TiB2-Based Composites for Ultra-High-Temperature Devices, Fabricated by SHS, Combining Strong and Weak Exothermic Reactions
Abstract
:1. Introduction
2. Experimental Procedure
| Composite | Carbon precursor (μm) | Intended TiB2:B4C ratio | Mixing/milling time (min) | Combustion | |
|---|---|---|---|---|---|
| Atomic ratio | Volume ratio | ||||
| A | 10 | 0.96 | 40:60 | 40 | No |
| B | 10 | 2.16 | 60:40 | 40 | Yes |
| C | 10 | 5.76 | 80:20 | 40 | Yes |
| D | 2 | 2.16 | 60:40 | 180 | Yes |
| E | 2 | 3.36 | 70:30 | 180 | Yes |
| F | 2 | 5.76 | 80:20 | 180 | Yes |
| G | 2 | 12.95 | 90:10 | 180 | Yes |
3. Results and Discussion
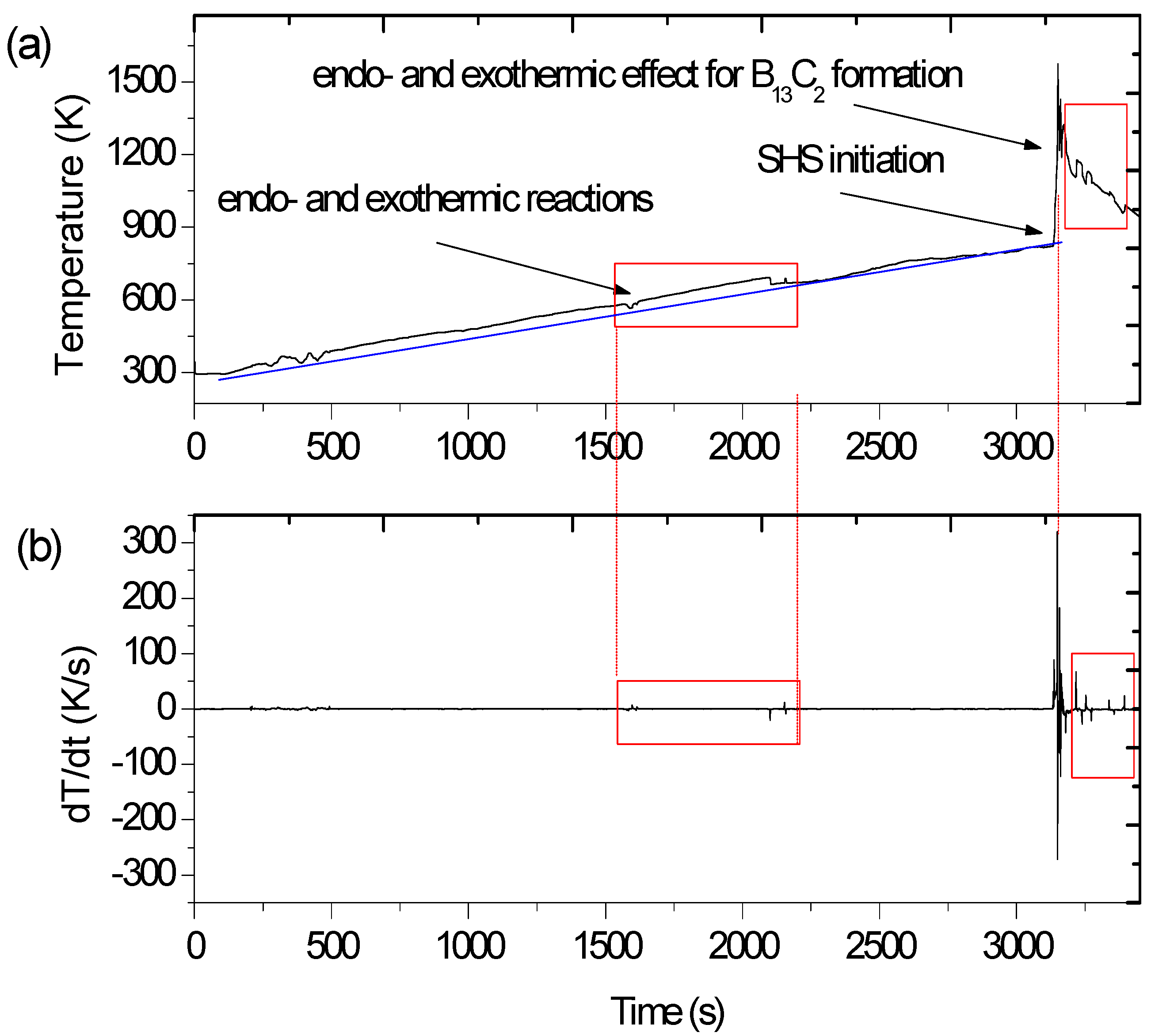
3.1. Heat Effects during SHS Process
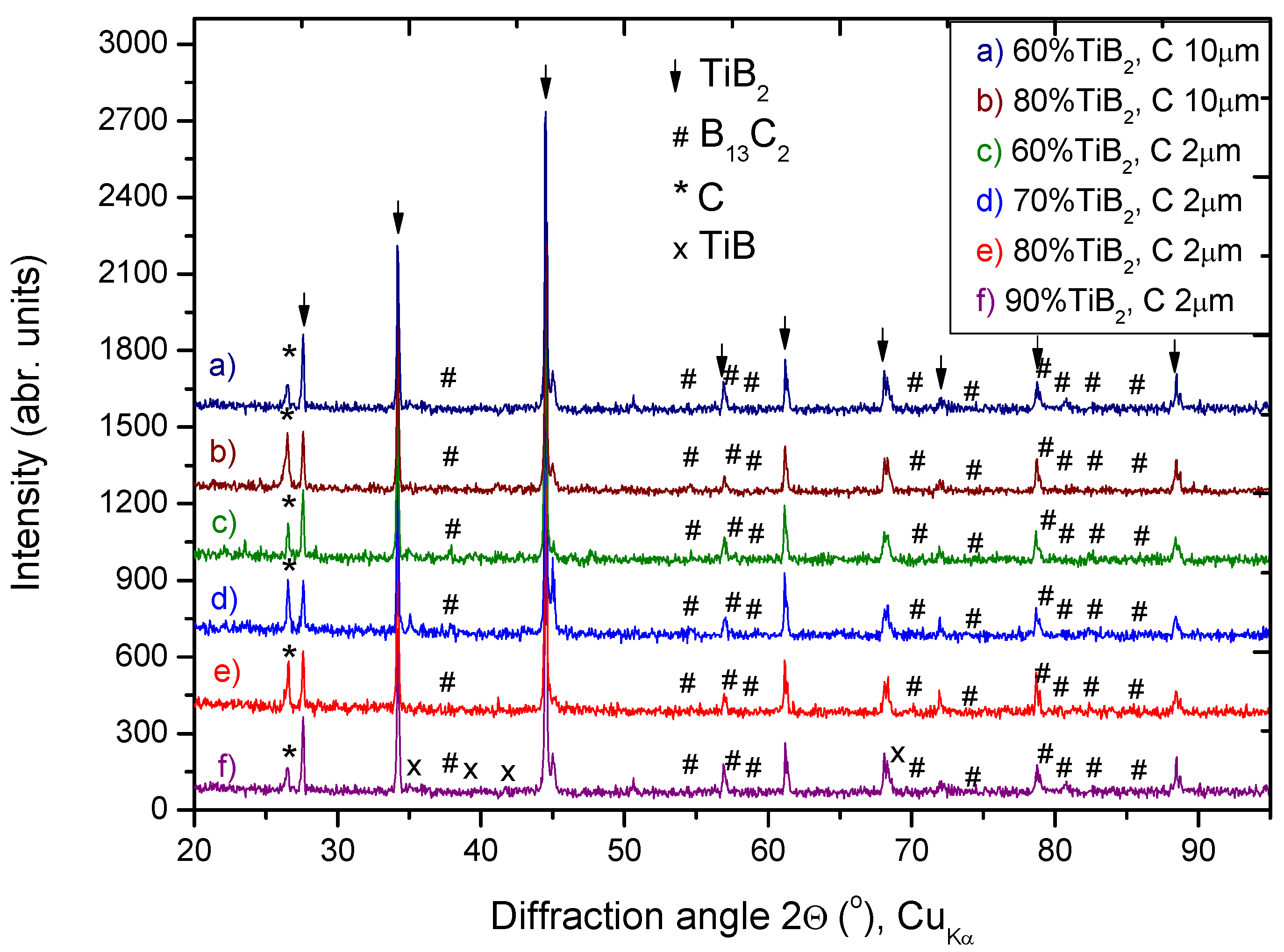
3.2. Phase Composition of Composites after SHS
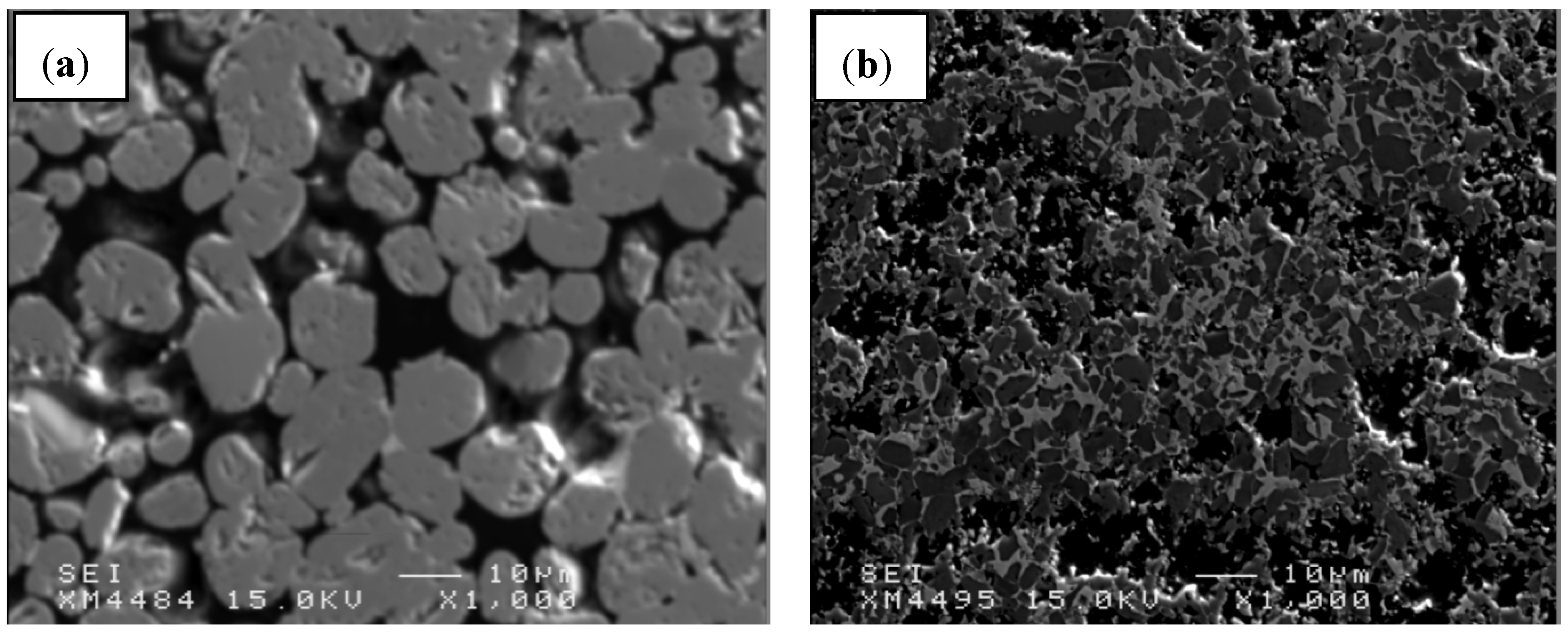
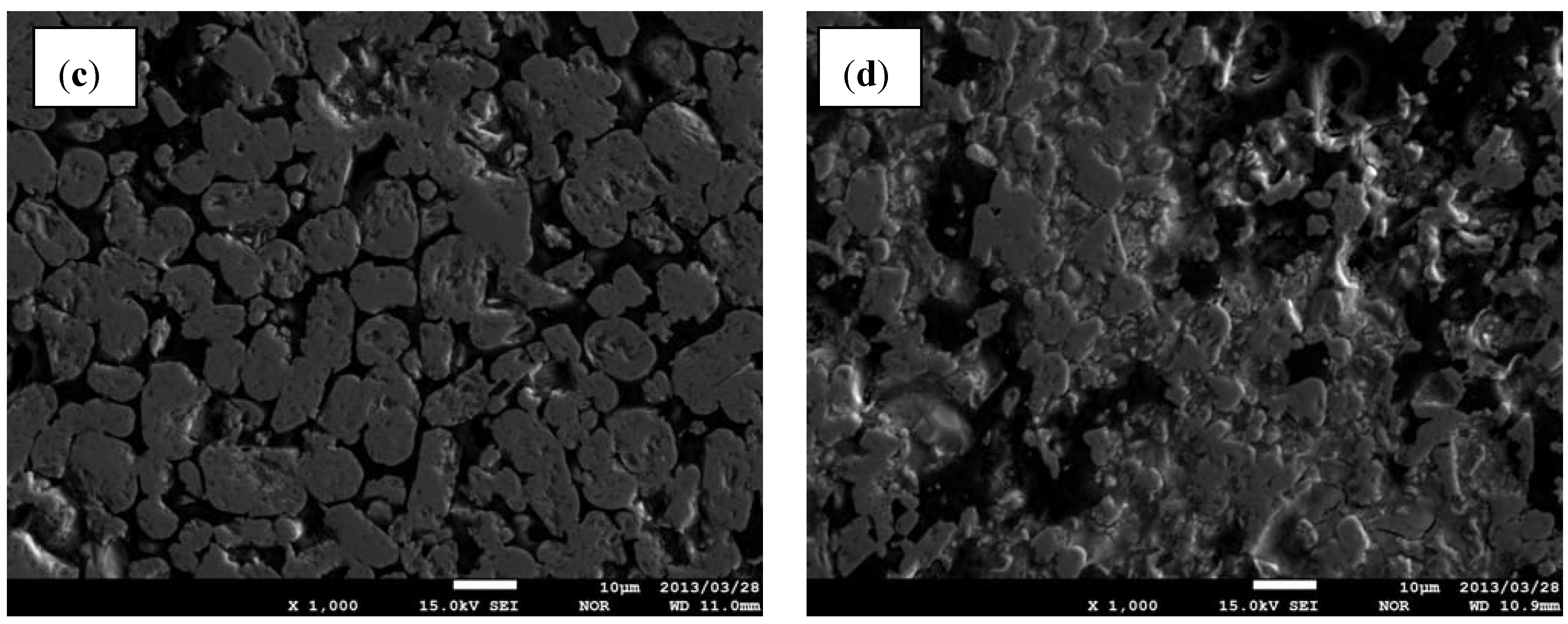
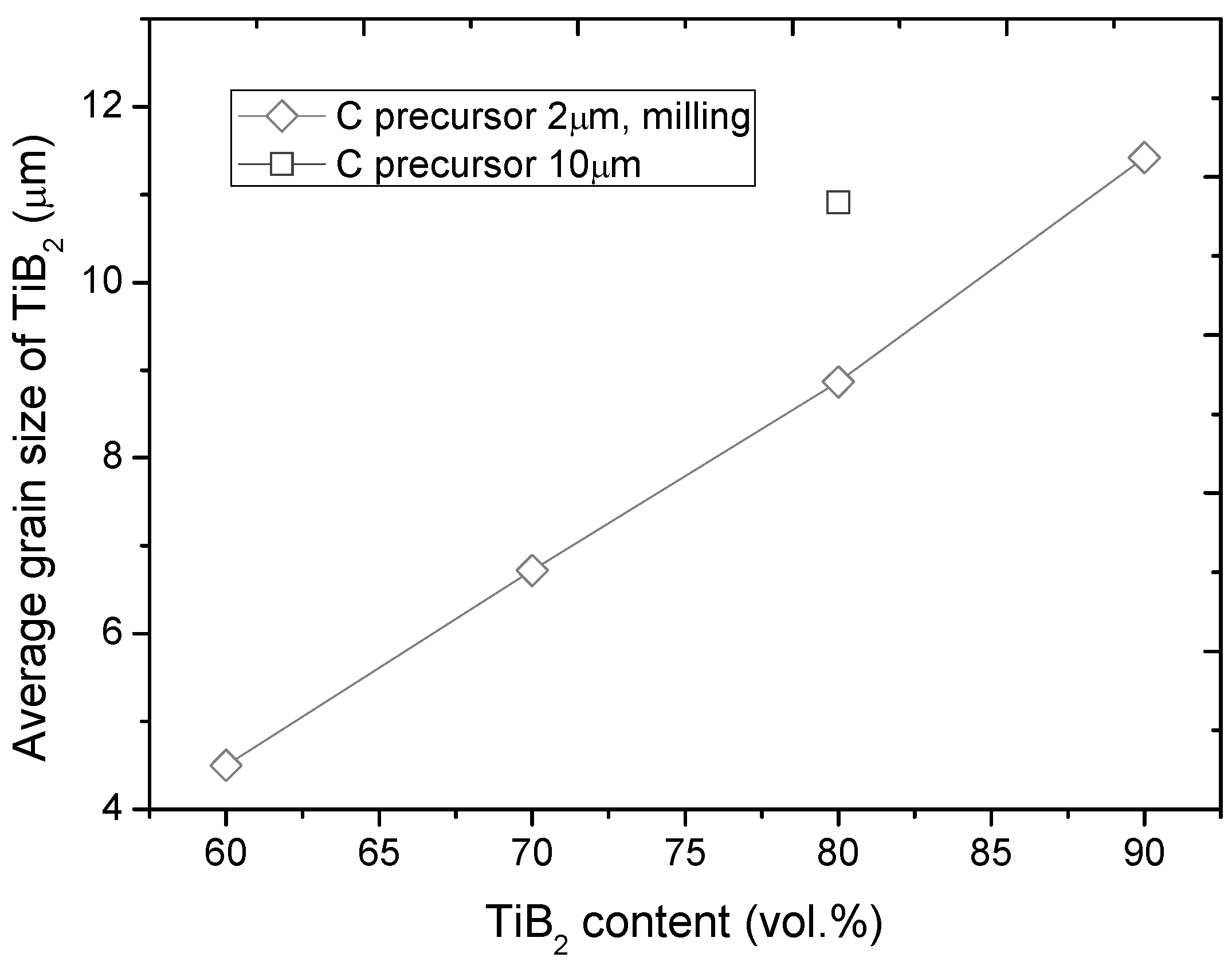
3.3. Microstructure


3.4. Dissertation on the Reaction Mechanism
- Formation of TiB, TiB2 nanolayers on the surface of titanium grains, by means of solid state reaction. Since the formation of TiB and TiB2 is strongly exothermic, locally the temperature increases immediately and the SHS process can be initiated. The study of Ti + 2B → TiB2 reaction has shown that this reaction begins to proceed at a noticeable rate long time before Ti starts to melt [40]. A low rate diffusion of carbon into boron is also predicted simultaneously with the preliminary formation of TiB and TiB2;
