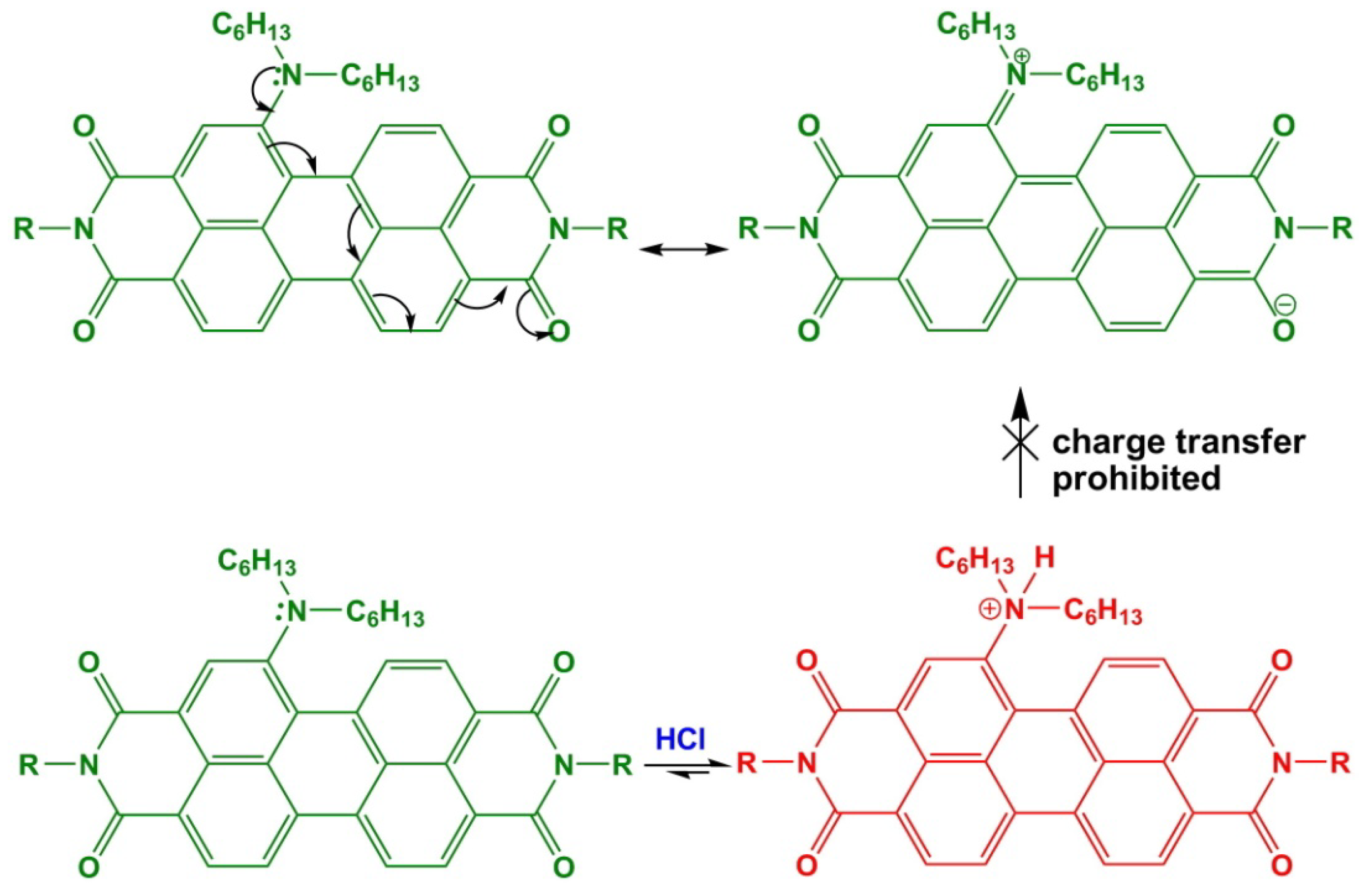
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jones, B.A.; Ahrens, M.J.; Yoon, M.H.; Facchetti, A.; Marks, T.J.; Wasielewski, M.R. High-mobility air-stable n-type semiconductors with processing versatility: Dicyanoperylene-3,4:9,10-bis(dicarboximides). Angew. Chem. Int. Ed. 2004, 43, 6363–6366. [Google Scholar] [CrossRef]
- Kim, F.S.; Guo, X.; Watson, M.D.; Jenekhe, S.A. High-mobility ambipolar transistors and high-gain inverters from a donor-acceptor copolymer semiconductor. Adv. Mater. 2009, 21, 1–5. [Google Scholar]
- Würthner, F.; Stolte, M. Naphthalene and perylene diimides for organic transistors. Chem. Commun. 2011, 47, 5109–5115. [Google Scholar] [CrossRef]
- Reghu, R.R.; Bisoyi, H.K.; Grazulevicius, J.V.; Anjukandi, P.; Gaidelis, V.; Jankauskas, V. Air stable electron-transporting and ambipolar bay substituted perylene bisimides. J. Mater. Chem. 2011, 21, 7811–7819. [Google Scholar] [CrossRef]
- Zaumseil, J.; Sirringhaus, H. Electron and ambipolar transport in organic field-effect transistors. Chem. Rev. 2007, 107, 1296–1323. [Google Scholar] [CrossRef]
- Locklin, J.; Li, D.; Mannsfeld, S.C.B.; Borkent, E.J.; Meng, H.; Advincula, R.; Bao, Z. Organic thin film transistors based on cyclohexyl-substituted organic semiconductors. Chem. Mater. 2005, 17, 3366–3374. [Google Scholar] [CrossRef]
- Kozma, E.; Kotowski, D.; Catellani, M.; Luzzati, S.; Famulari, A.; Bertini, F. Synthesis and characterization of new electron acceptor perylene diimide molecules for photovoltaic applications. Dyes Pigm. 2013, 99, 329–338. [Google Scholar] [CrossRef]
- Li, J.; Dierschke, F.; Wu, J.; Grimsdale, A.C.; Müllen, K. Poly(2,7-carbazole) and perylene tetracarboxydiimide: A promising donor/acceptor pair for polymer solar cells. J. Mater. Chem. 2006, 16, 96–100. [Google Scholar] [CrossRef]
- Dinçalp, H.; Aşkar, Z.; Zafer, C.; İçli, S. Effect of side chain substituents on the electron injection abilities of unsymmetrical perylene diimide dyes. Dyes Pigm. 2011, 91, 182–191. [Google Scholar] [CrossRef]
- Ramanan, C.; Semigh, A.L.; Anthony, J.E.; Marks, T.J.; Wasielewski, M.R. Competition between singlet fission and charge separation in solution-processed blend films of 6,13-bis(triisopropylsilylethynyl)-pentacene with sterically-encumbered perylene-3,4:9,10-bis(dicarboximide)s. J. Am. Chem. Soc. 2012, 134, 386–397. [Google Scholar]
- Shibano, Y.; Umeyama, T.; Matano, Y.; Imahori, H. Electron-donating perylene tetracarboxylic acids for dye-sensitized solar cells. Org. Lett. 2007, 9, 1971–1974. [Google Scholar] [CrossRef]
- Kozma, E.; Catellani, M. Perylene diimides based materials for organic solar cells. Dyes Pigm. 2013, 98, 160–179. [Google Scholar] [CrossRef]
