Effect of Ni Core Structure on the Electrocatalytic Activity of Pt-Ni/C in Methanol Oxidation
Abstract
:1. Introduction
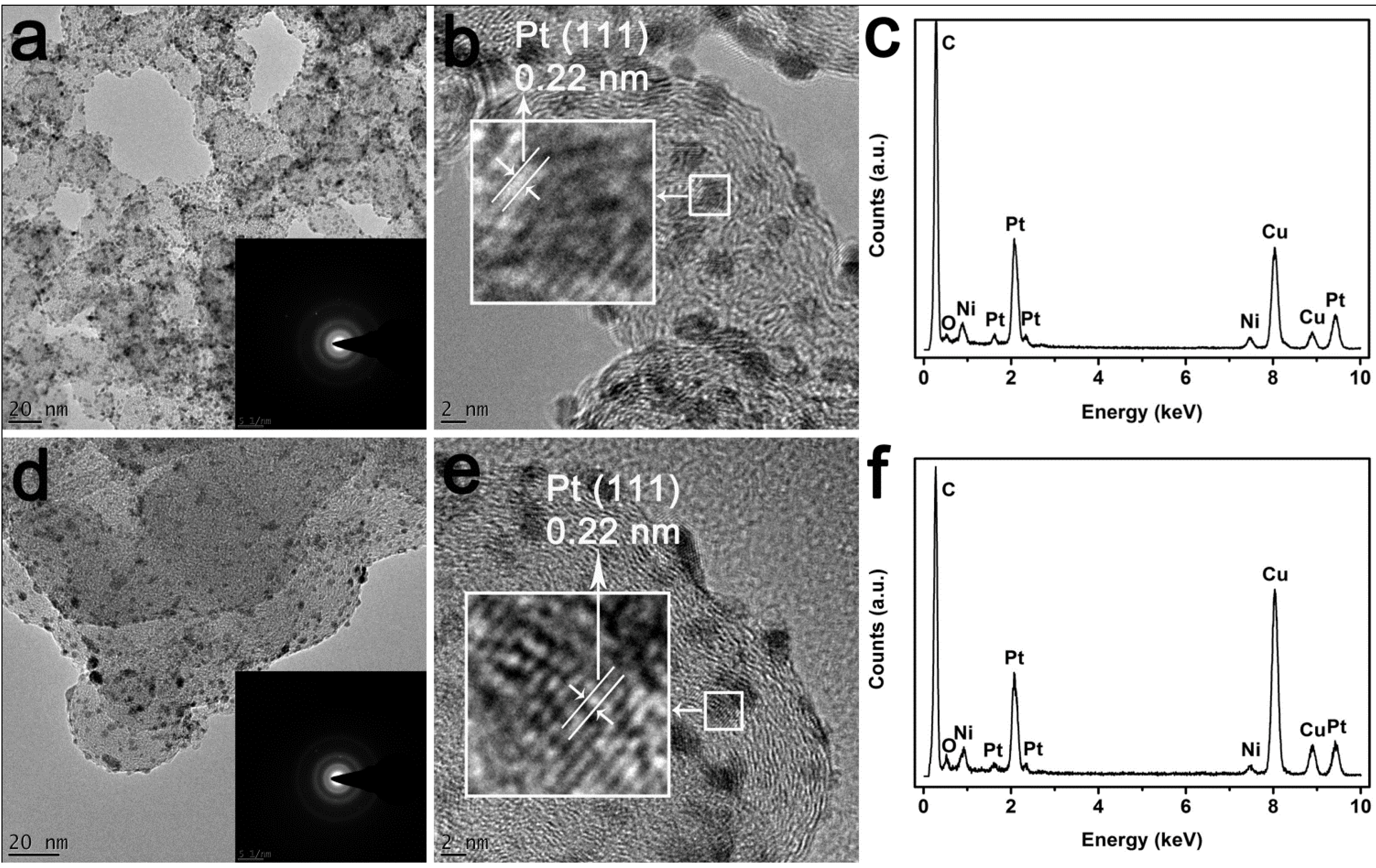
2. Results and Discussion

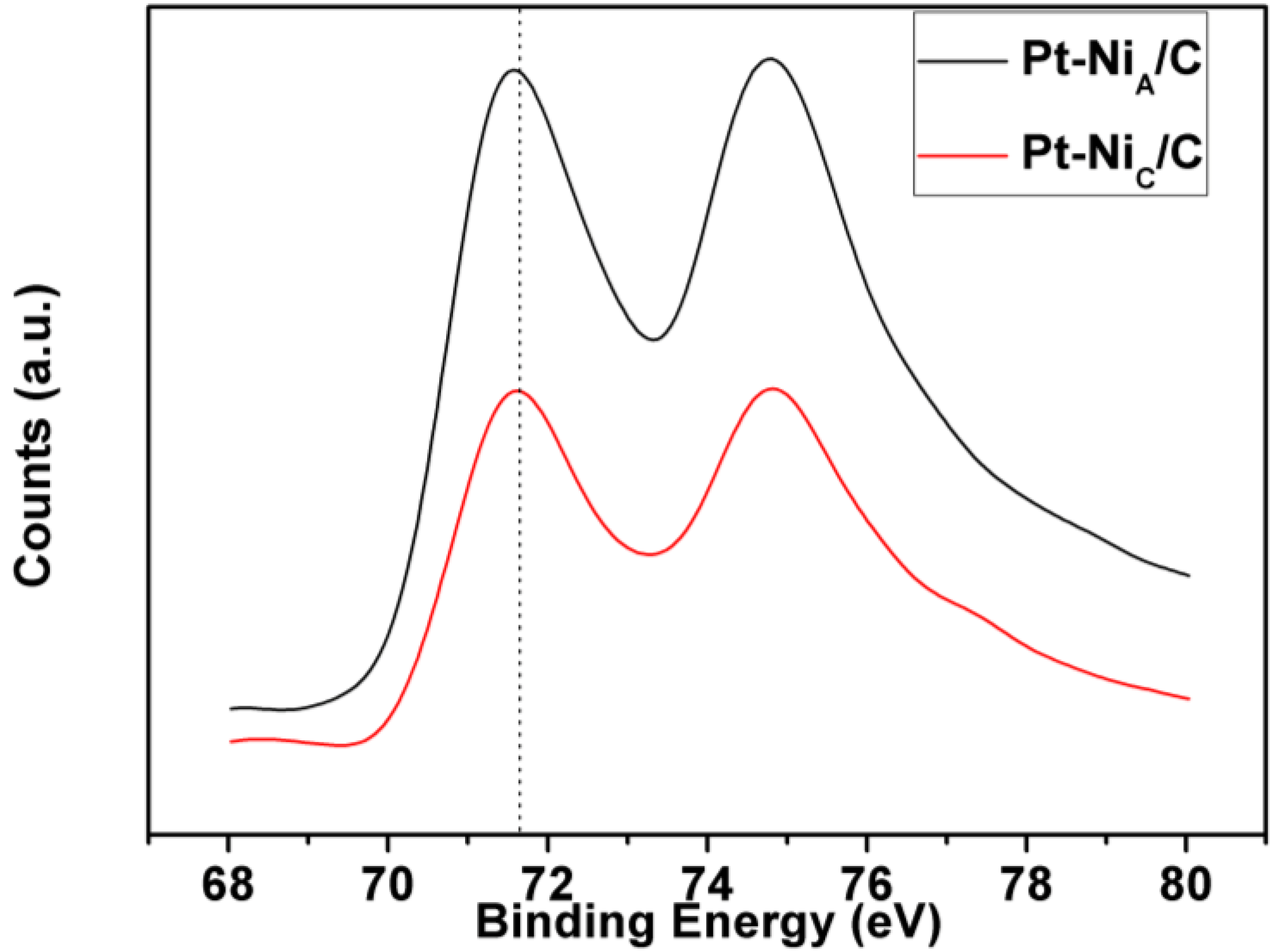
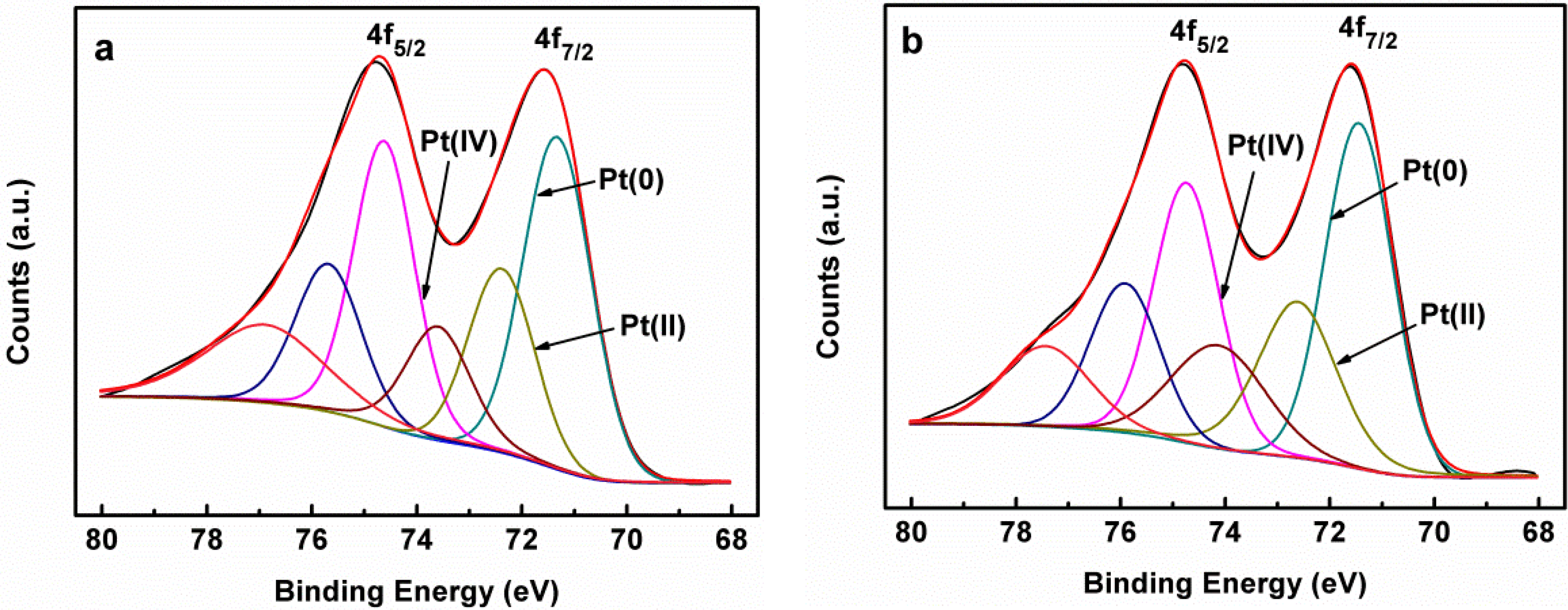
| Samples | Items | Pt 4f7/2 | ||
|---|---|---|---|---|
| Pt(0) | Pt(II) | Pt(IV) | ||
| Pt-NiC/C | Binding energy (eV) | 71.4 | 72.5 | 74.1 |
| Area ratio (%) | 49.6 | 29.2 | 21.2 | |
| Pt-NiA/C | Binding energy (eV) | 71.3 | 72.4 | 73.6 |
| Area ratio (%) | 53.0 | 28.0 | 19.0 | |

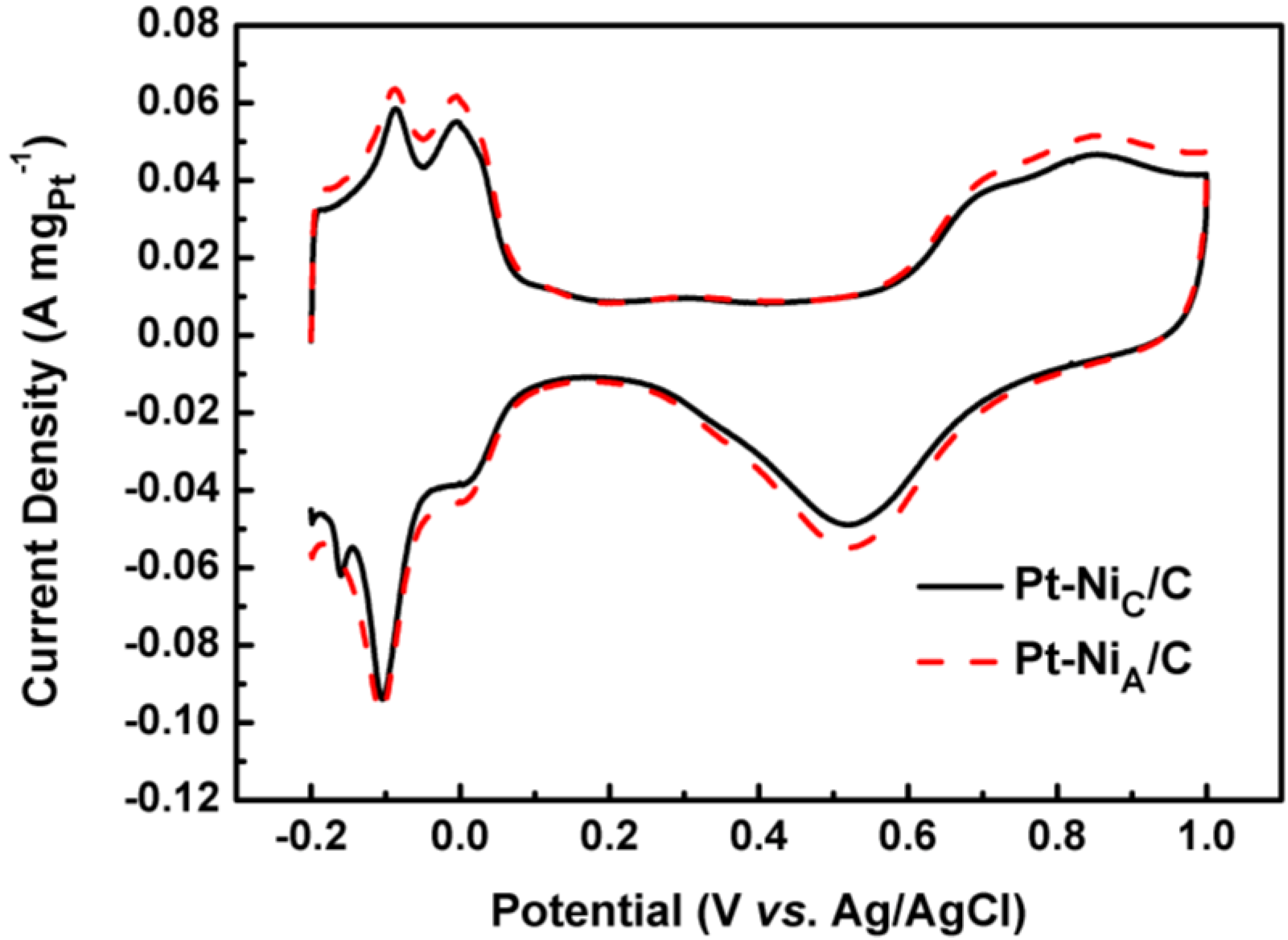
| Samples | If (A mgPt−1) | Ib(A mgPt−1) |
|---|---|---|
| Pt-NiA/C | 0.378 | 0.448 |
| Pt-NiC/C | 0.334 | 0.435 |
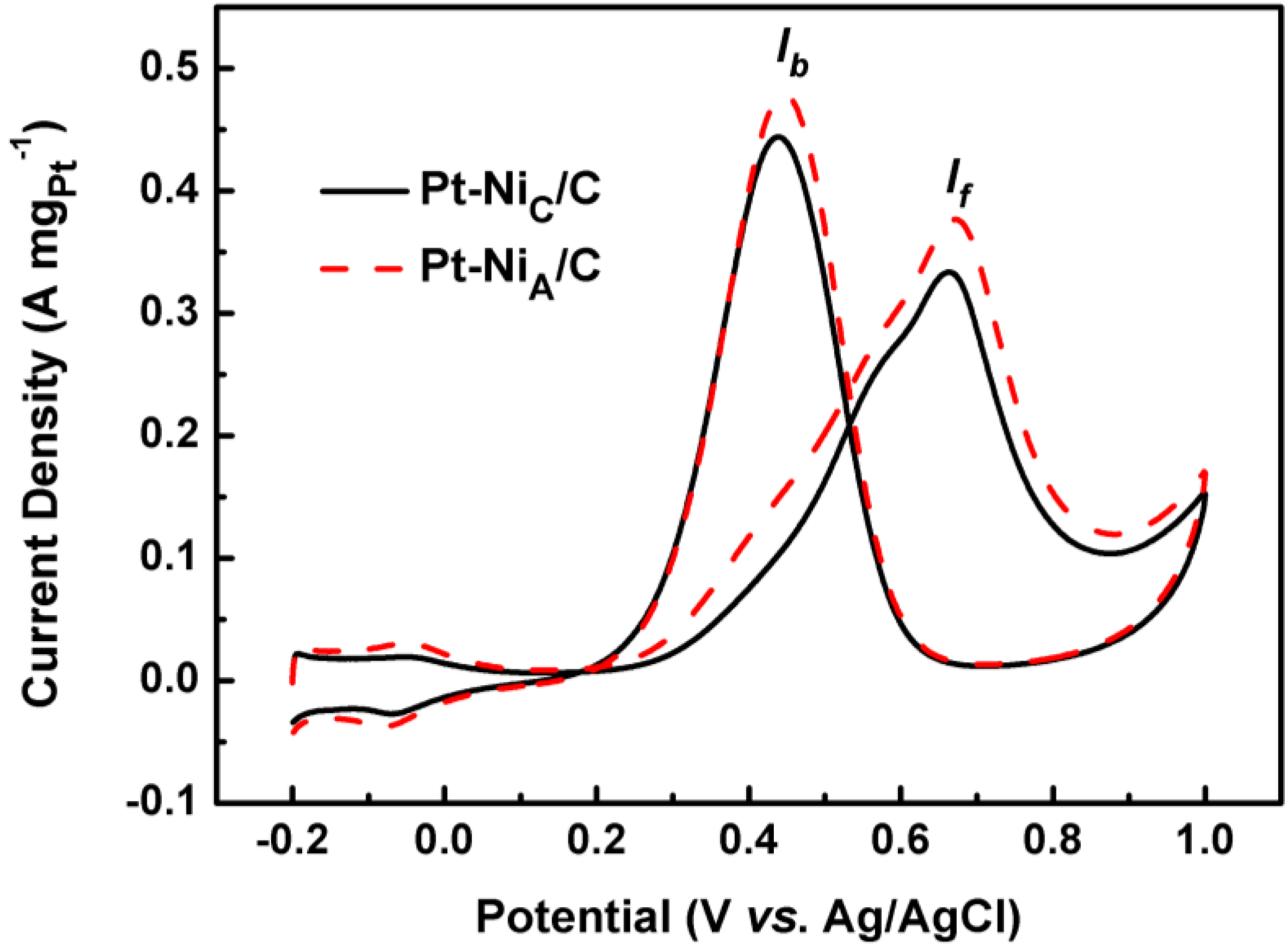

3. Experimental Section
3.1. Preparation of Carbon-Supported Crystalline and Amorphous Ni nanoparticles
3.2. Preparation of Pt-NiC/C and Pt-NiA/C Catalysts
3.3. Characterization
4. Conclusions
Acknowledgments
References
- Gan, L.; Du, H.-D.; Li, B.-H.; Kang, F.-Y. The effect of particle size on the interaction of Pt catalyst particles with a carbon black support. New Carbon Mater. 2010, 25, 53–59. [Google Scholar] [CrossRef]