- Initiation of SHS caused by heat released from the first portion of TiB and TiB2 causes improved diffusion which affects the accelerated formation of products. Significantly increased temperature causes melting of Ti unreacted in the first stage of the process. The boron and carbon present in the mixture can be partially dissolved in the liquid titanium and precipitate as B13C2 monolayers, along with TiB2 formation. The reaction rate depends on the heating rate before combustion. The slower the sample is heated and the longer it is held at high temperature before explosion, the higher the reaction rate in the run [40];
- Migrating thin-reaction-layer mechanism followed by precipitation from a liquid phase occurs while cooling.
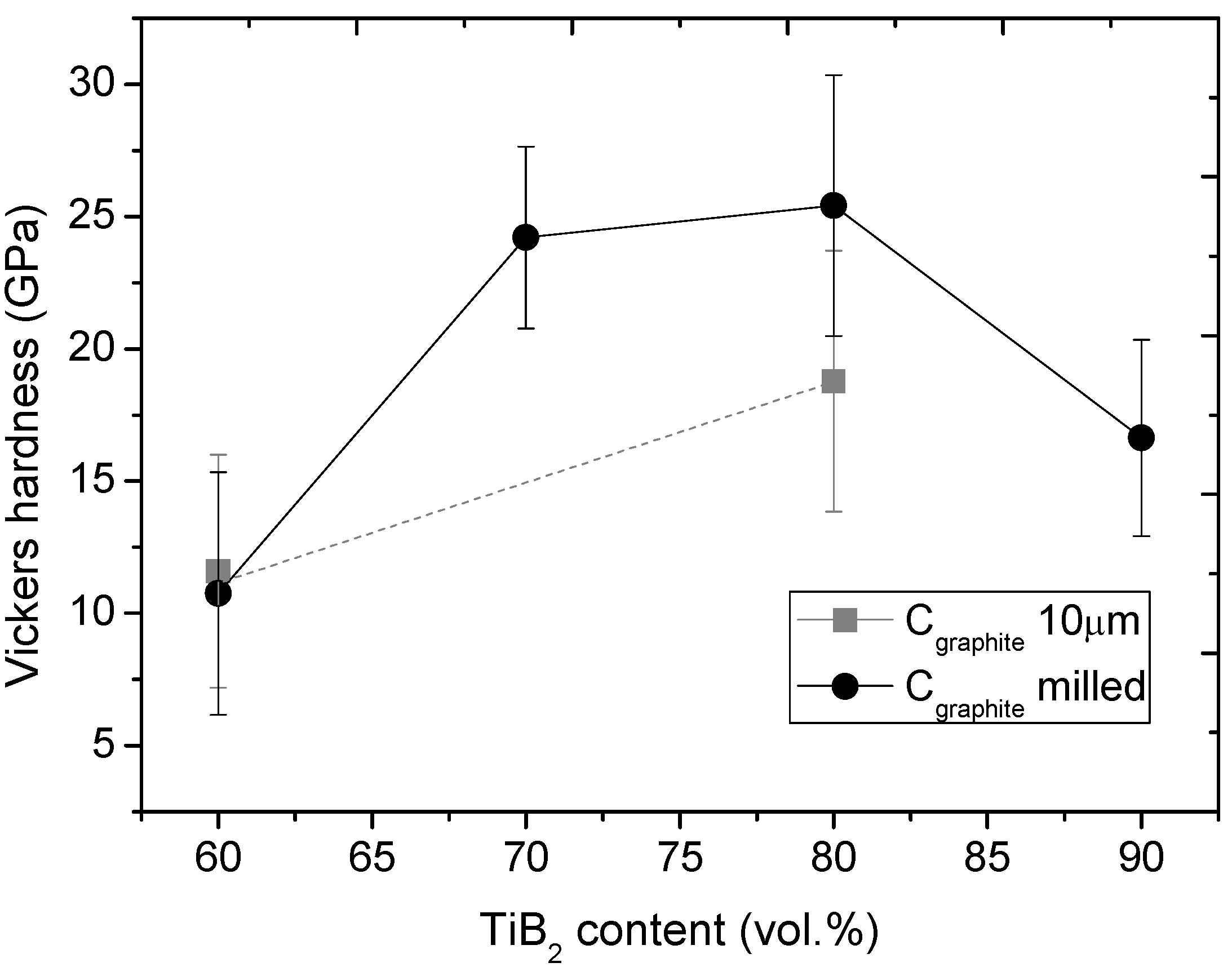
3.5. Hardness
4. Conclusions
- The exothermicity of TiB2 formation by means of the SHS process can be used in order to fabricate TiB2- B13C2 composites, when the volume fraction of TiB2 is above 60%;
- Despite B and C composed in the ratio corresponding to B4C, other stoichiometry boride B13C2 was detected as matrix phase in each composite. Perhaps, further annealing after synthesis could fulfill the diffusion needed for full saturation with carbon. However, a limited number of experimental data on diffusion and phase transformations has been reported so far and is essential for consideration;
- Several parameters, such as microstructure homogeneity, average grain size of TiB2 and hardness of the composites, proved that the grain size of C precursor is an important factor in effectiveness of B13C2 synthesis;
