Synthesis of Carbon Nanotubes of Few Walls Using Aliphatic Alcohols as a Carbon Source
Abstract
:1. Introduction
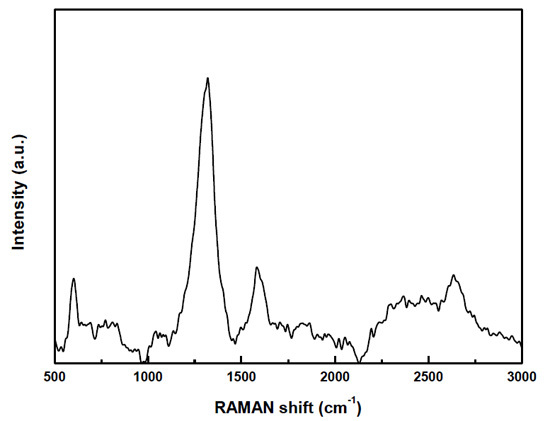
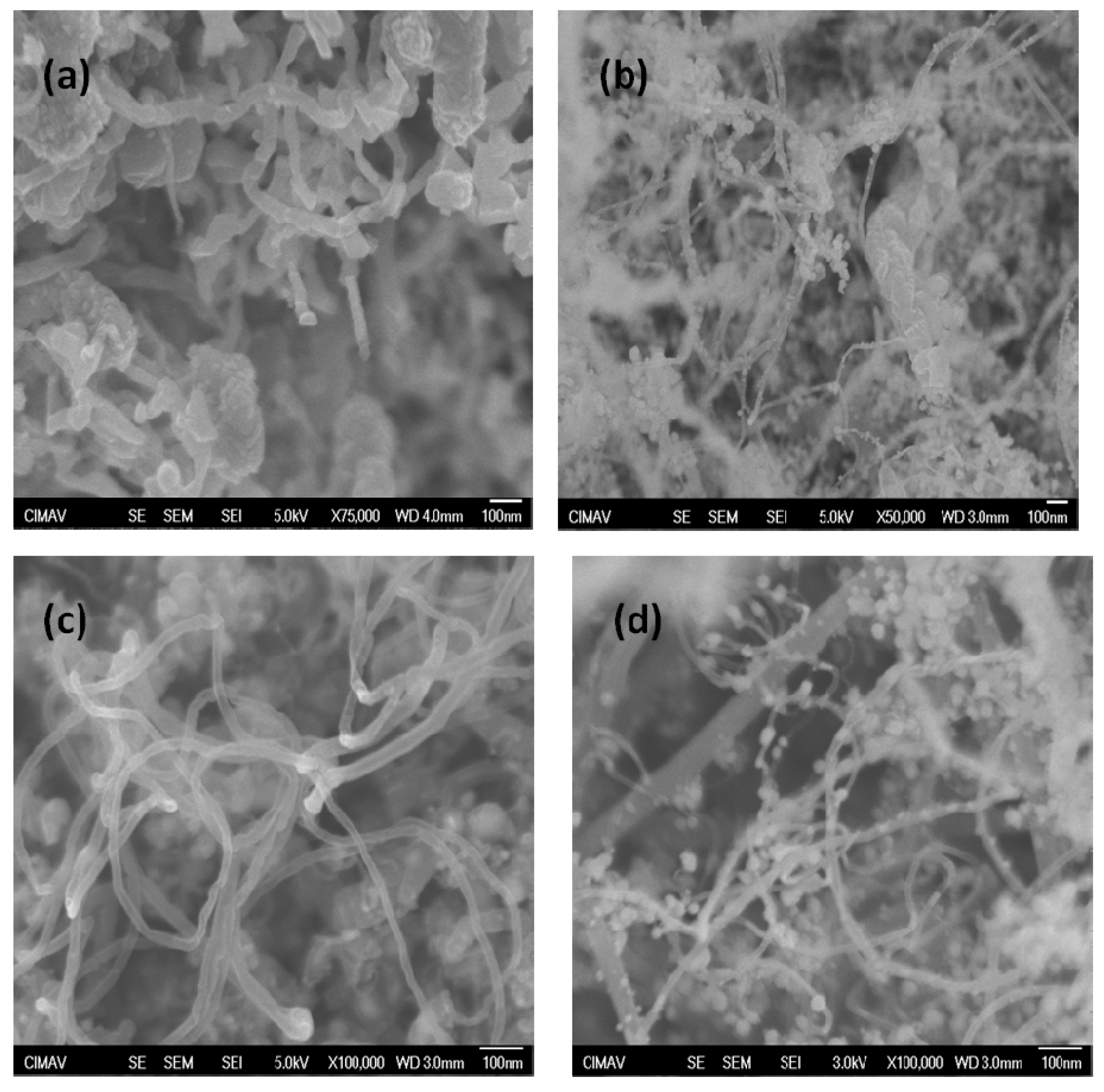
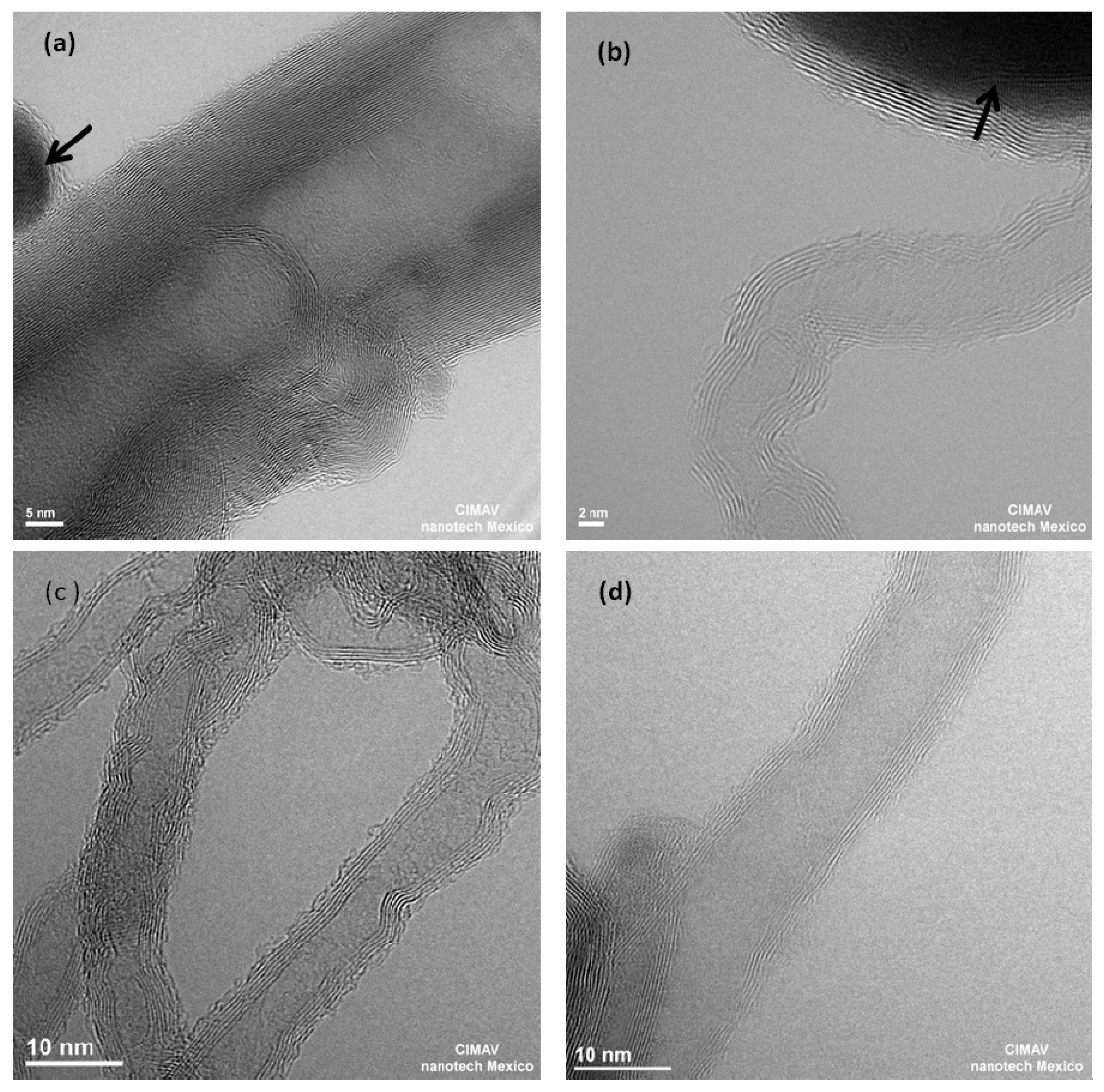
2. Results and Discussion
| alcohol | average external diameter | average length | average number of walls |
|---|---|---|---|
| nm | μm | ||
| Methanol (CH4O) | 24 | 0.5 | 35 |
| Ethanol (C2H6O) | 18 | 1.2 | 10 |
| Propanol (C3H8O) | 15 | 2.0 | 8 |
| Butanol (C4H10O) | 16 | 2.2 | 6 |
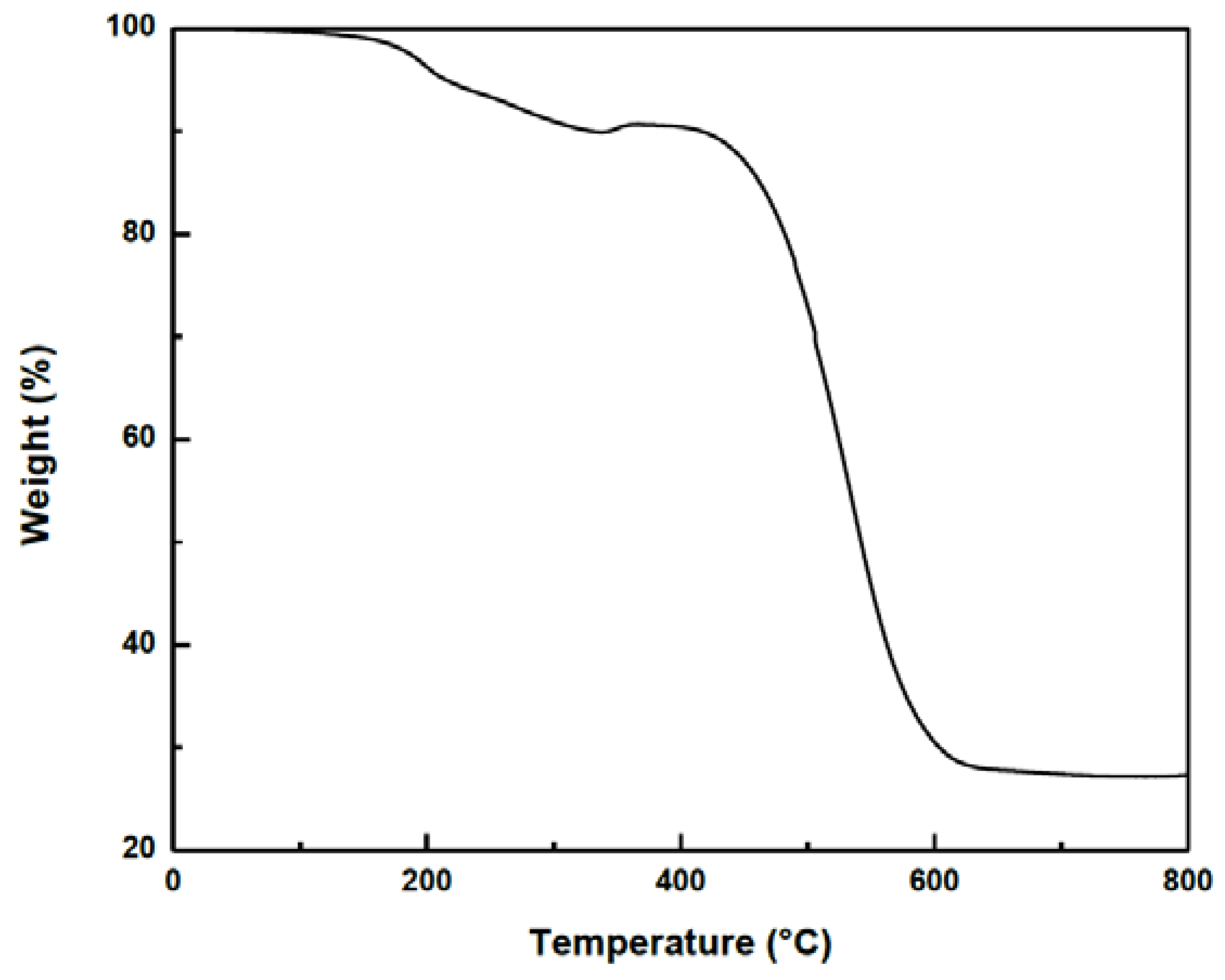

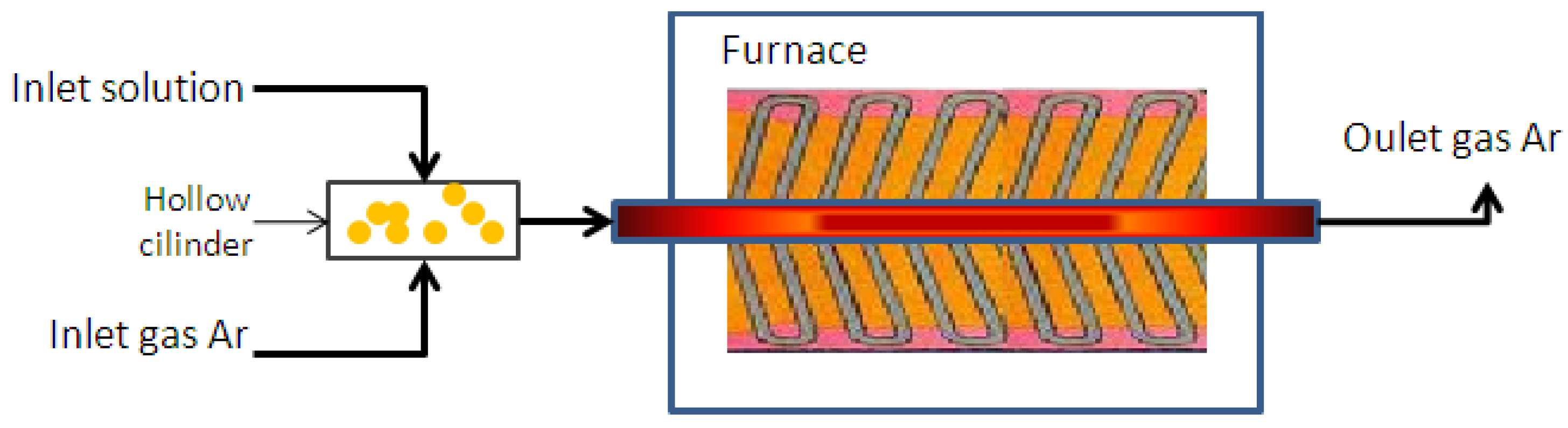
3. Experimental
4. Conclusions
Acknowledgments
References
- Bronikowski, M.J.; Willis, P.A.; Colbert, D.T.; Smith, K.A.; Smalley, R.E. Gas-phase production of carbon single-walled nanotubes from carbon monoxide via the HiPco process: A parametric study. J. Vac. Sci. Technol. A 2001, 19, 1800–1805. [Google Scholar] [CrossRef]
- Tsai, S.H.; Chao, C.W.; Lee, C.L.; Shih, H.C. Bias-enhanced nucleation and growth of the aligned carbon nanotubes with open ends under microwave plasma synthesis. Appl. Phys. Lett. 1999, 74, 3462–3464. [Google Scholar] [CrossRef]
- Hutchison, J.L.; Kiselev, N.A.; Krinichnaya, E.P.; Krestinin, A.V.; Loutfy, R.O.; Morawsky, A.P.; Muradyan, V.E.; Obraztsova, E.D.; Sloan, J.; Terekhov, S.V.; Zakharov, D.N. Double-walled carbon nanotubes fabricated by a hydrogen arc discharge method. Carbon 2001, 39, 761–770. [Google Scholar] [CrossRef]
