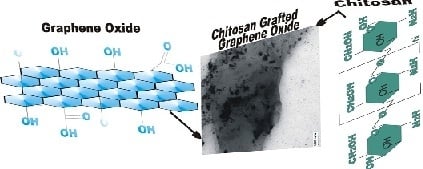
Covalently Bonded Chitosan on Graphene Oxide via Redox Reaction
Abstract
:1. Introduction
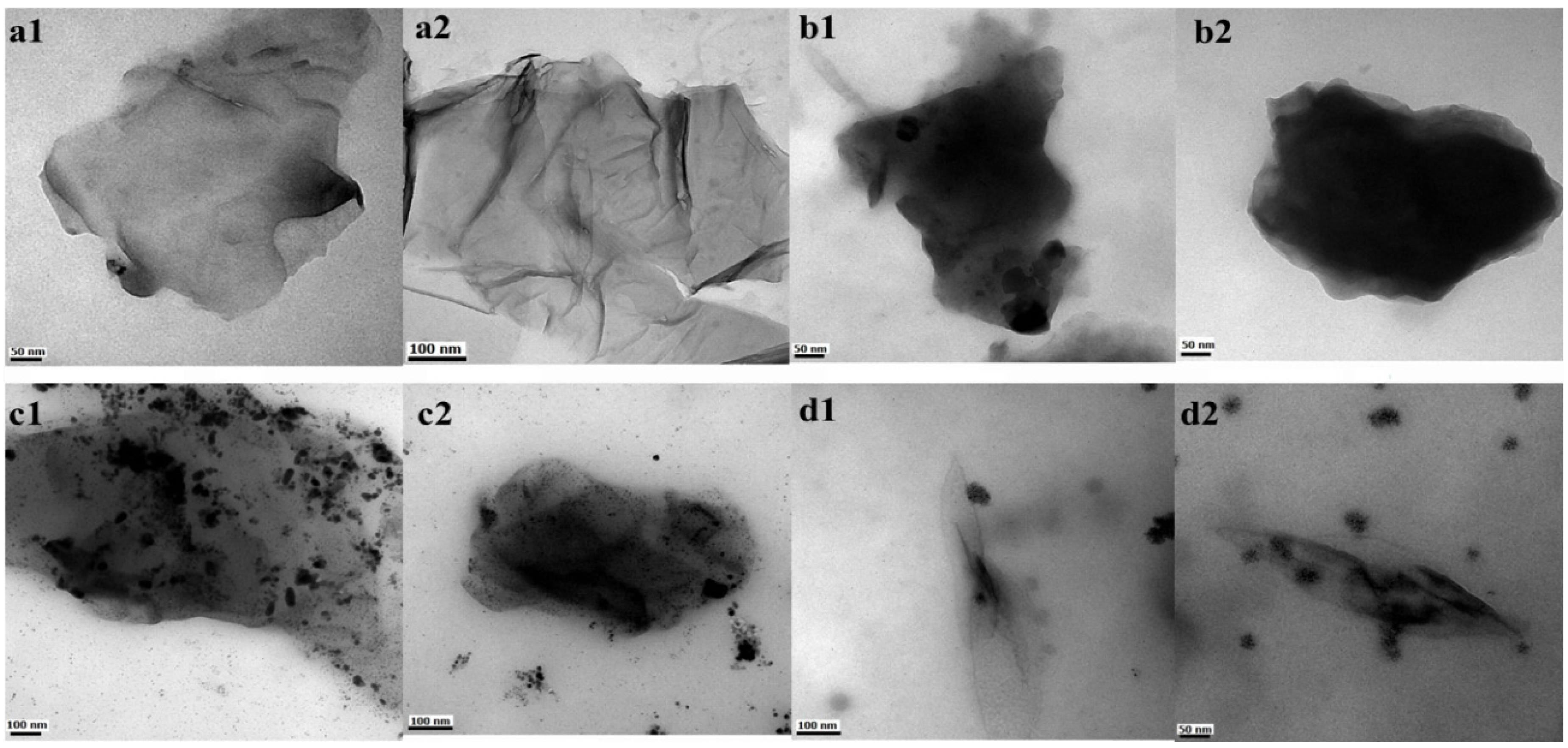
2. Results and Discussion
| Nomenclature | Description |
|---|---|
| GO | Graphite Oxide |
| GEO | Graphene Oxide |
| CGEO1 | Chitosan grafted on graphene oxide at 55–60° C |
| CGEO2 | Chitosan grafted on graphene oxide at 75–80° C |
| CGEO3 | Chitosan grafted on graphene oxide at 95–100° C |
| CGEO | Chitosan grafted on graphene oxide |



| Element | Graphene Oxide (Weight %) | CGEO1 (Weight %) | CGEO2 (Weight %) | CGEO3 (Weight %) |
| C | 60.45 | 26.06 | 23.63 | 24.32 |
| O | 38.59 | 72.07 | 71.69 | 71.54 |
| N | 0 | 0.12 | 1.6 | 2.95 |
| S | 0.97 | 1.75 | 2.73 | 0.78 |

3. Experimental Section
3.1. Materials
3.2. Graphene Oxide Sheet Preparation
3.3. Chitosan Grafting on Graphene Oxide
3.4. Analytical Characterization

3.5. Dispersion Study
4. Conclusions
Acknowledgments
References
- Wang, S.; Shen, L.; Zhang, W.; Tong, Y. Preparation and mechanical properties of chitosan/carbon nanotubes composites. Biomacromolecules 2005, 6, 3067–3072. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Feng, W.; Feng, Y.; Liu, Q.; Xu, X.; Sekino, T.; Fujii, A.; Ozaki, M. Preparation and characterization of chitosan-grafted multiwalled carbon nanotubes and their electrochemical properties. Carbon 2007, 45, 1212–1218. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S. Chitosan composites for bone tissue enginering—An overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, B.; Zeng, D.; Wang, D.; Wang, Y.; Zhang, L. Formation and properties of a novel complex composed of an amylose-grafted chitosan derivative and single-walled carbon nanotubes. Carbohydr. Polym. 2011, 85, 845–853. [Google Scholar] [CrossRef]
- Chung, T.; Limpanichpakdee, T.; Yang, M.; Tyan, Y. An electrode of quartz crystal microbalance decorated with CNT/Chitosan/fibronectin for investigating early adhesion and deforming morphology of rat mesenchymal stem cells. Carbohydr. Polym. 2011, 85, 726–732. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Water-Soluble graphene covalently functionalized by biocompatible Poly-L-Lysine. Langmuir 2009, 25, 12030–12033. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.; Rao, T.P.; Rao, K.S.; Rani, S.U.; Naidu, G.R.K.; Lee, H.Y.; Kawai, T. A review of DNA functionalized/grafted carbon nanotubes and their characterization. Sens. Actuators B 2007, 122, 672–682. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F.; Montazer-Rahmati, M.M.; Matsuura, T. Effect of chitosan as a functionalization agent on the performance and separation properties of polyimide/multi-walled carbon nanotubes mixed matrix flat sheet membranes. J. Membr. Sci. 2010, 364, 309–317. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Song, J.; Han, D.; Ivaska, A.; Niu, L. Direct electrochemistry of glucose oxidase and biosensing for glucose based on graphene. Anal. Chem. 2009, 81, 2378–2382. [Google Scholar] [CrossRef] [PubMed]