- Sinterability of TiB2 can be essentially improved by using boron carbide as sintering additive, but also reduced TiB2 grain growth was observed. The average grain size of TiB2 decreased from 11 to 5 μm when the TiB2 vol% was reduced from 90 to 60 vol%;
- The investigations revealed that the composite containing 80 vol% of TiB2 possessed the highest hardness of 25.5 GPa, low porosity, good homogeneity and compared to the composite with 90 vol% of TiB2, a more regular grain distribution, without agglomerates.
References
- White, R.M.; Dickey, E.C. The effect of residual stress distributions on indentation-induced microcracking in B4C-TiB2 eutectic ceramic composites. J. Am. Ceram. Soc. 2011, 94, 4032–4039. [Google Scholar] [CrossRef]
- Shapiro, M.; Gotman, I.; Dudko, V. Modeling of thermal explosion in constrained dies for B4C-Ti and BN-Ti powder blends. J. Eur. Ceram. Soc. 1999, 19, 2233–2239. [Google Scholar] [CrossRef]
- Aviles, M.A.; Chicardi, E.; Cordoba, J.M.; Sayagus, M.J.; Gotor, F.J. In situ synthesis of ceramic composite materials in the Ti-B-C-N system by a mechanically induced self-sustaining reaction. J. Am. Ceram. Soc. 2012, 95, 2133–2139. [Google Scholar] [CrossRef]
- Aizenshtein, M.; Frage, N.; Froumin, N.; Shapiro-Tsoref, E.; Dariel, M.P. Interface interaction in the (B4C + TiB2)/Cu system. J. Mater. Sci. 2006, 41, 5185–5189. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, S.; Yin, X.; Yang, L.; Xiao, C.; Zhou, H.; Zhang, J. In situ HIP synthesis of TiB2/SiC ceramic composites. J. Mater. Process. Technol. 1999, 89–90, 457–461. [Google Scholar]