- Tian, H.; Liu, P.H.; Zhu, W.; Gao, E.; Wu, D.J.; Cai, S. Synthesis of novel multi-chromophoric soluble perylene derivatives and their photosensitizing properties with wide spectral response for SnO2 nanoporous electrode. J. Mater. Chem. 2000, 10, 2708–2715. [Google Scholar] [CrossRef]
- Choi, H.; Paek, S.; Song, J.; Kim, C.; Cho, N.; Ko, J. Synthesis of annulated thiophene perylene bisimide analogues: Their applications to bulk heterojunction organic solar cells. Chem. Commun. 2011, 47, 5509–5511. [Google Scholar]
- Huang, C.; Barlow, S.; Marder, S.R. Perylene-3,4,9,10-tetracarboxylic acid diimides: Synthesis, physical properties, and use in organic electronics. J. Org. Chem. 2011, 76, 2386–2407. [Google Scholar] [CrossRef]
- Wang, H.Y.; Peng, B.; Wei, W. Solar cells based on perylene bisimide derivatives. Prog. Chem. 2008, 20, 1751–1760. [Google Scholar]
- Ventura, B.; Langhals, H.; Böck, B.; Flamigni, L. Phosphorescent perylene imides. Chem. Commun. 2012, 48, 4226–4228. [Google Scholar] [CrossRef] [Green Version]
- Matsui, M.; Wang, M.; Funabiki, K.; Hayakawa, Y.; Kitaguchi, T. Properties of novel perylene-3,4:9,10-tetracarboxidiimide-centred dendrimers and their application as emitters in organic electroluminescence devices. Dyes Pigm. 2007, 74, 169–175. [Google Scholar] [CrossRef]
- Damaceanu, M.D.; Constantin, C.P.; Bruma, N.; Pinteala, M. Tuning of the color of the emitted light from new polyperyleneimides containing oxadiazole and siloxane moieties. Dyes Pigm. 2013, 99, 228–239. [Google Scholar] [CrossRef]
- Lucenti, E.; Botta, C.; Cariati, E.; Righetto, S.; Scarpellini, M.; Tordin, E.; Ugo, R. New organic-inorganic hybrid materials based on perylene diimide–polyhedral oligomeric silsesquioxane dyes with reduced quenching of the emission in the solid state. Dyes Pigm. 2013, 96, 748–755. [Google Scholar] [CrossRef]
- Pan, J.; Zhu, W.; Li, S.; Zeng, W.; Cao, Y.; Tian, H. Dendron-functionalized perylene diimides with carrier-transporting ability for red luminescent materials. Polymer 2005, 46, 7658–7669. [Google Scholar] [CrossRef]
- Li, X.; Sinks, L.E.; Rybtchinski, B.; Wasielewski, M.R. Ultrafast aggregate-to-aggregate energy transfer within self-assembled light-harvesting columns of zinc phthalocyanine tetrakis(perylenediimide). J. Am. Chem. Soc. 2004, 126, 10810–10811. [Google Scholar] [CrossRef]
- Rybtchinski, B.; Sinks, L.E.; Wasielewski, M.R. Combining light-harvesting and charge separation in a self-assembled artificial photosynthetic system based on perylenediimide chromophores. J. Am. Chem. Soc. 2004, 126, 12268–12269. [Google Scholar] [CrossRef]
- Berberich, M.; Krause, A.M.; Orlandi, M.; Scandola, F.; Würthner, F. Toward fluorescent memories with nondestructive readout: Photoswitching of fluorescence by intramolecular electron transfer in a diaryl ethene-perylene bisimide photochromic system. Angew. Chem. Int. Ed. 2008, 47, 6616–6619. [Google Scholar] [CrossRef]