- Holt-Hindle, P.; Yi, Q.F.; Wu, G.S.; Koczkur, K.; Chen, A.C. Electrocatalytic activity of nanoporous Pt–Ir materials toward methanol oxidation and oxygen reduction. J. Electrochem. Soc. 2008, 155, K5–K9. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Lin, J.-Y. Fabrication of bimetallic Pt–M (M = Fe, Co, and Ni) nanoparticle/carbon nanotube electrocatalysts for direct methanol fuel cells. J. Power Sources 2009, 188, 347–352. [Google Scholar] [CrossRef]
- Zhu, J.; Cheng, F.; Tao, Z.; Chen, J. Electrocatalytic methanol oxidation of Pt0.5Ru0.5−xSnx/C (x = 0–0.5). J. Phys. Chem. C 2008, 112, 6337–6345. [Google Scholar] [CrossRef]
- Gurau, B.; Viswanathan, R.; Liu, R.; Lafrenz, T.J.; Ley, K.L.; Smotkin, E.S.; Reddington, E.; Sapienza, A.; Chan, B.C.; Mallouk, T.E.; et al. Structural and electrochemical characterization of binary, ternary, and quaternary platinum alloy catalysts for methanol electro-oxidation1. J. Phys. Chem. B 1998, 102, 9997–10003. [Google Scholar] [CrossRef]
- Deivaraj, T.C.; Chen, W.; Lee, J.Y. Preparation of PtNi nanoparticles for the electrocatalytic oxidation of methanol. J. Mater. Chem. 2003, 13, 2555–2560. [Google Scholar] [CrossRef]