- Kang, E.S.; Kim, C.H. Improvement in mechanical properties of TiB2 by the dispersion of B4C particles. J. Mater. Sci. 1990, 25, 580–584. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, J.; Feng, Y.; Ding, Z. Microstructure and mechanical properties of hot-pressed B4C/(W,Ti)C ceramic composites. Ceram. Int. 2002, 28, 425–430. [Google Scholar] [CrossRef]
- Benton, S.T.; Masters, D.R. Method for Preparing Boron-Carbide Articles. US Patent 3,914,371, 21 October 1975. [Google Scholar]
- Mohammad Shafiri, E.; Karimzadeh, F.; Enayati, M.H. Mechanochemical assisted synthesis of B4C nanoparticles. Adv. Powder Technol. 2011, 22, 354–358. [Google Scholar]
- Pei, L.; Xiao, H. B4C/TiB2 composite powders prepared by carbothermal reduction method. J. Mater. Process. Technol. 2009, 209, 2122–2127. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, J.; Zhuang, H.; Li, W. Combustion of Na2B4O7 + Mg + C to synthesis B4C powders. J. Nucl. Mater. 2009, 393, 487–491. [Google Scholar] [CrossRef]
- Baharvandi, H.R.; Tazabzadeh, N.; Ehsani, N.; Aghand, F. Synthesis of B4C-nano TiB2 composite powder by sol-gel method. J. Mater. Eng. Perform. 2009, 18, 273–277. [Google Scholar] [CrossRef]
- Singh, M. Thermodynamic analysis for the combustion synthesis of SiC-B4C composites. Scr. Mater. 1996, 34, 923–927. [Google Scholar] [CrossRef]
- Vallauri, D.; Atias Adrian, I.C.; Chrysanthou, A. TiC-TiB2 composites: A review of phase relationships, processing and properties. J. Eur. Ceram. Soc. 2008, 28, 1697–1713. [Google Scholar] [CrossRef]