- Tan, W.; Li, X.; Zhang, J.; Tian, H. A photochromic diarylethene dyad based on perylene diimide. Dyes Pigm. 2011, 89, 260–265. [Google Scholar] [CrossRef]
- Weiss, E.A.; Ahrens, M.J.; Sinks, L.E.; Gusev, A.V.; Ratner, M.A.; Wasielewski, M.R. Making a molecular wire: Charge and spin transport through para-phenylene oligomers. J. Am. Chem. Soc. 2004, 126, 5577–5584. [Google Scholar]
- Wilson, T.M.; Tauber, M.J.; Wasielewski, M.R. Toward an n-type molecular wire: Electron hopping within linearly linked perylenediimide oligomers. J. Am. Chem. Soc. 2009, 131, 8952–8957. [Google Scholar]
- Choi, J.; Lee, W.; Sakong, C.; Yuk, S.B.; Park, J.S.; Kim, J.P. Facile synthesis and characterization of novel coronene chromophores and their application to LCD color filters. Dyes Pigm. 2012, 94, 34–39. [Google Scholar] [CrossRef]
- Sakong, C.; Kim, Y.D.; Choi, J.H.; Yoon, C.; Kim, J.P. The synthesis of thermally-stable red dyes for LCD color filters and analysis of their aggregation and spectral properties. Dyes Pigm. 2011, 88, 166–173. [Google Scholar] [CrossRef]
- Lu, X.; Guo, Z.; Sun, C.; Tian, H.; Zhu, W. Helical assembly induced by hydrogen bonding from chiral carboxylic acids based on perylene bisimides. J. Phys. Chem. B 2011, 115, 10871–10876. [Google Scholar]
- Würthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 2004, 14, 1564–1579. [Google Scholar] [CrossRef]
- Wasielewski, M.R. Self-assembly strategies for integrating light harvesting and charge separation in artificial photosynthetic systems. Acc. Chem. Res. 2009, 42, 1910–1921. [Google Scholar] [CrossRef]
- Kaur, B.; Bhattacharya, S.N.; Henry, D.J. Interpreting the near-infrared reflectance of a series of perylene pigments. Dyes Pigm. 2013, 99, 502–511. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Sakr, A.R.; Bojinov, V.B. Design and synthesis of novel fluorescence sensing perylene diimides based on photoinduced electron transfer. Dyes Pigm. 2011, 91, 332–339. [Google Scholar] [CrossRef]
- Langhals, H.; Kirner, S. Novel fluorescent dyes by the extension of the core of perylenetetracarboxylic bisimides. Eur. J. Org. Chem. 2000, 2, 365–380. [Google Scholar]
- Liang, Y.; Wang, H.; Wang, D.; Liu, H.; Feng, S. The synthesis, morphology and liquid-crystalline property of polysiloxane-modified perylene derivative. Dyes Pigm. 2012, 95, 260–267. [Google Scholar] [CrossRef]
- Cazacu, M.; Vlad, A.; Airinei, A.; Nicolescu, A.; Stoica, I. New imides based on perylene and siloxane derivatives. Dyes Pigm. 2011, 90, 106–113. [Google Scholar] [CrossRef]
- Kaur, B.; Quazi, N.; Ivanov, I.; Bhattacharya, S.N. Near-infrared reflective properties of perylene derivatives. Dyes Pigm. 2012, 92, 1108–1113. [Google Scholar] [CrossRef]
- Boobalan, G.; Imran, P.S.; Nagarajan, S. Synthesis of highly fluorescent and water soluble perylene bisimide. Chin. Chem. Lett. 2012, 23, 149–153. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, Y.; Liu, Y.; Yang, G.; Liu, L.; Fu, H.; Li, Z.; Wang, S.; Wang, Z.; Chen, Y. PEGylated nanoparticles of diperylene bisimides with high efficiency of 1O2 generation. Dyes Pigm. 2013, 97, 129–133. [Google Scholar] [CrossRef]