- Kang, J.; Ma, W.; Wu, J.; Pan, M. A novel catalyst Pt@NiPcTs/C: Synthesis, structural and electro-oxidation for methanol. Catal. Commun. 2009, 10, 1271–1274. [Google Scholar] [CrossRef]
- Page, T.; Johnson, R.; Hormes, J.; Noding, S.; Rambabu, B. A study of methanol electro-oxidation reactions in carbon membrane electrodes and structural properties of Pt alloy electro-catalysts by EXAFS. J. Electroanal. Chem. 2000, 485, 34–41. [Google Scholar] [CrossRef]
- Yang, H.; Coutanceau, C.; Léger, J.-M.; Alonso-Vante, N.; Lamy, C. Methanol tolerant oxygen reduction on carbon-supported Pt–Ni alloy nanoparticles. J. Electroanal. Chem. 2005, 576, 305–313. [Google Scholar] [CrossRef]
- Park, K.-W.; Choi, J.-H.; Kwon, B.-K.; Lee, S.-A.; Sung, Y.-E. Chemical and electronic Effects of Ni in Pt/Ni and Pt/Ru/Ni Alloy Nanoparticles in Methanol Electrooxidation. J. Phys. Chem. B 2002, 106, 1869–1877. [Google Scholar] [CrossRef]
- Martz, N.; Roth, C.; Fue, H. Characterization of different Pt/Metal/Complex catalysts as anode catalysts for the PEM fuel cell. J. Appl. Electrochem. 2005, 35, 85–90. [Google Scholar] [CrossRef]
- Godínez-Salomón, F.; Hallen-López, M.; Solorza-Feria, O. Enhanced electroactivity for the oxygen reduction on Ni@Pt core-shell nanocatalysts. Int. J. Hydrog. Energy 2012, 37, 14902–14910. [Google Scholar] [CrossRef]