- Upalov, Y.P.; Valova, E.E.; Ordanyan, S.S. Preparation and abrasive properties of eutectic compositions in the system B4C-SiC-TiB2. Refractories 1995, 36, 233–234. [Google Scholar] [CrossRef]
- Zakaryan, D.A.; Kartuzov, V.V.; Khachatryan, A.V. Pseudopotencial method for calculating the euthectic temperaturę and concentration of the components of the B4C-TiB2, TiB2-SiC, and B4C-SiC systems. Powder Metall. Met. Ceram. 2009, 48, 588–594. [Google Scholar] [CrossRef]
- Velikanova, T.Y.; Turchanin, M.A.; Korniyenko, K.Y.; Bondar, A.A.; Agraval, P.G.; Kartuzov, V.V. Phase equilibria in metal systems and properties of alloys; Phase equilibria in the Ti-Si-B-C quaternary system as a basis for developing new ceramic materials. Powder Metall. Met. Ceram. 2011, 50, 385–396. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Fu, Z. Influence of Fe/Ni/Al additive on the sintering behaviors of TiB2 cermets. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 879–882. [Google Scholar] [CrossRef]
- Telle, R.; Petzow, G. Strengthening and toughening of boride and carbide hard material composites. Mater. Sci. Eng. A 1988, 105–106, 97–104. [Google Scholar] [CrossRef]
- Deng, J.; Sun, J. Sand erosion performance of B4C based ceramic nozzles. Int. J. Refract. Met. Hard Mater. 2008, 26, 128–134. [Google Scholar] [CrossRef]
- Heian, E.M.; Khalsa, S.K.; Lee, J.W.; Munir, Z.A.; Yamamoto, T.; Ohyanagi, M. Synthesis of dense, high-defect-concentration B4C through mechanical activation and field-assisted combustion. J. Am. Ceram. Soc. 2004, 87, 779–783. [Google Scholar] [CrossRef]