- Wang, R.; Shi, Z.; Zhang, C.; Zhang, A.; Chen, J.; Guo, W.; Sun, Z. Facile synthesis and controllable bromination of asymmetrical intermediates of perylene monoanhydride/monoimide diester. Dyes Pigm. 2013, 98, 450–458. [Google Scholar] [CrossRef]
- Luo, M.H.; Chen, K.Y. Asymmetric perylene bisimide dyes with strong solvatofluorism. Dyes Pigm. 2013, 99, 456–464. [Google Scholar] [CrossRef]
- Kang, H.; Jiang, W.; Wang, Z. Construction of well-defined butadiynylene-linked perylene bisimide arrays via cross-coupling. Dyes Pigm. 2013, 97, 244–249. [Google Scholar] [CrossRef]
- Shin, I.S.; Hirsch, T.; Ehrl, B.; Jang, D.H.; Wolfbeis, O.S.; Hong, J.I. Efficient fluorescence “turn-on” sensing of dissolved oxygen by electrochemical switching. Anal. Chem. 2012, 84, 9163–9168. [Google Scholar]
- Habuchi, S.; Fujiwara, S.; Yamamoto, T.; Vacha, M.; Tezuka, Y. Single-molecule study on polymer diffusion in a melt state: Effect of chain topology. Anal. Chem. 2013, 85, 7369–7376. [Google Scholar] [CrossRef]
- Daimon, T.; Nihei, E. Fabrication of a poly(3-octylthiophene-2,5-diyl) electrochemiluminescence device assisted by perylene. Materials 2013, 6, 1704–1717. [Google Scholar] [CrossRef]
- Sharma, G.D.; Kurchania, R.; Ball, R.J.; Roy, M.S.; Mikroyannidis, J.A. Effect of deoxycholic acid on the performance of liquid electrolyte dye-sensitized solar cells using a perylene monoimide derivative. Int. J. Photoenergy 2012, 2012. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Chang, C.W.; Chen, K.Y. 1,6- and 1,7-Regioisomers of asymmetric and symmetric perylene bisimides: Synthesis, characterization and optical properties. Molecules 2014, 19, 327–341. [Google Scholar] [CrossRef]
- El-Daly, S.A.; Alamry, K.A.; Asiri, A.M.; Hussein, M.A. Spectral characteristics and fluorescence quenching of N,N’-bis(4-pyridyl)-3,4:9,10-perylenebis(dicarboximide) (BPPD). J. Lumin. 2012, 132, 2747–2752. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Chen, K.Y. Synthesis and optical properties of novel asymmetric perylene bisimides. J. Lumin. 2014, 149, 103–111. [Google Scholar] [CrossRef]
- Yang, T.; Guan, Q.; Guo, X.; Meng, L.; Du, M.; Jiao, K. Direct and freely switchable detection of target genes engineered by reduced graphene oxide-poly(m-aminobenzenesulfonic acid) nanocomposite via synchronous pulse electrosynthesis. Anal. Chem. 2013, 85, 1358–1366. [Google Scholar] [CrossRef]
- Liao, D.; Chen, J.; Zhou, H.; Wang, Y.; Li, Y.; Yu, C. In situ formation of metal coordination polymer: A strategy for fluorescence turn-on assay of acetylcholinesterase activity and inhibitor screening. Anal. Chem. 2013, 85, 2667–2672. [Google Scholar] [CrossRef]