- Aguilar Elguezabal, A.; Antúnez, W.; Alonso, G.; Paraguay Delgado, F.; Espinosa Magaña, F.; Miki Yoshida, M. Study of carbon nanotubes synthesis by spray pyrolysis and model of growth. Diam. Relat. Mater. 2006, 15, 1329–1335. [Google Scholar]
- Galván, D.H.; Aguilar Elguezabal, A.; Alonso, G. High resolution TEM studies of carbon nanotubes produced by spray pyrolysis. Opt. Mater. 2006, 29, 140–143. [Google Scholar] [CrossRef]
- Bystrzejewski, M.; Huczko, A.; Byszewski, P.; Domanska, M.; Rummeli, M.H.; Gemming, T.; Lange, H. Systematic Studies on Carbon Nanotubes Synthesis from Aliphatic Alcohols by the CVD Floating Catalyst Method. Fuller. Nanotubes Carbon Nanostruct. 2009, 17, 298–307. [Google Scholar] [CrossRef]
- Bepete, G.; Tetana, Z.N.; Linder, S.; Rummeli, M.H.; Chiguvare, A.; Coville, N. The use of aliphatic alcohol chain length to control the nitrogen type and content in nitrogen doped carbon nanotubes. Carbon 2013, 52, 316–325. [Google Scholar] [CrossRef]
- Segura Cardenas, E.; Reyes Reyes, M.; Lopez Sandoval, R. The Effects of Varying the Content of Alcohol in the Reaction Mixture on the Graphitization of MWCNTs and their surface Functionalization. J. Phys. Chem. C 2012, 116, 9783–9792. [Google Scholar]