- Wakeland, S.; Martinez, R.; Grey, J.K.; Luhrs, C.C. Production of graphene from graphite oxide using urea as expansion-reduction agent. Carbon 2010, 48, 3463–3470. [Google Scholar] [CrossRef]
- Young, R.J.; Kinloch, I.A.; Gong, L.; Novoselov, K.S. The mechanics of graphene nanocomposites: A review. Compos. Sci. Technol. 2012, 72, 1459–1476. [Google Scholar] [CrossRef]
- Shokrieh, M.M.; Rafiee, R. Prediction of Young’s modulus of graphene sheets and carbon nanotubes using nanoscale continuum mechanics approach. Mater. Des. 2010, 31, 790–795. [Google Scholar] [CrossRef]
- Veca, L.M.; Lu, F.; Meziani, M.J.; Cao, L.; Zhang, P.; Qi, G.; Qu, L.; Shrestha, M.; Sun, Y. Polymer functionalization and solubilization of carbon nanosheets. Chem. Commun. 2009, 2565–2567. [Google Scholar]
- Nguyen, D.A.; Lee, Y.R.; Raghu, A.V.; Jeong, H.M.; Shin, C.M.; Kim, B.K. Morphological and physical properties of a thermoplastic polyurethane reinforced with functionalized graphene sheet. Polym. Int. 2009, 58, 412–417. [Google Scholar] [CrossRef]
- Min, S.K.; Kim, W.Y.; Cho, Y.; Kim, K.S. Fast DNA sequencing with a graphene-based nanochannel device. Nat. Nanotech. 2011, 6, 162–165. [Google Scholar] [CrossRef]
- Myung, S.; Yin, P.T.; Kim, C.; Park, J.; Solanki, A.; Reyes, P.I.; Lu, Y.; Kim, K.S.; Lee, K.B. Label-free polypeptide-based enzyme detections using a grapheme-nanoparticle hybrid sensor. Adv. Mater. 2012, 24, 6081–6087. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.; Kim, K.S. Waater-Dispersable magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano. 2010, 7, 3979–3986. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, J.; Kim, J.; Kim, K.S.; Hong, B.H.; Cho, K. Control of graphene field-effect transistors by interfacial hydrophilic self-assembled monolayers. Adv. Mater. 2011, 23, 3460–3464. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Park, J.; Sim, S.H.; Lim, S.; Kim, K.S.; Hong, B.H.; Cho, K. Surface-Directed molecular assembly of pentacene on monolayer graphene fir high-performance organic transistors. J. Am. Chem. Soc. 2011, 133, 4447–4454. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotech. 2012, 5, 574–578. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong., B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef] [PubMed]
- Bourlinos, A.B.; Gournis, D.; Petridis, D.; Szabò, T.; Szeri, A.; Dèkány, I. Graphite oxide: Chemical reduction to graphite and surface modification with primary aliphatic amines and amino acids. Langmuir 2003, 19, 6050–6055. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Mukherjee, A.; Chakraborty, S.; Kang, J.; Loos, J.P.; Kelly, K.F.; Schmidt, H.K.; Billups, W.E. Exfoliated soluble graphite. Carbon 2009, 47, 2945–2949. [Google Scholar] [CrossRef]