- Naveenraj, S.; Raj, M.R.; Anandan, S. Binding interaction between serum albumins and perylene-3,4,9,10-tetracarboxylate—A spectroscopic investigation. Dyes Pigm. 2012, 94, 330–337. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Yu, J.; Zhang, G.; Cai, X.; Wu, Y.; Wang, L. A colorimetric and fluorescent sensor based on PBIs for palladium detection. Tetrahedron Lett. 2013, 54, 4019–4022. [Google Scholar] [CrossRef]
- Boobalan, G.; Imran, P.M.; Ramkumar, S.G.; Nagarajan, S. Fabrication of luminescent perylene bisimide nanorods. J. Lumin. 2014, 146, 387–393. [Google Scholar] [CrossRef]
- Weitz, R.T.; Amsharov, K.; Zschieschang, U.; Villas, E.B.; Goswami, D.K.; Burghard, M.; Dosch, H.; Jansen, M.; Kern, K.; Klauk, H. Organic n-channel transistors based on core-cyanated perylene carboxylic diimide derivatives. J. Am. Chem. Soc. 2008, 130, 4637–4645. [Google Scholar] [CrossRef]
- Jones, B.A.; Facchetti, A.; Wasielewski, M.R.; Marks, T.J. Tuning orbital energetics in arylene diimide semiconductors. Materials design for ambient stability of n-type charge transport. J. Am. Chem. Soc. 2007, 129, 15259–15278. [Google Scholar]
- Chen, K.Y.; Chow, T.J. 1,7-Dinitroperylene bisimides: Facile synthesis and characterization as n-type organic semiconductors. Tetrahedron Lett. 2010, 51, 5959–5963. [Google Scholar] [CrossRef]
- Chen, Z.J.; Wang, L.M.; Zou, G.; Zhang, L.; Zhang, G.J.; Cai, X.F.; Teng, M.S. Colorimetric and ratiometric luorescent chemosensor for fluoride ion based on perylene diimide derivatives. Dyes Pigm. 2012, 94, 410–415. [Google Scholar] [CrossRef]
- Kong, X.; Gao, J.; Ma, T.; Wang, M.; Zhang, A.; Shi, Z.; Wei, Y. Facile synthesis and replacement reactions of mono-substituteded perylene bisimide dyes. Dyes Pigm. 2012, 95, 450–454. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, J.; Xiao, Y.; Li, Z.; Qian, X. Core-perfluoroalkylated perylene diimides and naphthalene diimides: Versatile synthesis, solubility, electrochemistry, and optical properties. J. Org. Chem. 2010, 75, 3007–3016. [Google Scholar] [CrossRef]
- Li, Y.; Tan, L.; Wang, Z.; Qian, H.; Shi, Y.; Hu, W. Air-stable n-type semiconductor: Core-perfluoroalkylated perylene bisimides. Org. Lett. 2008, 10, 529–532. [Google Scholar]
- Chao, C.C.; Leung, M.K. Photophysical and electrochemical properties of 1,7-diaryl-substituted perylene diimides. J. Org. Chem. 2005, 70, 4323–4331. [Google Scholar] [CrossRef]
- Miasojedovasa, A.; Kazlauskasa, K.; Armonaitea, G.; Sivamuruganb, V.; Valiyaveettilb, S.; Grazuleviciusc, J.V.; Jursenasa, S. Concentration effects on emission of bay-substituted perylene diimide derivatives in a polymer matrix. Dyes Pigm. 2012, 92, 1285–1291. [Google Scholar] [CrossRef]
- Goretzki, G.; Davies, E.S.; Argent, S.P.; Warren, J.E.; Blake, A.J.; Champness, N.R. Building multistate redox-active architectures using metal-complex functionalized perylene bis-imides. Inorg. Chem. 2009, 48, 10264–10274. [Google Scholar] [CrossRef]