- Li, Y.; Kim, W.; Zhang, Y.; Rolandi, M.; Wang, D.; Hongjie, D. Growth of Single-Walled Carbon Nanotubes from Discrete Catalytic Nanoparticles of Various Sizes. J. Phys. Chem. B 2001, 105, 11424–11431. [Google Scholar] [CrossRef]
- Unalan, H.; Chhowalla, M. Investigation of single-walled carbon nanotube growth parameters using alcohol chemical vapor deposition. Nanotechnology 2005, 16, 2153–2155. [Google Scholar] [CrossRef]
- Li, Y.; Cui, R.; Ding, L.; Liu, Y.; Zhou, W.; Zhang, Y.; Jin, Z.; Peng, F.; Liu, J. How catalysts affect the growth of single-walled carbon nanotubes on substrates. Adv. Mater. 2010, 22, 1508–1515. [Google Scholar] [CrossRef]
- Basaev, A.S.; Blagov, E.V.; Galperin, V.A.; Pavlov, A.A.; Shaman, U.P.; Shamanaev, A.A.; Shamanaev, S.V.; Prihodko, A.S. Specificities of growth of topological arrays of carbon nanotubes. Nanotechnol. Russ. 2012, 7, 22–27. [Google Scholar] [CrossRef]
- Oida, S.; Sakai, A.; Nakatsuka, O.; Ogawa, M.; Zaima, S. Effect of alcohol sources on synthesis of single-walled carbon nanotubes. Appl. Surf. Sci. 2008, 254, 7697–7702. [Google Scholar] [CrossRef]
- Inoue, S.; Kikuchi, Y.; Matsumara, Y. Effect of catalyst combination on growth of single-walled carbon nanotubes. Diam. Relat. Mater. 2008, 17, 1888–1890. [Google Scholar] [CrossRef]
- Okamoto, A.; Shinohara, H. Control of Diameter Distribution of Single-walled Carbon Nanotubes. R D Rev. Toyota CRDL 2005, 40, 22–28. [Google Scholar]
