Simple Preparation of Novel Metal-Containing Mesoporous Starches †
Abstract
:1. Introduction

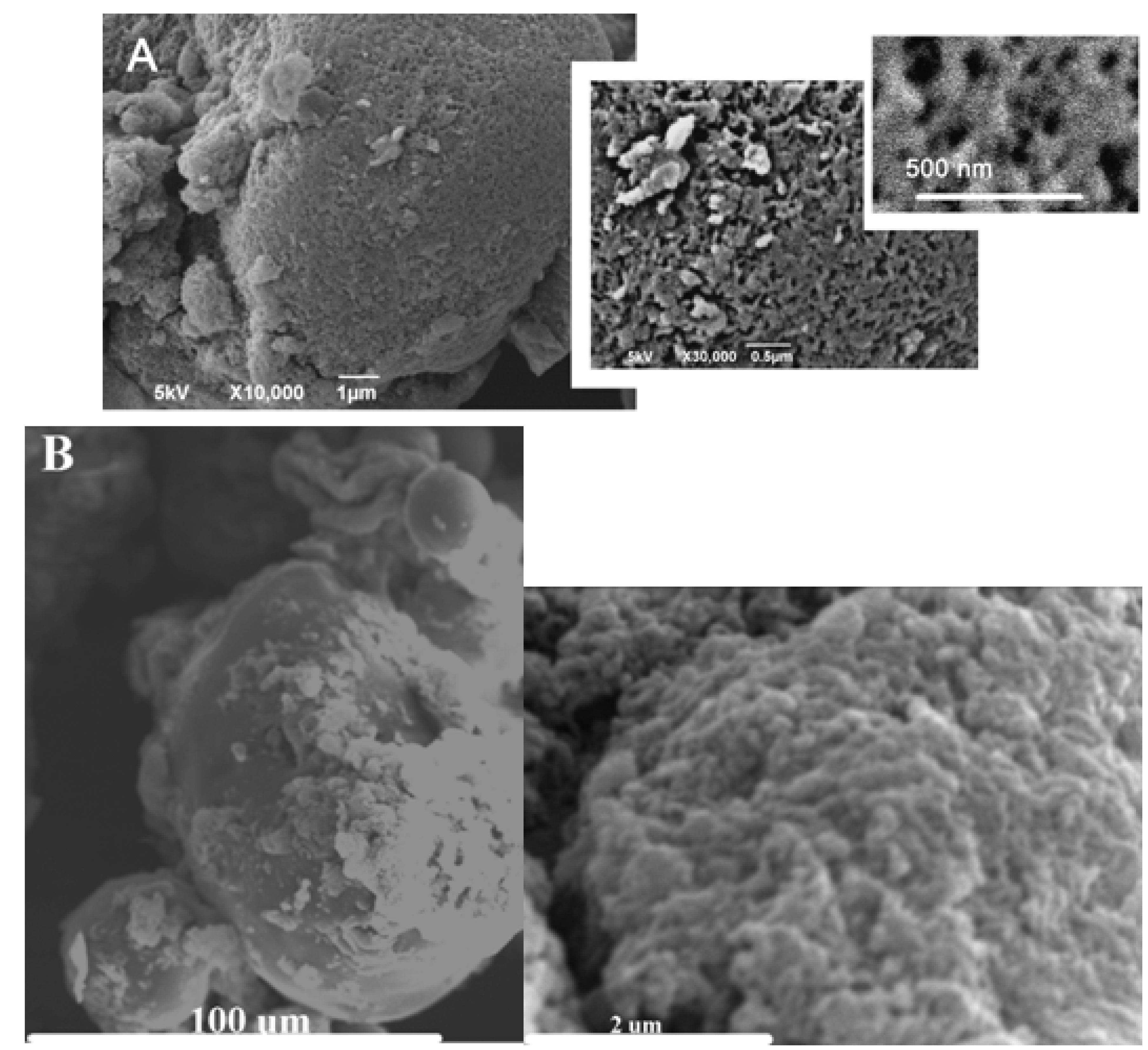
2. Results and Discussion
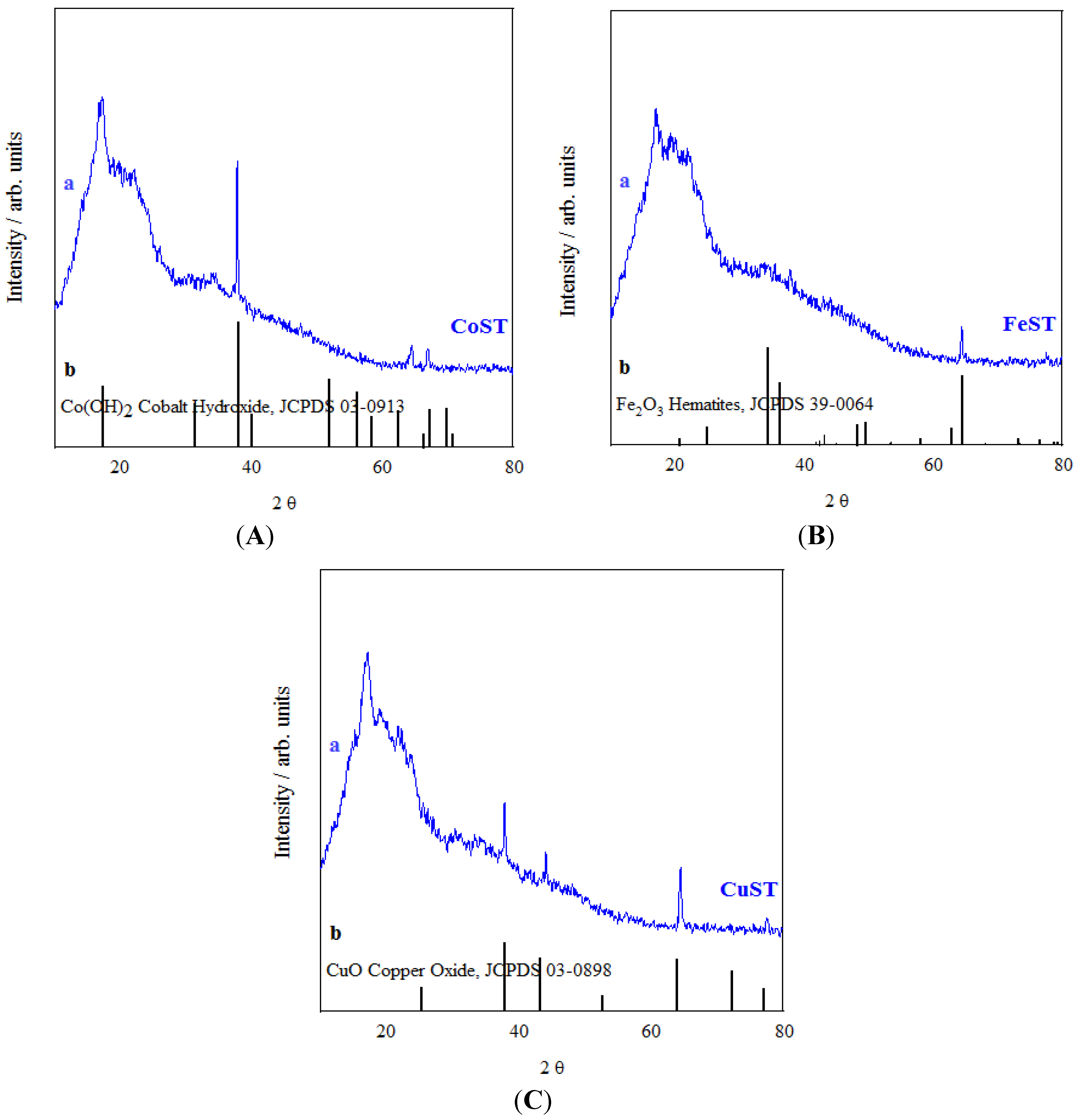
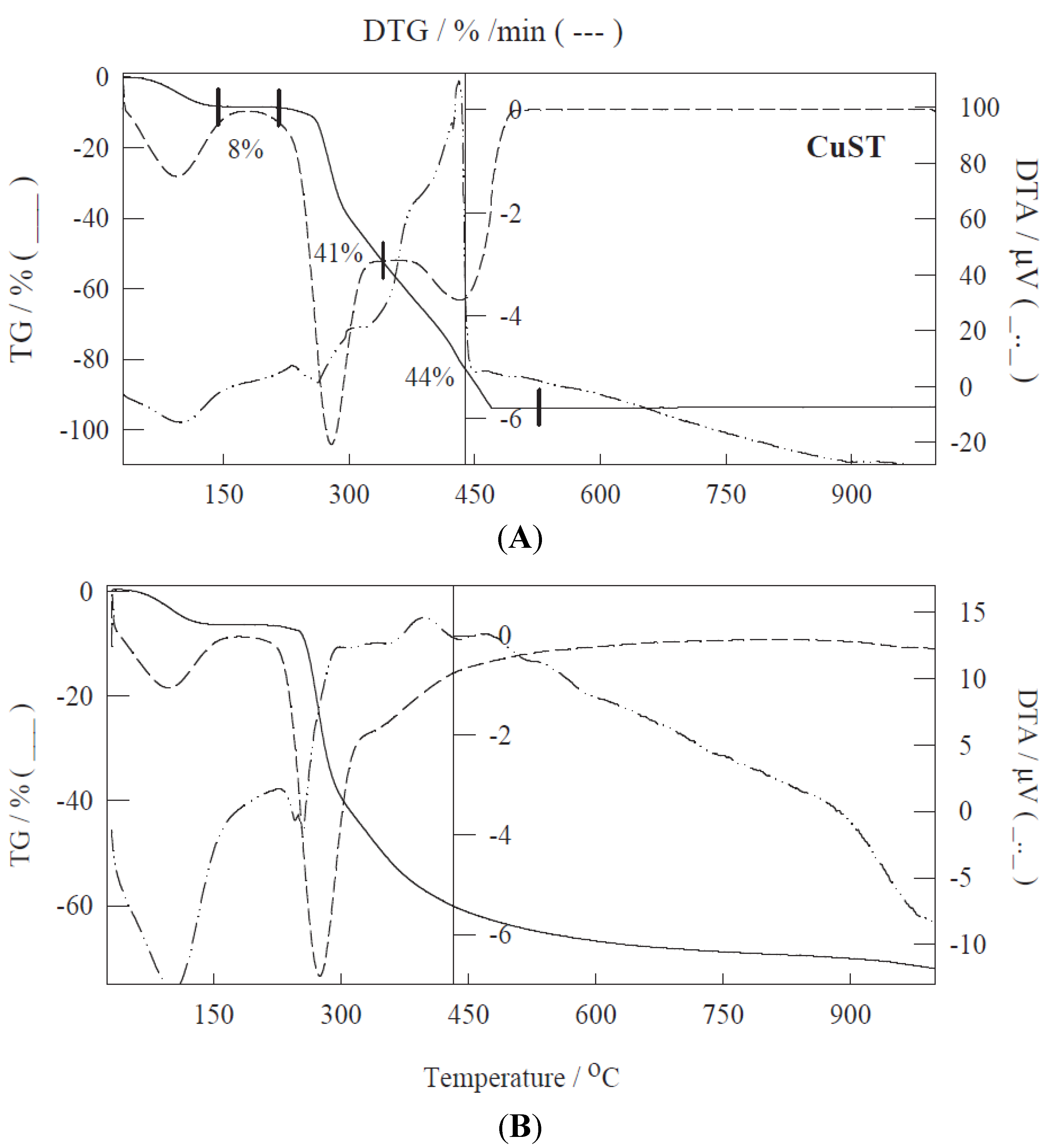
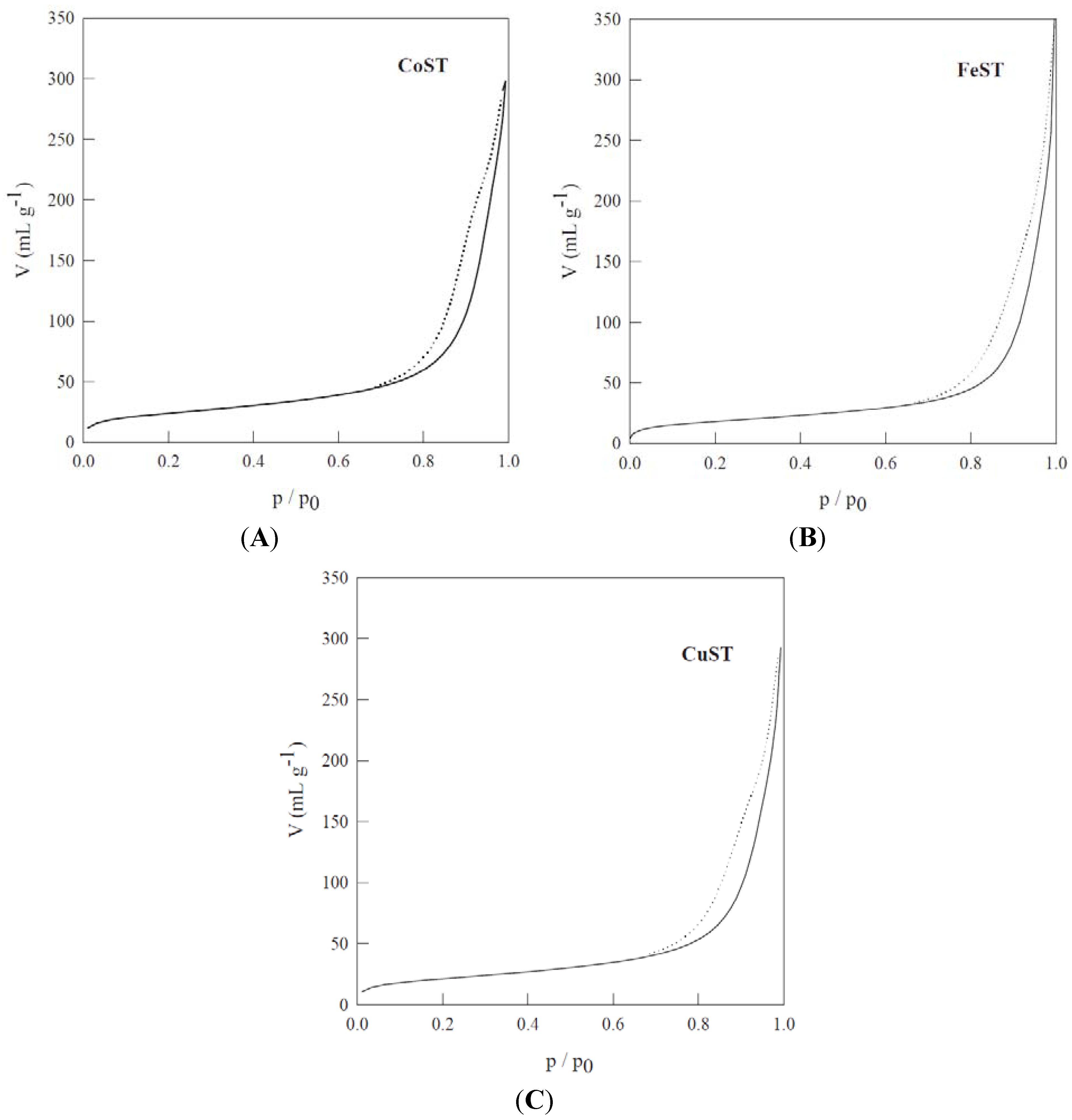
| Materials | SBET a (m2/g) | DBJH b (nm) | VBJH c (mL/g) |
|---|---|---|---|
| CoST | 87 | 14.3 | 0.32 |
| CuST | 76 | 13.7 | 0.31 |
| FeST | 66 | 11.2 | 0.30 |
| Co-STB-uncontrolled calcination | <5 | – | – |
| Co-STB-controlled calcination | 112 | 13.6 | 0.35 |
| Materials | Theoretical metal content (%) | ICP–MS metal content (%) | SEM–EDX metal content (%) |
|---|---|---|---|
| Fe-ST | 5 | 4.8 | 2.9 |
| Co-ST | 1.9 | 0.18 | – |
| Cu-ST | 3.9 | 0.2 | – |


3. Experimental Section
3.1. Materials Synthesis
- Biopolymer expansion (key process stage), via aqueous polysaccharide gel preparation assisted by a simple and efficient microwave irradiation methodology;
- Incorporation of the metal via addition of the metal precursor to the aqueous gel;
- Production of porous polysaccarides, via solvent exchange/drying.