- Di Noto, V.; Negro, E. Pt–Fe and Pt–Ni Carbon nitride-based “core–shell” ORR electrocatalysts for polymer electrolyte membrane fuel cells. Fuel Cells 2010, 10, 234–244. [Google Scholar]
- Wang, X.L.; Wang, H.; Wang, R.F.; Wang, Q.Z.; Lei, Z.Q. Carbon-supported platinum-decorated nickel nanoparticles for enhanced methanol oxidation in acid media. J. Solid State Electrochem. 2012, 16, 1049–1054. [Google Scholar] [CrossRef]
- Papadimitriou, S.; Armyanov, S.; Valova, E.; Hubin, A.; Steenhaut, O.; Pavlidou, E.; Kokkinidis, G.; Sotiropoulos, S. Methanol wxidation at Pt–Cu, Pt–Ni, and Pt–Co electrode coatings prepared by a galvanic replacement process. J. Phys. Chem. C 2010, 114, 5217–5223. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, Z.; Sun, Q.; Xie, J.; Jing, J. Synthetic core-shell Ni@Pd nanoparticles supported on graphene and used as an advanced nanoelectrocatalyst for methanol oxidation. New J. Chem. 2012, 36, 2533–2540. [Google Scholar] [CrossRef]
- Du, B.; Rabb, S.A.; Zangmeister, C.; Tong, Y. A volcano curve: Optimizing methanol electro-oxidation on Pt-decorated Ru nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 8231–8239. [Google Scholar] [CrossRef]