- Liu, B.C.; Lyu, S.C.; Jung, S.I.; Kang, H.K.; Yang, C.W.; Park, J.W.; Park, C.Y.; Lee, C.J. Single-walled carbon nanotubes produced by catalytic chemical vapor deposition of acetylene over Fe–Mo/MgO catalyst. Chem. Phys. 2004, 383, 104–108. [Google Scholar]
- Cele, L.M.; Neil, J.C. The negative effects of alcohols on carbon nanotubes synthesis in a nebulised spray pyrolysis process. Carbon 2009, 47, 1824–1832. [Google Scholar] [CrossRef]
- Botello-Méndez, A.; Campos-Delgado, J.; Morelos-Gómez, A.; Romero-Herrera, J.M.; Rodríguez, A.G.; Navarro, H.; Vidal, M.A.; Terrones, H.; Terrones, M. Controlling the dimensions, reactivity and cristallinity of multiwalled carbon nanotubes using low ethanol concentrations. Chem. Phys. Lett. 2008, 453, 55–61. [Google Scholar] [CrossRef]
- Zamolo Valeria, A.; Vazquez, E.; Prato, M. Carbon Nanotubes: Synthesis, Structure, Functionalization and Characterization. Top. Curr. Chem. 2013. [Google Scholar] [CrossRef]
- Pócsik, I.; Hundhausen, M.; Koós, M.; Ley, L. Origin of the D peak in the Raman spectrum of microcrystalline graphite. J. Non-Cryst. Solids 1998, 227, 1083–1086. [Google Scholar] [CrossRef]
- Matthews, M.J.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Endo, M. Origin of dispersive effects of the Raman D band in carbon materials. Phys. Rev. B 1999, 59, R6585–R6588. [Google Scholar] [CrossRef]
