Adsorption Properties of Doxorubicin Hydrochloride onto Graphene Oxide: Equilibrium, Kinetic and Thermodynamic Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of GO and DOX/GO
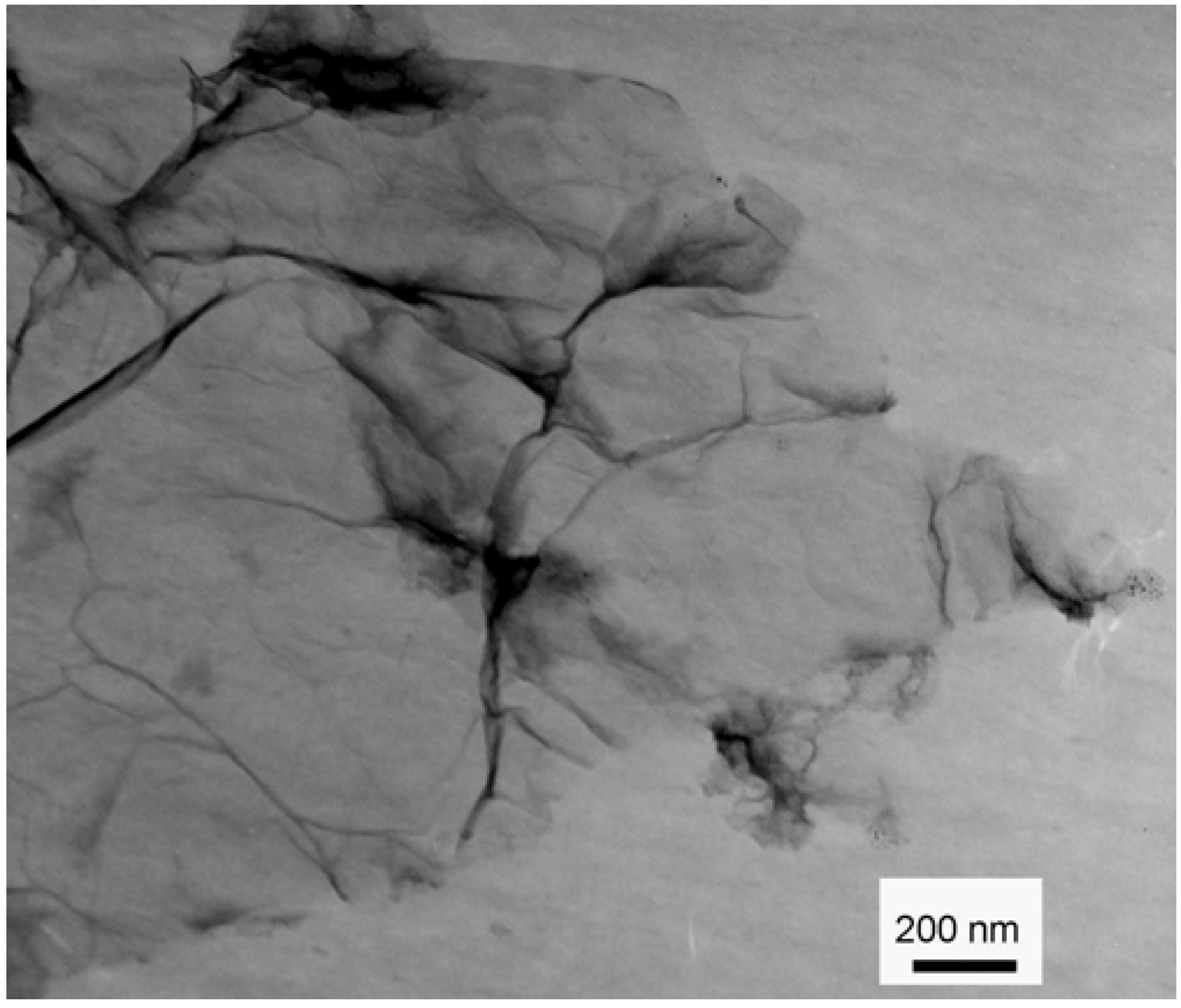
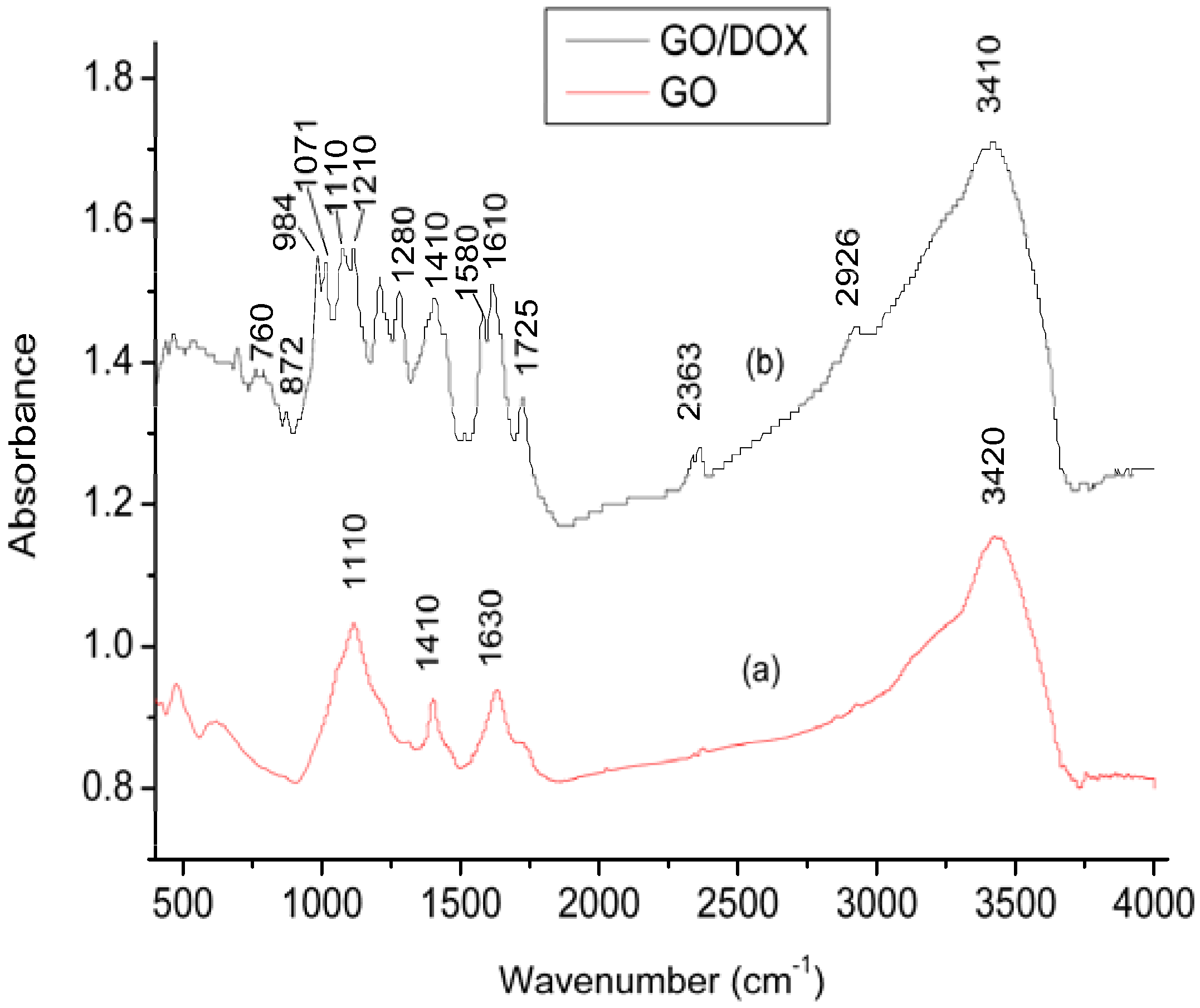
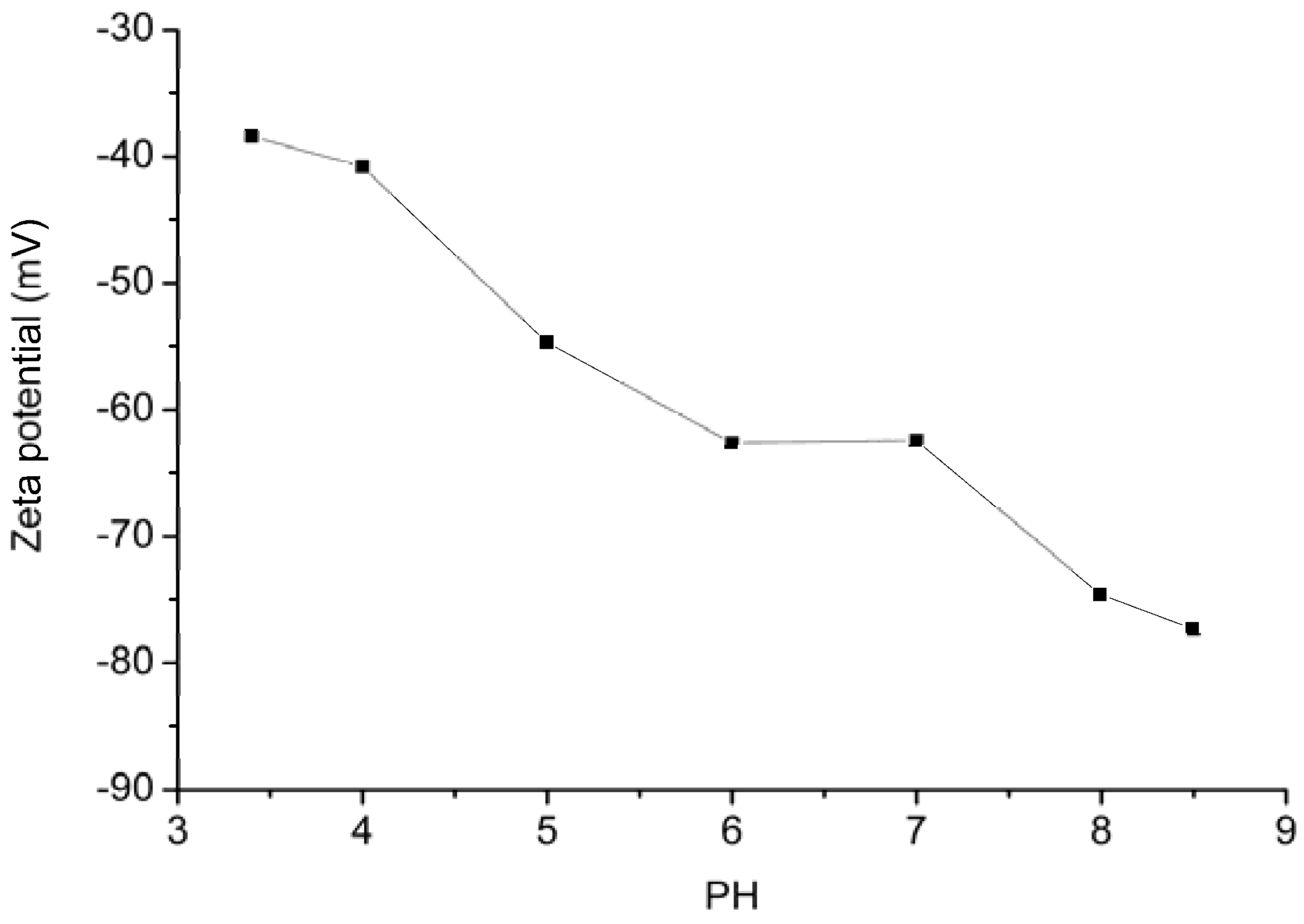
2.2. Effect of pH
2.3. Effect of Adsorbent Dosage
2.4. Effect of Contact Time

2.5. Effect of Temperature
2.6. Kinetic Studies
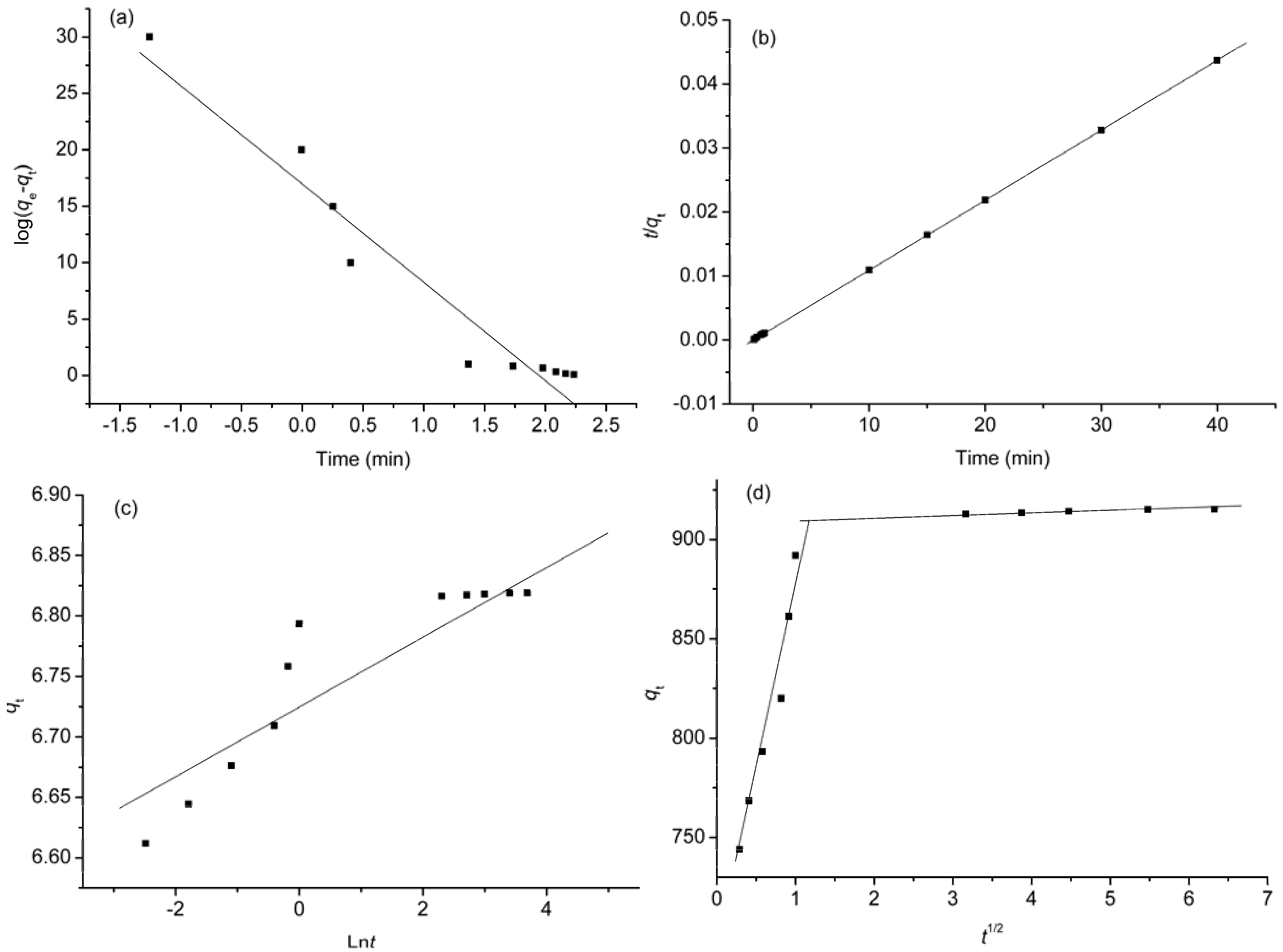
| Kinetic model | Parameters | Values |
|---|---|---|