- Dhokale, B.; Gautam, P.; Misra, R. Donor-acceptor perylenediimide-ferrocene conjugates: Synthesis, photophysical, and electrochemical properties. Tetrahedron Lett. 2012, 53, 2352–2354. [Google Scholar] [CrossRef]
- Dey, S.; Efimov, A.; Lemmetyinen, H. Bay region borylation of perylene bisimides. Eur. J. Org. Chem. 2011, 30, 5955–5958. [Google Scholar]
- Handa, N.V.; Mendoza, K.D.; Shirtcliff, L.D. Syntheses and properties of 1,6 and 1,7 perylene diimides and tetracarboxylic dianhydrides. Org. Lett. 2011, 13, 4724–4727. [Google Scholar] [CrossRef]
- Fin, A.; Petkova, I.; Doval, D.A.; Sakai, N.; Vauthey, E.; Matile, S. Naphthalene- and perylenediimides with hydroquinones, catechols, boronic esters and imines in the core. Org. Biomol. Chem. 2011, 9, 8246–8252. [Google Scholar] [CrossRef]
- Perrin, L.; Hudhomme, P. Synthesis, electrochemical and optical absorption properties of new perylene-3,4:9,10-bis(dicarboximide) and perylene-3,4:9,10-bis(benzimidazole) derivatives. Eur. J. Org. Chem. 2011, 28, 5427–5440. [Google Scholar] [CrossRef] [Green Version]
- Dinçalp, H.; Kızılok, Ş.; İçli, S. Fluorescent macromolecular perylene diimides containing pyrene or indole units in bay positions. Dyes Pigm. 2010, 86, 32–41. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Li, R.; Li, X.; Jiang, J. Di(alkoxy)- and di(alkylthio)-substituted perylene-3,4;9,10-tetracarboxy diimides with tunable electrochemical and photophysical properties. J. Org. Chem. 2007, 72, 2402–2410. [Google Scholar] [CrossRef]
- Ren, H.; Li, J.; Zhang, T.; Wang, R.; Gao, Z.; Liu, D. Synthesis and properties of novel perylenebisimide-cored dendrimers. Dyes Pigm. 2011, 91, 298–303. [Google Scholar]
- Zhang, X.; Pang, S.; Zhang, Z.; Ding, X.; Zhang, S.; He, S.; Zhan, C. Facile synthesis of 1-bromo-7-alkoxyl perylene diimide dyes: Toward unsymmetrical functionalizations at the 1,7-positions. Tetrahedron Lett. 2012, 53, 1094–1097. [Google Scholar] [CrossRef]
- Feng, J.; Wang, D.; Wang, S.; Zhang, L.; Li, X. Synthesis and properties of novel perylenetetracarboxylic diimide derivatives fused with BODIPY units. Dyes Pigm. 2011, 89, 23–28. [Google Scholar] [CrossRef]
- Chen, K.Y.; Fang, T.C.; Chang, M.J. Synthesis, photophysical and electrochemical properties of 1-aminoperylene bisimides. Dyes Pigm. 2011, 92, 517–523. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Chen, K.Y. 1,7-Diaminoperylene bisimides: Synthesis, optical and electrochemical properties. Dyes Pigm. 2013, 96, 319–327. [Google Scholar] [CrossRef]
- Ahrens, M.J.; Tauber, M.J.; Wasielewski, M.R. Bis(n-octylamino)perylene-3,4:9,10-bis(dicarboximide)s and their radical cations: Synthesis, electrochemistry, and ENDOR spectroscopy. J. Org. Chem. 2006, 71, 2107–2114. [Google Scholar] [CrossRef]