- Thomsen, C.; Reich, S. Double resonant Raman scattering in graphite. Phys. Rev. Lett. 2000, 85, 5214–5217. [Google Scholar] [CrossRef]
- Brown, S.D.M.; Jorio, A.; Dresselhaus, M.S.; Dresselhaus, G. Observations of the D-band feature in the Raman spectra of carbon nanotubes. Phys. Rev. B 2001, 64, 073403:1–073403:4. [Google Scholar]
- Bokobza, L.; Zhang, J. Raman spectroscopic characterization of multiwall carbon nanotubes and of composites. Express Polym. Lett. 2012, 6, 601–608. [Google Scholar] [CrossRef]
- Ouyanga, Y.; Conga, L.M.; Chena, L.; Liub, Q.X.; Fangc, Y. Raman study on single-walled carbon nanotubes and multi-walled carbon nanotubes with different laser excitation energies. Physica E 2008, 40, 2386–2389. [Google Scholar] [CrossRef]
- Jorio, A.; Pimienta, M.A.; Souza Filho, A.G.; Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Characterizing carbon nanotubes samples with resonance Raman scattering. New J. Phys. 2003, 5. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Hofmann, M. The big picture of Raman scattering in carbon nanotubes. Vib. Spectrosc. 2007, 45, 711–781. [Google Scholar] [CrossRef]
- Cheng, H.M.; Li, F.; Su, G.H.; Pan, Y.; He, L.L.; Sun, X.; Dresselhaus, M.S. Large-Scale and Low-Cost Synthesis of Single-Walled Carbon Nanotubes by the Catalytic Pyrolysis of Hydrocarbons. Appl. Phys. Lett. 1998, 72, 3282–3284. [Google Scholar] [CrossRef]