| Pseudo-first-order | qe (mg/g) × 1016 | 7.6 × 1016 |
| k1 (1/min) | 19.56 | |
| R2 | 0.9467 | |
| Pseudo-second-order | qe (mg/g) | 909.09 |
| K2 (g/mg min) | 0.03 | |
| R2 | 1.0000 | |
| Elovich | Lnα | 229.11 |
| Β | 34.60 | |
| R2 | 0.7801 | |
| Intra-particle diffusion | kI | 190.85 |
| CI | 685.82 | |
| R2 | 0.9529 | |
| kII | 0.84 | |
| CII | 910.28 | |
| R2 | 0.9353 |
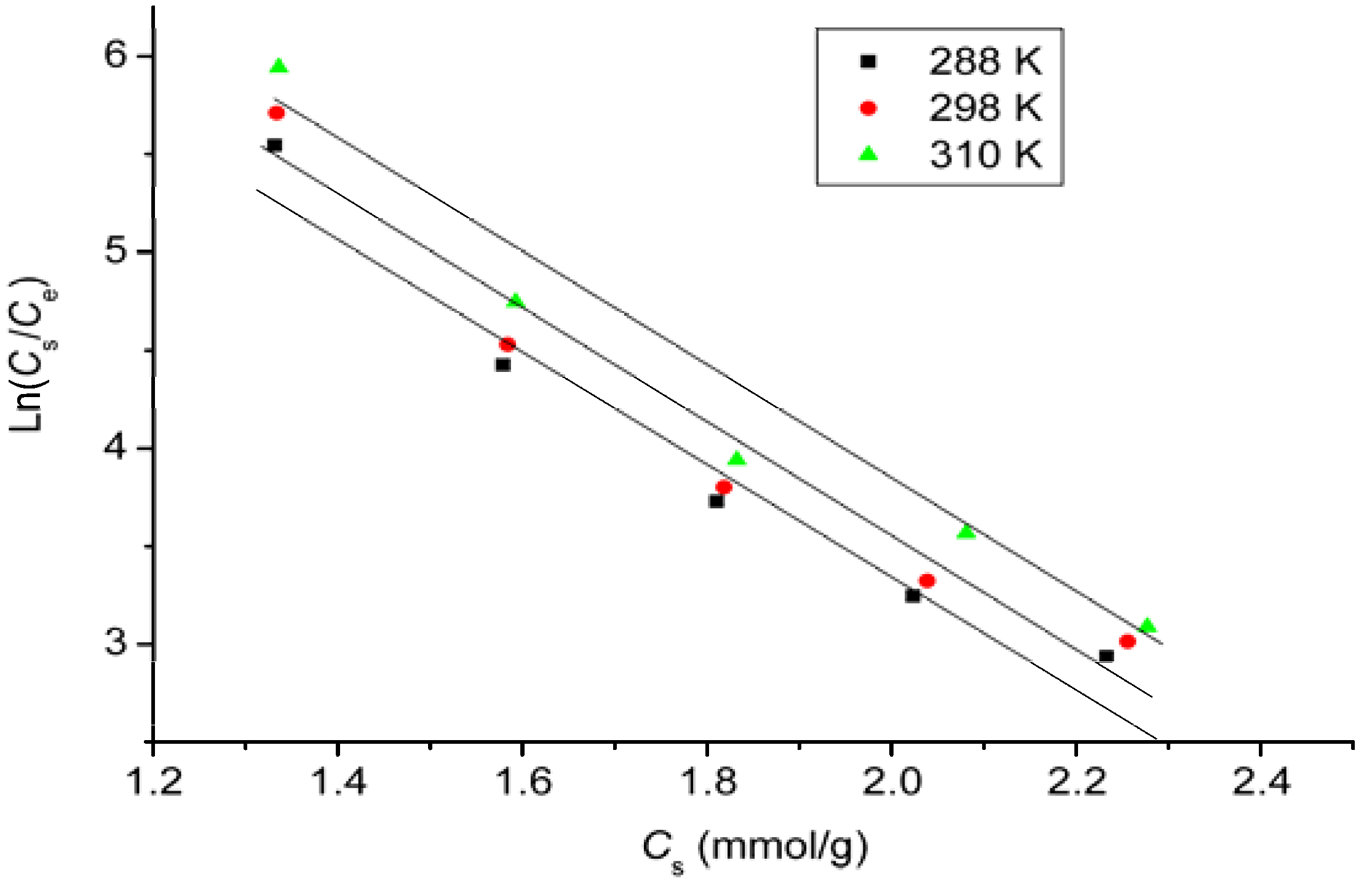
2.7. Adsorption Isotherms
| Temperature (K) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qmax | kL | R2 | 1/n | kF | R2 | |