- Okamoto, H. Boron-Carbon phase diagram. In ASM Handbook; Baker, H., Ed.; ASM International: Materials Park, OH, USA, 1992; Volume 3, p. 422. [Google Scholar]
- Widom, M.; Huhn, W.P. Prediction of orientational phase transition in boron carbide. Solid State Sci. 2012, 14, 1648–1652. [Google Scholar] [CrossRef]
- Will, G.; Kossobutzki, K.H. An X-ray structure analysis of boron carbide, B13C2. J. Less Common Met. 1976, 44, 87–97. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, W.; Wang, C.; Shen, Q.; Zhang, L. Synthesis and characterization of B13C2 boron carbide ceramic by pulsed electric current sintering. Ceram. Int. 2012, 38, 895–900. [Google Scholar] [CrossRef]
- Andrievski, R.A.; Baiman, I.F. Short-time creep investigation of TiB2-Fe composite. J. Mater. Sci. Lett. 1992, 11, 1661–1662. [Google Scholar] [CrossRef]
- Zou, B.; Shen, P.; Jiang, Q. Reaction synthesis of TiC-TiB2/Al composites from an Al-Ti-B4C system. J. Mater. Sci. 2007, 42, 9927–9933. [Google Scholar] [CrossRef]
- Ziemnicka-Sylwester, M. Superhard TiB2-based composites with different matrix fabricated from elemental powders by SHS-p-HIP. Adv. Sci. Technol. 2013, 77, 146–152. [Google Scholar] [CrossRef]
- Baharvandi, H.R.; Hadian, A.M. Pressureless sintering of TiB2-B4C ceramic matrix composite. J. Mater. Eng. Perform. 2008, 17, 838–841. [Google Scholar] [CrossRef]
- Grigorev, O.N.; Koval’chuk, V.V.; Zaporozhets, O.I.; Bega, N.D.; Galanov, B.A.; Prilutski, E.V.; Kotenko, V.A.; Kutran, T.N.; Dordienko, N.A. Synthesis and physicomechanical properties of B4C-VB2 composites. Powder Metall. Met. Ceram. 2006, 45, 47–58. [Google Scholar] [CrossRef]
