Applications of Carbon Nanotubes for Lithium Ion Battery Anodes
Abstract
:1. Introduction
2. Mechanism of Lithium Ion Intercalation and Adsorption in CNTs
3. CNTs (SWCNTs, MWCNTs): Structural Descriptions and Their Application in Anode Materials for LIBs
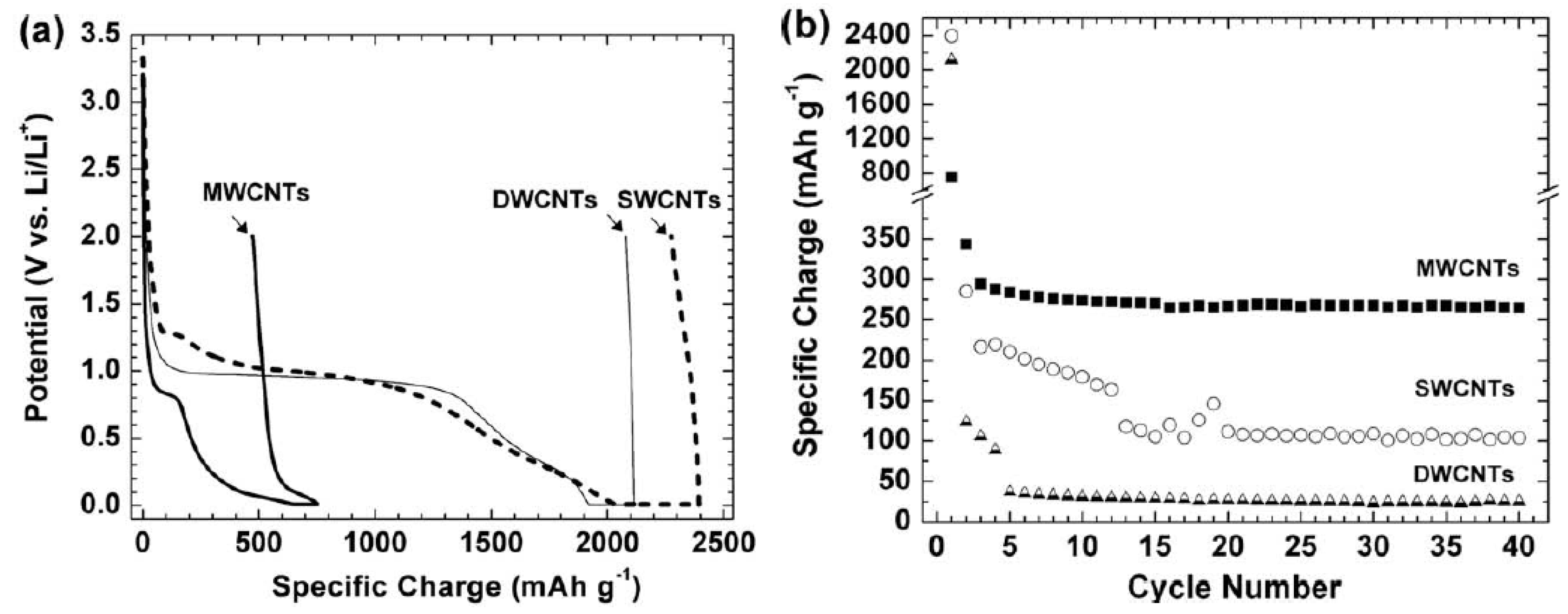
3.1. SWCNTs as Anodes for LIBs
3.2. MWCNT as Anode for LIBs