| 288 | 1428.57 | 0.20 | 0.9930 | 0.16 | 632.96 | 0.9884 |
| 298 | 1428.57 | 0.22 | 0.9924 | 0.16 | 651.06 | 0.9851 |
| 310 | 1428.57 | 0.26 | 0.9941 | 0.16 | 678.78 | 0.9866 |
2.8. Thermodynamic Study
| Thermodynamic constant | Temperature (K) | ||
|---|---|---|---|
| 288 | 298 | 310 | |
| K0 | 9256 | 10952 | 14657 |
| ΔG0 (kJ/mol) | −21.87 | −23.04 | −24.72 |
| ΔH0 (kJ/mol) | 15.52 | 15.52 | 15.52 |
| ΔS0 (J/mol·K) | 129.83 | 129.36 | 129.81 |
2.9. Adsorption Mechanisms
- (a)
- The unique structure of GO. GO is a one-atom-thick two-dimensional individual sheet. Every exposed carbon atom has the opportunity to contact and interact with DOX molecules. The adsorption isotherm data, fitted well with the Langmuir equation (Table 2) indicates that a monolayer of DOX molecules is adsorbed homogeneously on the edge and two sides of a GO sheet.
- (b)
- Electrostatic interaction. GO has a negatively charged surface. The positively charged DOX molecules can be easily attracted and adsorbed onto the surface of GO by means of electrostatic attraction [44].
- (c)
- (c) The role of hydrogen bonding. The edge of GO has many oxygen-containing functional groups such as −COOH, −C=O and −OH. The functional groups make GO more hydrophilic and suitable for the adsorption of relatively low molecular weight compounds [45] through the role of hydrogen bonding. The hydrogen bonding between DOX and GO maybe exists in four formats: (i) –COOH of GO and –OH of DOX; (ii) –COOH of GO and –NH2 of DOX; (iii) –OH of GO and –OH of DOX; and (iv) –OH of GO and –NH2 of DOX [25].