- Worsley, K.A.; Ramesh, P.; Mandal, S.K.; Niyogi, S.; Itkis, M.E.; Haddon, R.C. Soluble graphene derived from graphite fluoride. Chem. Phys. Lett. 2007, 445, 51–56. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Piner, R.D.; Jung, I.; Yang, D.; Velamakanni, A.; Nguyen, S.T.; Ruoff, R.S. Aqueous suspension and characterization of chemically modified graphene sheets. Chem. Mater. 2008, 20, 6592–6594. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Park, S.; Dikin, A.D.; Nguyen, I.S.; Ruoff, S.R. Graphene oxide sheets chemically cross-linked by polyallylamine. J. Phys. Chem. C 2009, 113, 15801–15804. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Hua, L.; Kai, W.; Yang, J.; Inoue, Y. A new poly(l-lactide)-grafted graphite oxide composite. Polym. Degrad. Stab. 2010, 95, 2619–2627. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Kang, X.; Wang, C.; Wang, D.; Liu, J.; Aksay, I.A.; Lin, Y. Glucose biosensor based on immobilization of glucose in platinum nanoparticles/graphene/chitosan nanocomposite film. Talanta 2009, 80, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Z.; Yin, J. Facile synthesis of soluble graphene via a green reduction of graphene oxide in tea solution and its biocomposites. Appl. Mater. Interfaces 2011, 3, 1127–1133. [Google Scholar] [CrossRef]
- Rodríguez-González, C.; Martínez-Hernández, A.L.; Castaño, V.M.; Kharissova, O.V.; Ruoff, R.S.; Velasco-Santos, C. Polysaccharide nanocomposites reinforced with graphene oxide and keratin-grafted graphene oxide. Ind. Eng. Chem. Res. 2012, 51, 3619–3629. [Google Scholar] [CrossRef]
- Rodríguez-González, C.; Martínez-Hernández, A.L.; Castaño, V.M.; Kharissova, O.V.; Velasco-Santos, C. Graphene oxide sheets covalently grafted with keratin obtained from chicken feathers. Digest J. Nanomater. Biostruct. 2013, 8, 127–138. [Google Scholar]
- Pan, Y.; Bao, H.; Li, L. Noncovalently functionalized multiwalled carbon nanotubes by Chitosan-grafted reduced graphene oxide and their synergistic reinforcing effects in Chitosan films. Appl. Mater. Interfaces 2011, 3, 4819–4830. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Li, X.; Lu, F.; Qiu, H.; Sun, M. Fabrication of novel magentic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215–216, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Pan, X.; Clarke, K.; Li, K. Covalent functionalization of graphene with polysaccharides. Ind. Eng. Chem. Res. 2012, 51, 310–317. [Google Scholar] [CrossRef]
- Bao, H.; Pan, Y.; Ping, Y.; Sahoo, N.G.; Wu, T.; Li, L.; Li, J.; Gan, L.H. Chitosan-Functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small 2011, 7, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tu, Y.; Li, L.; Shang, S.; Tao, X. Well-dispersed chitosan/graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Deepachitra, R.; CHamundeeswari, M.; Santhosh, K.B.; Krithiga, G.; Prabu, P.; Pandima, D.M.; Sastry, P.T. Osteo mineralization of fibrin-decorated graphene oxide. Carbon 2013, 56, 64–76. [Google Scholar] [CrossRef]
- Sun, Z.; Fu, H.; DEng, L.; Wang, J. Redox-Active thionine-graphene oxide hybrid nanosheet: One-Pot, rapid synthesis, and application as a sensing platform for uric acid. Anal. Chim. Acta 2013, 761, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Y.; Zheng, L.; Zhan, Y.; He, L. Graphene oxide/poly-l-lysine assembled layer for adhesion and electrochemical impedance detection of leukemia K562 cancer cells. Biosen. Bioelectron. 2013, 42, 112–118. [Google Scholar] [CrossRef]