4. CNTs of Different Morphologies as Anode for LIBS
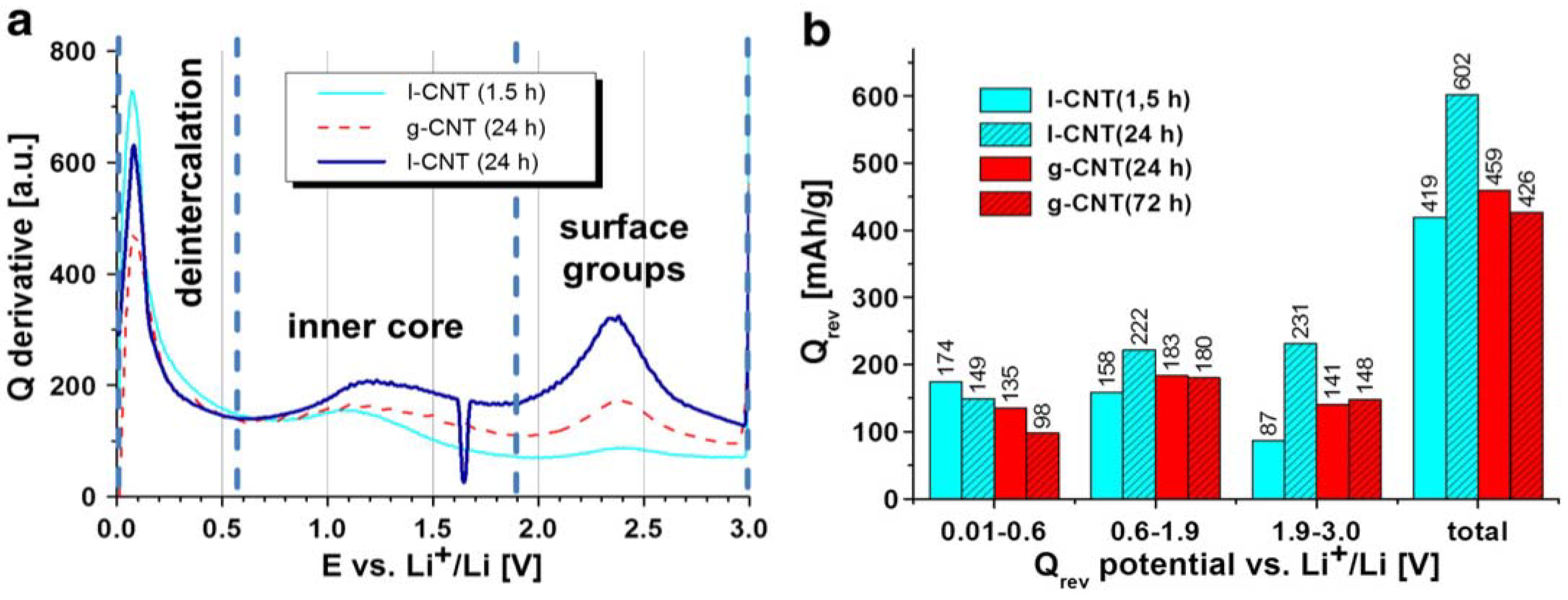
4.1. Defective CNTs as Anode Materials for LIBs.

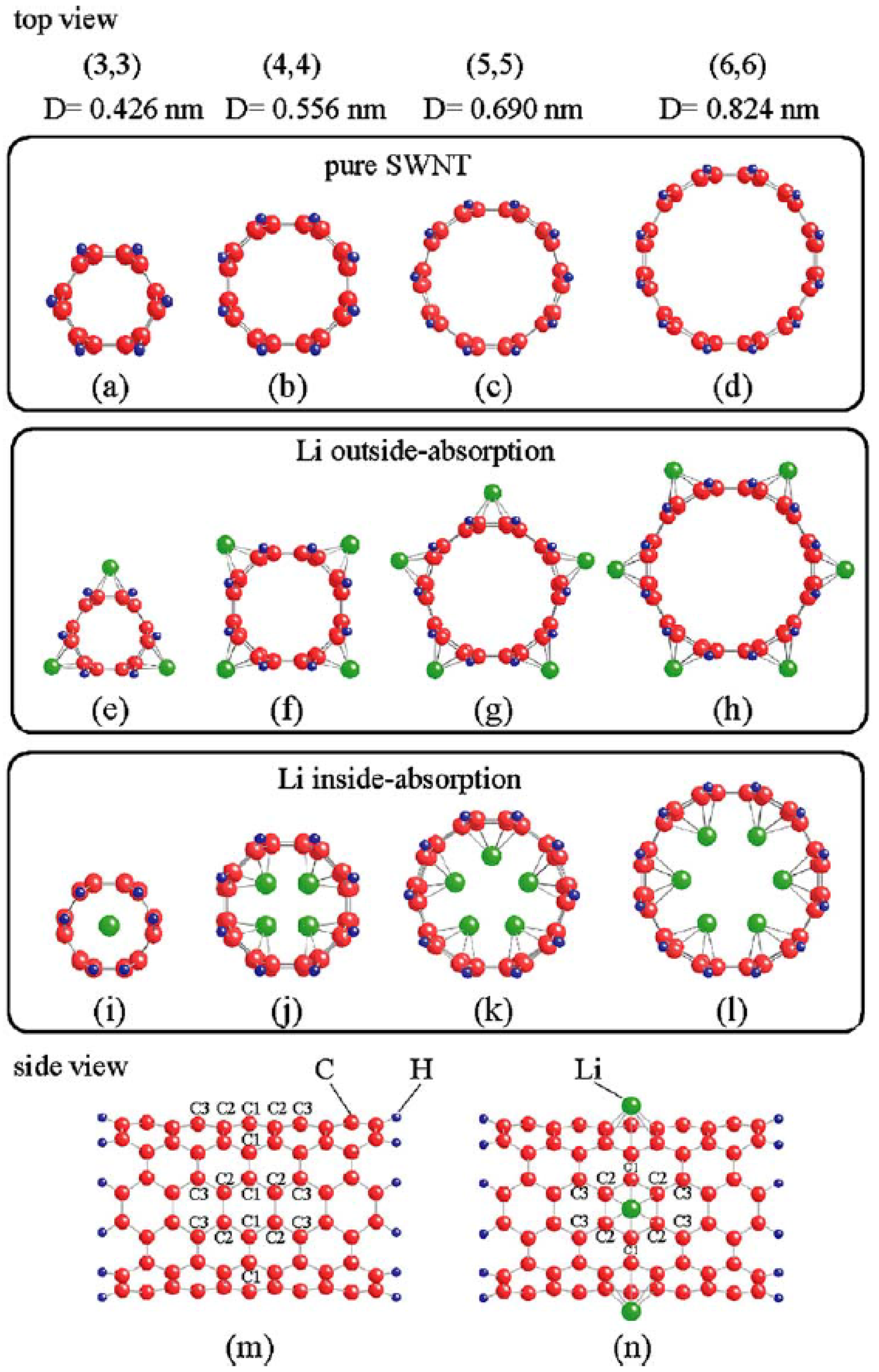