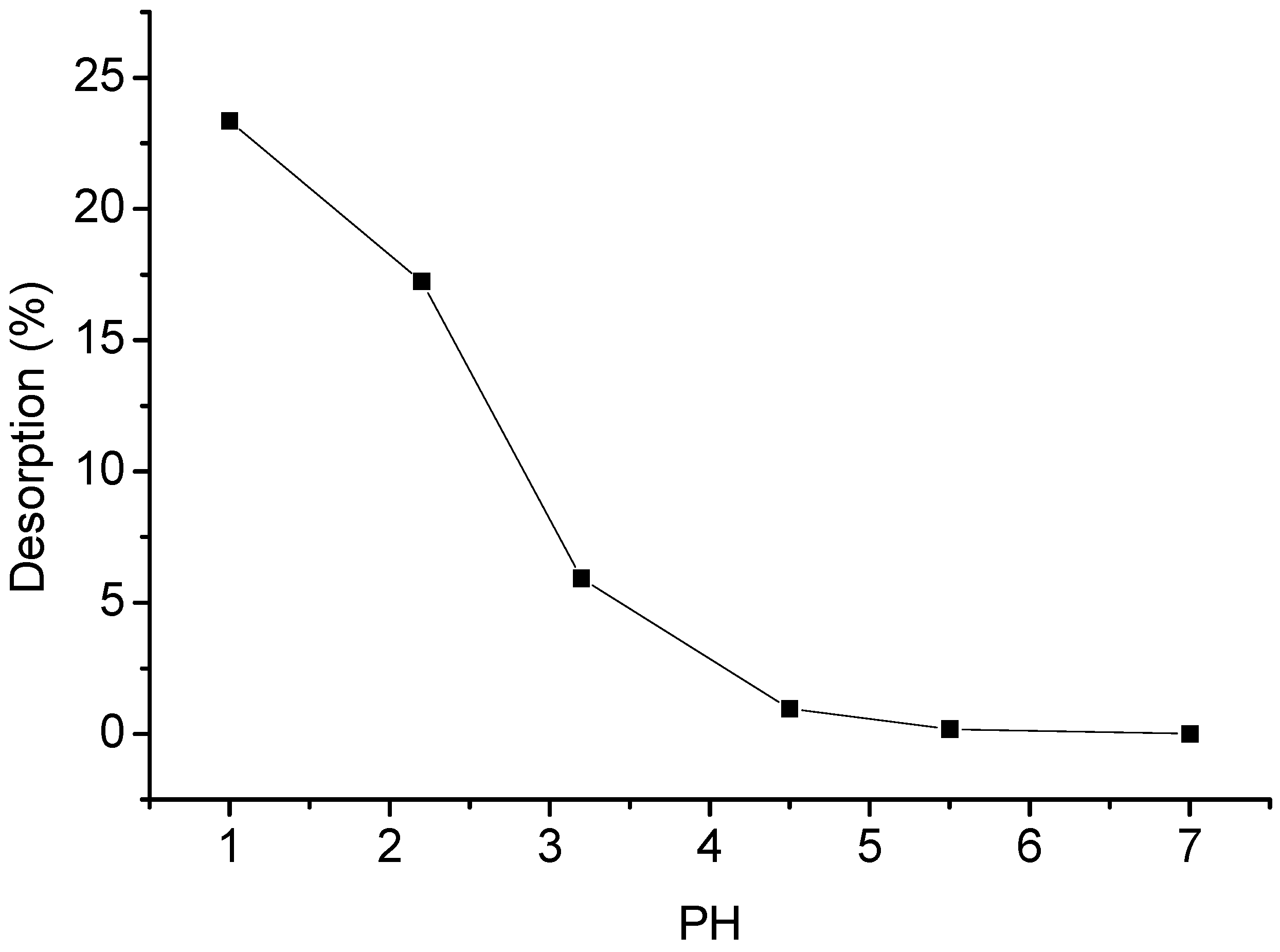
2.10. Desorption Studies
3. Experimental Section
3.1. Materials
3.2. Adsorbent Characterization
3.3. Adsorption and Desorption Experiments
4. Conclusions
Acknowledgments
References
- Vashist, S.K.; Zheng, D.; Pastorin, G.; Al-Rubeaan, K.; Luong, J.H.T.; Sheu, F.S. Delivery of drugs and biomolecules using carbon nanotubes. Carbon 2011, 49, 4077–4097. [Google Scholar] [CrossRef]
- Janes, K.A.; Fresneau, M.P.; Marazuela, A.; Fabra, A.; Alonso, M.J. Chitosan nanoparticles as delivery systems for doxorubicin. J. Control. Release 2011, 73, 255–267. [Google Scholar] [CrossRef]
- Niu, G.; Cogburn, B.; Hughes, J. Preparation and characterization of doxorubicin liposomes. Methods Mol. Biol. 2010, 624, 211–219. [Google Scholar] [PubMed]
- Yi, Y.; Kim, J.H.; Kang, H.W.; Oh, H.S.; Kim, S.W.; Seo, M.H. A polymeric nanoparticle consisting of mPEG-PLA-Toco and PLMA-COONa as a drug carrier: Improvements in cellular uptake and biodistribution. Pharm. Res. 2005, 22, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; He, Q.; Gao, Y.; Shi, J.; Li, Y. Mesoporous silica nanoparticles loading doxorubicin reverse multidrug resistance: Performance and mechanism. Nanoscale 2011, 3, 4314–4322. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.L.; Guo, Y.N.; Liu, J.; Liu, Y.L.; Huo, Q.H. Size-tunalbe and functional core-sheel structured silica nanoparticles for drug release. J. Phys. Chem. C 2010, 114, 2519–2523. [Google Scholar] [CrossRef]
- Chen, Z.; Pierre, D.; He, H.; Tan, S.H.; Pham-Huy, C.; Hong, H.; Huang, J.L. Adsorption behavior of epirubicin hydrochloride on carboxylated carbon nanotubes. Int. J. Pharm. 2011, 405, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, K.; Feng, L.Z.; Liu, Z. In vitro and in vivo behaviors of dextran functionalized graphene. Carbon 2011, 49, 4040–4049. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X.; Huang, F.; Li, K. Fabrication of high-concentration and stable aqueous suspensions of graphene nanosheets by noncovalent functionalization with lignin and cellulose derivatives. J. Phys. Chem. C 2010, 114, 3811–3816. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Fang, Y.; Dong, S. Reducing Sugar: New functional molecules for the green synthesis of graphene nanosheets. ACS Nano 2010, 4, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Robinson, J.T.; Sun, X.M.; Dai, H.J. PEGylated nano-graphene oxide for delivery of water insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.L.; Yang, S.T.; Liu, J.H.; Dong, E.Y.; Wang, Y.W.; Cao, A.N.; Liu, Y.F.; Wang, H.F. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2010, 200, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.J.; Lü, L.L.; Li, H.W.; Luo, F. The adsorption properties of Pb(II) and Cd(II) on functionalized graphene prepared by electrolysis method. J. Hazard. Mater. 2010, 183, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Chang, Y.L.; Wang, H.F.; Liu, G.B.; Chen, S.; Wang, Y.W.; Liu, Y.F.; Cao, A.N. Folding/aggregation of graphene oxide and its application in Cu2+ removal. J. Colloid Interface Sci. 2010, 351, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Dwivedi, V.; Chi, C.Y.; Wu, J.S. Graphene oxide/ferric hydroxide composites for efficient arsenate removal from drinking water. J. Hazard. Mater. 2010, 182, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.H.; Li, Y.H.; Du, Q.J.; Sun, J.K.; Jiao, Y.Q.; Yang, G.M.; Wang, Z.H.; Xia, Y.Z.; Zhang, W.; Wang, K.; et al. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf. B 2012, 90, 197–203. [Google Scholar] [CrossRef]