- Alvino, A.; Franceschin, M.; Cefaro, C.; Borioni, S.; Ortaggi, G.; Bianco, A. Synthesis and spectroscopic properties of highly water-soluble perylene derivatives. Tetrahedron 2007, 63, 7858–7865. [Google Scholar] [CrossRef]
- Wang, H.; Kaiser, T.E.; Uemura, S.; Würthner, F. Perylene bisimide J-aggregates with absorption maxima in the NIR. Chem. Commun. 2008, 10, 1181–1183. [Google Scholar]
- Dubey, R.K.; Efimov, A.; Lemmetyinen, H. 1,7- and 1,6-regioisomers of diphenoxy and dipyrrolidinyl substituted perylene diimides: Synthesis, separation, characterization, and comparison of electrochemical and optical properties. Chem. Mater. 2011, 23, 778–788. [Google Scholar] [CrossRef]
- Zhao, Y.; Wasielewski, M.R. 3,4:9,10-Perylenebis(dicarboximide) chromophores that function as both electron donors and acceptors. Tetrahedron Lett. 1999, 40, 7047–7050. [Google Scholar] [CrossRef]
- Ma, Y.S.; Wang, C.H.; Zhao, Y.J.; Yu, Y.; Han, C.X.; Qiu, X.J.; Shi, Z. Perylene diimide dyes aggregates: Optical properties and packing behavior in solution and solid state. Supramol. Chem. 2007, 19, 141–149. [Google Scholar] [CrossRef]
- Fan, L.; Xu, Y.; Tian, H. 1,6-Disubstituted perylene bisimides: Concise synthesis and characterization as near-infrared fluorescent dyes. Tetrahedron Lett. 2005, 46, 4443–4447. [Google Scholar] [CrossRef]
- Rohr, U.; Kohl, C.; Müllen, K.; van de Craats, A.; Warman, J. Liquid crystalline coronene derivatives. J. Mater. Chem. 2001, 11, 1789–1799. [Google Scholar] [CrossRef]
- Rajasingh, P.; Cohen, R.; Shirman, E.; Shimon, L.J.W.; Rybtchinski, B. Selective bromination of perylene diimides under mild conditions. J. Org. Chem. 2007, 72, 5973–5979. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 2nd ed.; Plenum: New York, NY, USA, 1999. [Google Scholar]
- Luo, M.H.; Tsai, H.Y.; Lin, H.Y.; Fang, S.K.; Chen, K.Y. Extensive spectral tuning of the proton transfer emission from green to red via a rational derivatization of salicylideneaniline. Chin. Chem. Lett. 2012, 23, 1279–1282. [Google Scholar] [CrossRef]
- Fang, T.C.; Tsai, H.Y.; Luo, M.H.; Chang, C.W.; Chen, K.Y. Excited-state charge coupled proton transfer reaction via the dipolar functionality of salicylideneaniline. Chin. Chem. Lett. 2013, 24, 145–148. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chang, C.-W.; Tsai, H.-Y.; Chen, K.-Y. Green Perylene Bisimide Dyes: Synthesis, Photophysical and Electrochemical Properties. Materials 2014, 7, 5488-5506. https://doi.org/10.3390/ma7085488
Chang C-W, Tsai H-Y, Chen K-Y. Green Perylene Bisimide Dyes: Synthesis, Photophysical and Electrochemical Properties. Materials. 2014; 7(8):5488-5506. https://doi.org/10.3390/ma7085488
Chicago/Turabian StyleChang, Che-Wei, Hsing-Yang Tsai, and Kew-Yu Chen. 2014. "Green Perylene Bisimide Dyes: Synthesis, Photophysical and Electrochemical Properties" Materials 7, no. 8: 5488-5506. https://doi.org/10.3390/ma7085488