- Zhang, X.T.; Wang, H.; Key, J.L.; Linkov, V.; Ji, S.; Wang, X.L.; Lei, Z.Q.; Wang, R.F. Strain effect of core-shell Co@Pt/C nanoparticle catalyst with enhanced electrocatalytic activity for methanol oxidation. J. Electrochem. Soc. 2012, 159, B270–B276. [Google Scholar] [CrossRef]
- Lee, K.-S.; Yoo, S.J.; Ahn, D.; Jeon, T.-Y.; Choi, K.H.; Park, I.-S.; Sung, Y.-E. Surface structures and electrochemical activities of Pt overlayers on Ir nanoparticles. Langmuir 2011, 27, 3128–3137. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Yan, J.-M.; Han, S.; Shioyama, H.; Xu, Q. Magnetically recyclable Fe@Pt core−shell nanoparticles and their use as electrocatalysts for ammonia borane oxidation: the role of crystallinity of the core. J. Am. Chem. Soc. 2009, 131, 2778–2779. [Google Scholar] [CrossRef]
- Mihailov, L.; Spassov, T.; Bojinov, M. Effect of microstructure on the electrocatalytic activity for hydrogen evolution of amorphous and nanocrystalline Zr–Ni alloys. Int. J. Hydrog. Energy 2012, 37, 10499–10506. [Google Scholar] [CrossRef]