- Zhao, G.X.; Jiang, L.; He, Y.H.; Li, J.H.; Dong, H.L.; Wang, X.K.; Hu, W.P. Sulfonated graphene for persistent aromatic pollutant management. Adv. Mater. 2011, 23, 3959–3963. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.X.; Li, J.X.; Wang, X.K. Kinetic and thermodynamic study of 1-naphthol adsorption from aqueous solution to sulfonated graphene nanosheets. Chem. Eng. J. 2011, 173, 185–190. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.J.; Shah, M.S.; Su, X.G. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.B.; Shi, Z.G.; Gao, Q.; Feng, Y.Q. Magnetic retrieval of graphene: Extraction of sulfonamide antibiotics from environmental water samples. J. Chromatogr. A 2011, 1218, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Liu, T.H.; Du, Q.J.; Sun, J.K.; Xia, Y.Z.; Wang, Z.; Zhang, W.; Wang, K.; Zhu, H.; Wu, D. Adsorption of cationic red X-GRL from aqueous solutions by graphene: Equilibrium, kinetics and thermodynamics study. Chem. Biochem. Eng. Q. 2011, 25, 483–491. [Google Scholar]
- Bradder, P.; Ling, S.K.; Wang, S.; Liu, S. Dye adsorption on layered graphite oxide. J. Chem. Eng. Data 2011, 56, 138–141. [Google Scholar] [CrossRef]
- Murugan, A.V.; Muraliganth, T.; Manthiram, A. Facile microwave-solvothermal synthesis of graphene nanosheets and their polyaniline nanocomposites for energy storage. Chem. Mater. 2009, 21, 5004–5006. [Google Scholar] [CrossRef]
- Wu, M.; Kempaiah, R.; Huang, P.J.J.; Maheshwari, V.; Liu, J. Adsorption and desorption of DNA on graphene oxide studied by fluorescently labeled oligonucleotides. Langmuir 2011, 27, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Depan, D.; Shah, J.; Misra, R.D.K. Controlled release of drug from folate-decorated and graphene mediated drug delivery system: Synthesis, loading efficiency, and drug release response. Mater. Sci. Eng. C 2011, 31, 1305–1312. [Google Scholar] [CrossRef]
- Kayal, S.; Ramanujan, R.V. Doxorubicin loaded PVA coated iron oxide nanoparticles for targeted drug delivery. Mater. Sci. Eng. C 2010, 30, 484–490. [Google Scholar] [CrossRef]
- Yuan, Q.; Sha, J.; Hein, S.; Misra, R.D. Controlled and extended drug release behavior of chitosan-based nanoparticle carrier. Acta Biomater. 2010, 6, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Du, Q.J.; Wang, X.D.; Zhang, P.; Wang, D.C.; Wang, Z.H.; Xia, Y.Z. Removal of lead from aqueous solution by activated carbon prepared from enteromorpha prolifera by zinc chloride activation. J. Hazard. Mater. 2010, 183, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Wang, S.G.; Cao, A.Y.; Zhao, D.; Zhang, X.F.; Xu, C.L.; Luan, Z.K.; Ruan, D.B.; Liang, J.; Wu, D.H.; et al. Adsorption of fluoride from water by amorphous alumina supported on carbon nanotubes. Chem. Phys. Lett. 2001, 350, 412–416. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.M.; Nakayama-Ratchford, N.; Dai, H.J. Superamolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano 2007, 1, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Balasubramanian, R.; Iyer, C.S. Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu (II) from aqueous solutions. Bioresour. Technol. 2007, 98, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Unuabonah, E.I.; Adebowale, K.O.; Olu-Owolabi, B.I.; Yang, L.Z.; Kong, L.X. Adsorption of Pb(II) and Cd(II) from aqueous solutions onto sodium tetraborate modified kaolinite clay: Equilibrium and thermodynamic studies. Hydrometallurgy 2008, 63, 1–9. [Google Scholar] [CrossRef]
- Gerçel, Ö.; Gerçel, H.F. Adsorption of lead (II) ions from aqueous solutions by activated carbon prepared from biomass plant material of Euphorbia rigida. Chem. Eng. J. 2007, 132, 289–297. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour. Technol. 2007, 98, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Liu, X.; Han, M.; Ma, W. Adsorption kinetic character of copper ions onto a modified chitosan transparent thin membrane from aqueous solution. J. Hazard. Mater. 2010, 182, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die Adsorption in Lösungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Sanson, C.; Schatz, C.; Le Meins, J.F.; Soum, A.; Thévenot, J.; Garanger, E.; Lecommandoux, S. A simple method to achieve high doxorubicin loading in biodegradable polymersomes. J. Control. Release 2010, 147, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.H.; Le, H.C.; Lin, J.Y.; Golzarian, J. Golzarian, Doxorubicin loading and eluting characteristics of bioresorbable hydrogel microspheres: In vitro study. Int. J. Pharm. 2011, 409, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.N.; Yao, P.; He, F.; Yu, C.L.; Huang, C. Nanoparticles with dextran/chitosan shell and BSA/chitosan core-doxorubicin loading and delivery. Int. J. Pharm. 2010, 393, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Adsorption of phenolic compounds by carbon nanotubes: Role of aromaticity and substitution of hydroxyl groups. Environ. Sci. Technol. 2008, 42, 7254–7259. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, S.; Zhao, X.; Li, Y.; Du, Q.; Sun, J.; Wang, Y.; Wang, X.; Xia, Y.; Wang, Z.; Xia, L. Adsorption Properties of Doxorubicin Hydrochloride onto Graphene Oxide: Equilibrium, Kinetic and Thermodynamic Studies. Materials 2013, 6, 2026-2042. https://doi.org/10.3390/ma6052026
Wu S, Zhao X, Li Y, Du Q, Sun J, Wang Y, Wang X, Xia Y, Wang Z, Xia L. Adsorption Properties of Doxorubicin Hydrochloride onto Graphene Oxide: Equilibrium, Kinetic and Thermodynamic Studies. Materials. 2013; 6(5):2026-2042. https://doi.org/10.3390/ma6052026
Chicago/Turabian StyleWu, Shaoling, Xindong Zhao, Yanhui Li, Qiuju Du, Jiankun Sun, Yonghao Wang, Xin Wang, Yanzhi Xia, Zonghua Wang, and Linhua Xia. 2013. "Adsorption Properties of Doxorubicin Hydrochloride onto Graphene Oxide: Equilibrium, Kinetic and Thermodynamic Studies" Materials 6, no. 5: 2026-2042. https://doi.org/10.3390/ma6052026