- Pampuch, R. Advanced HT ceramic materials via solid combustion. J. Eur. Ceram. Soc. 1999, 19, 2395–2404. [Google Scholar] [CrossRef]
- Nikzad, L.; Licheri, R.; Vaezi, M.R.; Orru, R.; Cao, G. Chemically and mechanically activated combustion synthesis of B4C-TiB2 composites. Int. J. Refract. Met. Hard Mater. 2012, 35, 41–48. [Google Scholar] [CrossRef]
- Xue, H.; Munir, Z.A. Extending the compositional limit of combustion-synthesized B4C-TiB2 composites by field activation. Metall. Mater. Trans. B 1996, 27, 475–480. [Google Scholar] [CrossRef]
- Holt, J.B.; Kingman, D.D.; Bianchini, G.M. Kinetics of the combustion synthesis of TiB2. Mater. Sci. Eng. 1985, 71, 321–327. [Google Scholar] [CrossRef]
- Zhang, G.; Xiao, G.; Fan, Q. Numerical modeling of field-activated combustion synthesis process of the B4C system. Mater. Res. Bull. 2011, 46, 345–349. [Google Scholar] [CrossRef]
- Munir, Z.A.; Aselmi-Tamburini, U. Self-propagating exothermic reactions: The synthesis of high-temperature materials by combustion. Mater. Sci. Rep. 1989, 3, 227–365. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Chen, K.; Zhou, H. Combustion synthesis of (TiC + SiC) composite powders by coupling strong and weak exothermic reactions. J. Alloy. Compd. 2010, 492, L82–L86. [Google Scholar] [CrossRef]
- Panek, Z. The synthesis of SiC-B4C ceramics by combustion during hot-pressing. J. Eur. Ceram. Soc. 1993, 11, 231–236. [Google Scholar] [CrossRef]