- Wang, H.; Jiao, X.; Chen, D. monodispersed nickel nanoparticles with tunable phase and size: Synthesis, characterization, and magnetic properties. J. Phys. Chem. C 2008, 112, 18793–18797. [Google Scholar] [CrossRef]
- Alagiri, M.; Muthamizhchelvan, C.; Ponnusamy, S. Structural and magnetic properties of iron, cobalt and nickel nanoparticles. Synth. Metals 2011, 161, 1776–1780. [Google Scholar] [CrossRef]
- Corcoran, C.J.; Tavassol, H.; Rigsby, M.A.; Bagus, P.S.; Wieckowski, A. Application of XPS to study electrocatalysts for fuel cells. J. Power Sources 2010, 195, 7856–7879. [Google Scholar] [CrossRef]
- Shui, J.-L.; Zhang, J.-W.; Li, J.C.M. Making Pt-shell Pt30Ni70 nanowires by mild dealloying and heat treatments with little Ni loss. J. Mater. Chem. 2011, 21, 6225–6229. [Google Scholar] [CrossRef]
- Zhou, W.-P.; Lewera, A.; Bagus, P.S.; Wieckowski, A. Electrochemical and electronic properties of platinum deposits on Ru(0001): Combined XPS and cyclic voltammetric study. J. Phys. Chem. C 2007, 111, 13490–13496. [Google Scholar] [CrossRef]
- Wang, Z.-B.; Yin, G.-P.; Lin, Y.-G. Synthesis and characterization of PtRuMo/C nanoparticle electrocatalyst for direct ethanol fuel cell. J. Power Sources 2007, 170, 242–250. [Google Scholar] [CrossRef]