3.2. Materials Characterisation
4. Conclusions
Acknowledgments
References
- White, R.J.; Budarin, V.; Luque, R.; Clark, J.H.; Macquarrie, D.J. Tuneable porous carbonaceous materials from renewable resources. Chem. Soc. Rev. 2009, 38, 3401–3418. [Google Scholar] [CrossRef] [PubMed]
- Robitzer, M.; Tourrette, A.; Horga, R.; Valentin, R.; Boissiere, M.; Devoisselle, J.M.; di Renzo, F.; Quignard, F. Nitrogen sorption as a tool for the characterisation of polysaccharide aerogels. Carbohydr. Polym. 2011, 85, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Valentin, R.; Molvinger, K.; Viton, C.; Domard, A.; Quignard, F. From hydrocolloids to high specific surface area porous supports for catalysis. Biomacromolecules 2005, 6, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Quignard, F.; di Renzo, F.; Guibal, E. From natural polysaccharides to materials for catalysis, adsorption, and remediation. Top. Curr. Chem. 2010, 294, 165–197. [Google Scholar] [PubMed]
- Primo, A.; Liebel, M.; Quignard, F. Palladium coordination biopolymer: A versatile access to highly porous dispersed catalyst for suzuki reaction. Chem. Mater. 2009, 21, 621–627. [Google Scholar] [CrossRef]
- Budarin, V.; Clark, J.H.; Hardy, J.J.E.; Luque, R.; Macquarrie, D.J.; Milkowski, K.; Tavener, S.J.; Wilson, A.J. Starbons: New starch-derived mesoporous carbonaceous materials with tunable properties. Angew. Chem. Int. Ed. 2006, 45, 3782–3786. [Google Scholar] [CrossRef]
- White, R.J.; Budarin, V.L.; Clark, J.H. Pectin-derived porous materials. Chem. Eur. J. 2010, 16, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Budarin, V.L.; Clark, J.H. Tuneable mesoporous materials from alpha-D-polysaccharides. ChemSusChem 2008, 1, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.H.; Falco, C.; Weber, J.; White, R.J.; Howe, J.Y.; Titirici, M.M. Carbohydrate-derived hydrothermal carbons: A thorough characterization study. Langmuir 2012, 28, 12373–12383. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.L.; Hunt, A.J.; Budarin, V.L.; Shuttleworth, P.S.; Millar, K.L.; Clark, J.H. The importante of being porous: Polysaccharide-derived porous materials for use in dye adsorption. RSC Adv. 2012, 2, 8992–8997. [Google Scholar] [CrossRef]
- White, R.J.; Budarin, V.L.; Moir, J.W.B.; Clark, J.H. A sweet killer: Mesoporous polysaccharide confined silver nanoparticles for antibacterial applications. Int. J. Mol. Sci. 2011, 12, 5782–5796. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Antonio, C.; Budarin, V.L.; Bergstrom, E.; Thomas-Oates, J.; Clark, J.H. Polysaccharide-derived carbons for polar analyte separations. Adv. Funct. Mater. 2010, 20, 1834–1841. [Google Scholar] [CrossRef]
- Budarin, V.; Clark, J.H.; Deswarte, F.E.I.; Hardy, J.J.E.; Hunt, A.J.; Kerton, F.M. Delicious not siliceous: Expanded carbohydrates as renewable separation media for column chromatography. Chem. Commun. 2005, 2903–2905. [Google Scholar]
- Budarin, V.L.; Clark, J.H.; Luque, R.; Macquarrie, D.J.; White, R.J. Palladium nanoparticles on polysaccharide-derived mesoporous materials and their catalytic performance in C–C coupling reactions. Green Chem. 2008, 10, 382–387. [Google Scholar] [CrossRef]
- Budarin, V.; Clark, J.H.; Luque, R.; Macquarrie, D.J. Versatile mesoporous carbonaceous materials for acid catalysed reactions. Chem. Commun. 2007, 634–635. [Google Scholar]
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-based completely biodegradable polymer materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Shuttleworth, P.S.; Clark, J.H.; Mantle, R.; Stansfield, N. Switchable adhesives for carpet tiles: A major breakthrough in sustainable flooring. Green Chem. 2010, 12, 798–803. [Google Scholar] [CrossRef]
- Kosslick, H.; Lischke, G.; Landmesser, H.; Parlitz, B.; Storek, W.; Fricke, R. Acidity and catalytic behavior of substituted MCM-48. J. Catal. 1998, 176, 102–114. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press Inc.: London, UK, 1982. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ojeda, M.; Budarin, V.; Shuttleworth, P.S.; Clark, J.H.; Pineda, A.; Balu, A.M.; Romero, A.A.; Luque, R. Simple Preparation of Novel Metal-Containing Mesoporous Starches. Materials 2013, 6, 1891-1902. https://doi.org/10.3390/ma6051891
Ojeda M, Budarin V, Shuttleworth PS, Clark JH, Pineda A, Balu AM, Romero AA, Luque R. Simple Preparation of Novel Metal-Containing Mesoporous Starches. Materials. 2013; 6(5):1891-1902. https://doi.org/10.3390/ma6051891
Chicago/Turabian StyleOjeda, Manuel, Vitaliy Budarin, Peter S. Shuttleworth, James H. Clark, Antonio Pineda, Alina M. Balu, Antonio A. Romero, and Rafael Luque. 2013. "Simple Preparation of Novel Metal-Containing Mesoporous Starches" Materials 6, no. 5: 1891-1902. https://doi.org/10.3390/ma6051891