- Murayana, S.; Kojima, R.; Miyauchi, Y.; Chiashi, S.; Kohno, M.I. Low-temperature synthesis of high-purity single-walled carbon nanotubes from alcohol. Chem. Phys. Lett. 2002, 360, 229–234. [Google Scholar] [CrossRef]
- Khavarian, M.; Chai, S.P.; Tan, S.H.; Mohamed, A.R. Effect of Different Parameters on the Morphology of Carbon Nanotubes Structures Grown by Floating Catalyst Method. J. Appl. Sci. 2011, 11, 2382–2387. [Google Scholar] [CrossRef]
- Asaro, M.; Smith, R.M. Gas to Liquid Technologies. Foss. Energy 2013, 247–310. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ordoñez-Casanova, E.G.; Román-Aguirre, M.; Aguilar-Elguezabal, A.; Espinosa-Magaña, F. Synthesis of Carbon Nanotubes of Few Walls Using Aliphatic Alcohols as a Carbon Source. Materials 2013, 6, 2534-2542. https://doi.org/10.3390/ma6062534
Ordoñez-Casanova EG, Román-Aguirre M, Aguilar-Elguezabal A, Espinosa-Magaña F. Synthesis of Carbon Nanotubes of Few Walls Using Aliphatic Alcohols as a Carbon Source. Materials. 2013; 6(6):2534-2542. https://doi.org/10.3390/ma6062534
Chicago/Turabian StyleOrdoñez-Casanova, Elsa G., Manuel Román-Aguirre, Alfredo Aguilar-Elguezabal, and Francisco Espinosa-Magaña. 2013. "Synthesis of Carbon Nanotubes of Few Walls Using Aliphatic Alcohols as a Carbon Source" Materials 6, no. 6: 2534-2542. https://doi.org/10.3390/ma6062534