- Liu, Z.; Lee, J.Y.; Chen, W.; Han, M.; Gan, L.M. Physical and Electrochemical characterizations of microwave-assisted polyol preparation of carbon-supported PtRu nanoparticles. Langmuir 2004, 20, 181–187. [Google Scholar] [CrossRef]
- Ding, L.X.; Li, G.R.; Wang, Z.L.; Liu, Z.Q.; Liu, H.; Tong, Y.X. Porous Ni@Pt core-shell nanotube array electrocatalyst with high activity and stability for methanol oxidation. Chemistry 2012, 18, 8386–8391. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, D.-Y.; Han, H.; Shul, Y.-G. PtRu/C-Au/TiO2 electrocatalyst for a direct methanol fuel cell. J. Power Sources 2006, 159, 484–490. [Google Scholar] [CrossRef]
- Chetty, R.; Kundu, S.; Xia, W.; Bron, M.; Schuhmann, W.; Chirila, V.; Brandl, W.; Reinecke, T.; Muhler, M. PtRu nanoparticles supported on nitrogen-doped multiwalled carbon nanotubes as catalyst for methanol electrooxidation. Electrochim. Acta 2009, 54, 4208–4215. [Google Scholar] [CrossRef]
- Mintsouli, I.; Georgieva, J.; Armyanov, S.; Valova, E.; Avdeev, G.; Hubin, A.; Steenhaut, O.; Dille, J.; Tsiplakides, D.; Balomenou, S.; et al. Pt-Cu electrocatalysts for methanol oxidation prepared by partial galvanic replacement of Cu/carbon powder precursors. Appl. Catal. B Environ. 2013, 136–137, 160–167. [Google Scholar] [CrossRef]