- Podila, R.; Moore, T.; Alexis, F.; Rao, M.A. Graphene coatings for enhanced hemo-compatibility of nitinol stents. RSC Adv. 2013, 3, 1660–1665. [Google Scholar] [CrossRef]
- El-Hefian, E.A.; Elgannoudi, E.S.; Mainal, A.; Yahaya, A.H. Characterization of chitosan in acetic acid: Rheological and thermal studies. Turk. J. Chem. 2010, 34, 47–56. [Google Scholar]
- Vikram-Singh, A. A DSC study of some biomaterials relevant to pharmaceutical industry. J. Therm. Anal. Calorim. 2012. [Google Scholar] [CrossRef]
- Kumar, S.; Koh, J. Physiochemical and optical study of chitosan–terephthaldehyde derivative for biomedical applications. Int. J. Biol. Macromol. 2012, 51, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [PubMed]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Velasco-Santos, C.; Martínez-Hernández, A.L.; Lozada-Cassou, M.; Alvarez-Castillo, A.; Castaño, V.M. Chemical functionalization of carbon nanotubes through an organosilane. Nanotechnology 2002, 13, 495–498. [Google Scholar] [CrossRef]
- Cuong, T.V.; Pham, V.H.; Tran, Q.T.; Hahn, S.H.; Chung, J.S.; Shin, E.W.; Kim, E.J. Photoluminescence and Raman studies of graphene thin films prepared by reduction of graphene oxide. Mater. Lett. 2010, 64, 399–401. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. graphene oxide papers modified by divalent ions—Enhancing mechanical properties via chemical cross-linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.I.; Villar-Rodil, S.; Martinez-Alonso, A.; Tascon, J.M.D. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.A. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 47, 51–87. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hoffman, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Pawlak, J.J.; Venditti, A.R.; El-tahlawy, K. Synthesis and characterization of starch citrate-chitosan foam with superior water and saline absorbance properties. Biomacromolecules 2010, 11, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernandez, A.L.; Velasco-Santos, C.; de icaza, M.; Castaño, M.V. Grafting of methyl methacrylate onto natural keratin. e-Polymers 2003, 16, 1–11. [Google Scholar]
- Martínez-Hernandez, A.L.; Santiago-Valtierra, A.L.; Alvarez-Ponce, M.J. Chemical modification of keratin biofibres by graft polymerisation of methyl methacrylate using redox initiation. Mater. Res. Innov. 2008, 12, 184–191. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bustos-Ramírez, K.; Martínez-Hernández, A.L.; Martínez-Barrera, G.; Icaza, M.D.; Castaño, V.M.; Velasco-Santos, C. Covalently Bonded Chitosan on Graphene Oxide via Redox Reaction. Materials 2013, 6, 911-926. https://doi.org/10.3390/ma6030911
Bustos-Ramírez K, Martínez-Hernández AL, Martínez-Barrera G, Icaza MD, Castaño VM, Velasco-Santos C. Covalently Bonded Chitosan on Graphene Oxide via Redox Reaction. Materials. 2013; 6(3):911-926. https://doi.org/10.3390/ma6030911
Chicago/Turabian StyleBustos-Ramírez, Karina, Ana L. Martínez-Hernández, Gonzalo Martínez-Barrera, Miguel De Icaza, Víctor M. Castaño, and Carlos Velasco-Santos. 2013. "Covalently Bonded Chitosan on Graphene Oxide via Redox Reaction" Materials 6, no. 3: 911-926. https://doi.org/10.3390/ma6030911