- Ziemnicka-Sylwester, M.; Matsuura, K.; Ohno, M. Phase evolution, microstructure and hardness of TiB2-based Co-containing composites by SHS under pseudo-isostatic pressure. ISIJ Int. 2012, 52, 1698–1704. [Google Scholar] [CrossRef]
- Shteinberg, A.S.; Knyazik, V.A. Microkinetics of high-temperature heterogenous reactions: SHS aspects. Pure Appl. Chem. 1992, 64, 965–976. [Google Scholar] [CrossRef]
- Thevenot, F. Boron carbide—Comprehensive review. J. Eur. Ceram. Soc. 1990, 6, 205–225. [Google Scholar] [CrossRef]
- Gorsse, S.; Chaminade, J.P.; Le Petitcorps, Y. In situ preparation of Ti-based composites reinforced by TiB single crystals using a powder metallurgy technique. Compos. Part A Appl. Sci. Manuf. 1998, 29, 1229–1234. [Google Scholar] [CrossRef]
- Niihara, K.; Nakahira, A.; Hirai, T. The effect of stoichiometry on the mechanical properties of boron carbide. J. Am. Ceram. Soc. 1984, 67, C13–C14. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ziemnicka-Sylwester, M. TiB2-Based Composites for Ultra-High-Temperature Devices, Fabricated by SHS, Combining Strong and Weak Exothermic Reactions. Materials 2013, 6, 1903-1919. https://doi.org/10.3390/ma6051903
Ziemnicka-Sylwester M. TiB2-Based Composites for Ultra-High-Temperature Devices, Fabricated by SHS, Combining Strong and Weak Exothermic Reactions. Materials. 2013; 6(5):1903-1919. https://doi.org/10.3390/ma6051903
Chicago/Turabian StyleZiemnicka-Sylwester, Marta. 2013. "TiB2-Based Composites for Ultra-High-Temperature Devices, Fabricated by SHS, Combining Strong and Weak Exothermic Reactions" Materials 6, no. 5: 1903-1919. https://doi.org/10.3390/ma6051903