- Mintsouli, I.; Georgieva, J.; Valova, E.; Armyanov, S.; Kakaroglou, A.; Hubin, A.; Steenhaut, O.; Dille, J.; Papaderakis, A.; Kokkinidis, G.; et al. Pt–Ni carbon-supported catalysts for methanol oxidation prepared by Ni electroless deposition and its galvanic replacement by Pt. J. Solid State Electrochem. 2013, 17, 435–443. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kang, J.; Wang, R.; Wang, H.; Liao, S.; Key, J.; Linkov, V.; Ji, S. Effect of Ni Core Structure on the Electrocatalytic Activity of Pt-Ni/C in Methanol Oxidation. Materials 2013, 6, 2689-2700. https://doi.org/10.3390/ma6072689
Kang J, Wang R, Wang H, Liao S, Key J, Linkov V, Ji S. Effect of Ni Core Structure on the Electrocatalytic Activity of Pt-Ni/C in Methanol Oxidation. Materials. 2013; 6(7):2689-2700. https://doi.org/10.3390/ma6072689
Chicago/Turabian StyleKang, Jian, Rongfang Wang, Hui Wang, Shijun Liao, Julian Key, Vladimir Linkov, and Shan Ji. 2013. "Effect of Ni Core Structure on the Electrocatalytic Activity of Pt-Ni/C in Methanol Oxidation" Materials 6, no. 7: 2689-2700. https://doi.org/10.3390/ma6072689