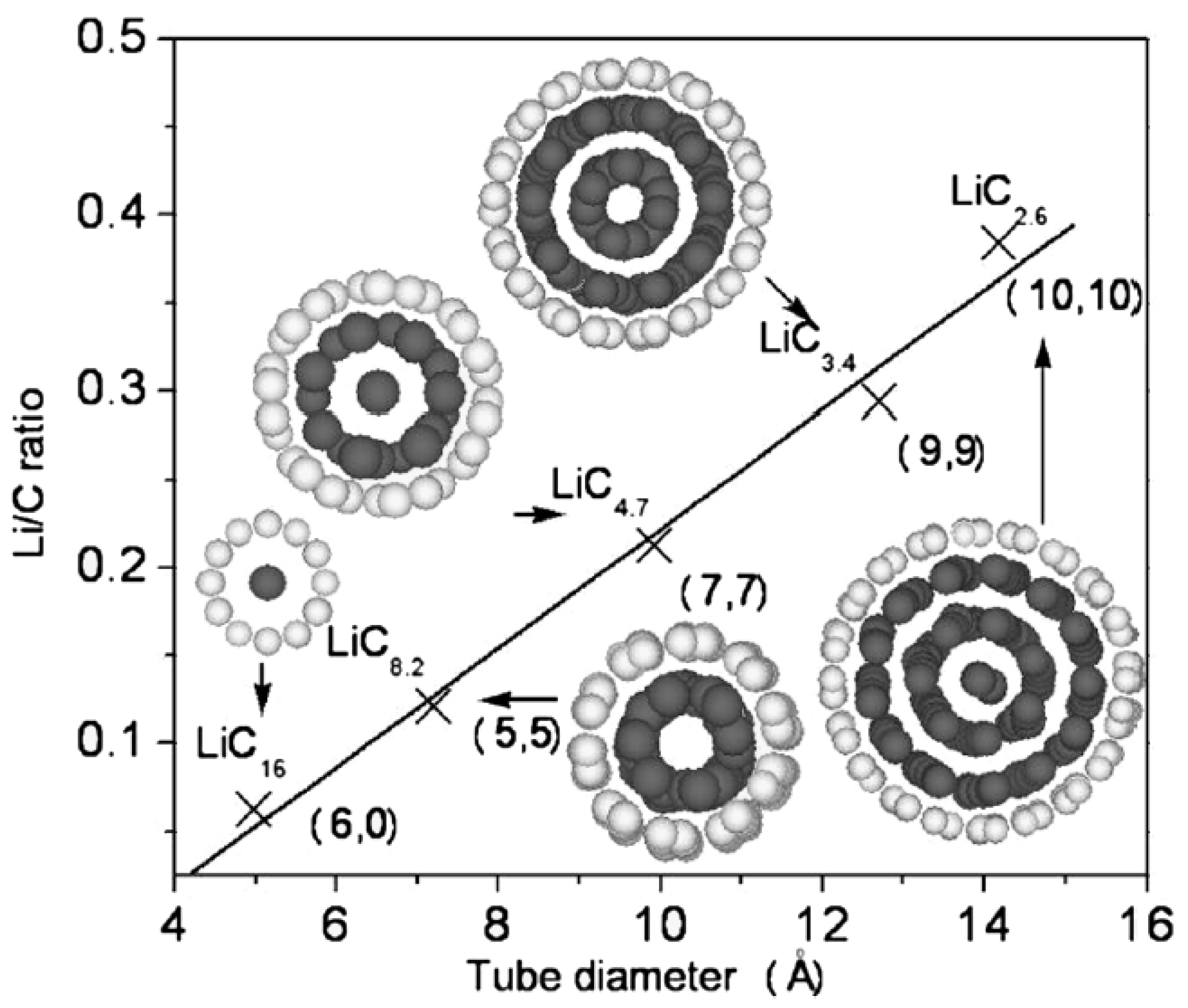
4.2. CNTs of Different Diameters as Anode Materials for LIBs
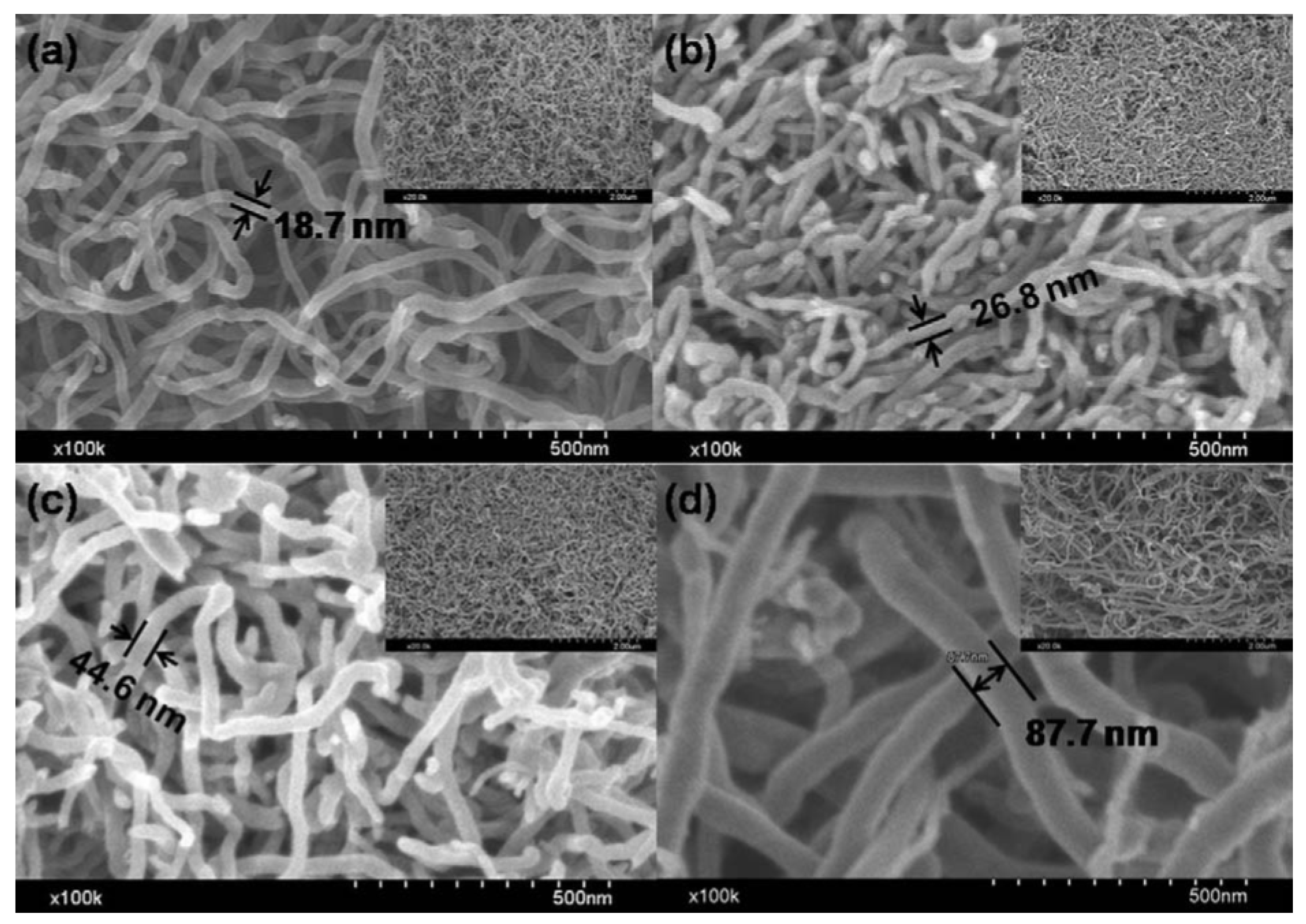
4.3. CNTs of Different Lengths for LIB Anode Materials
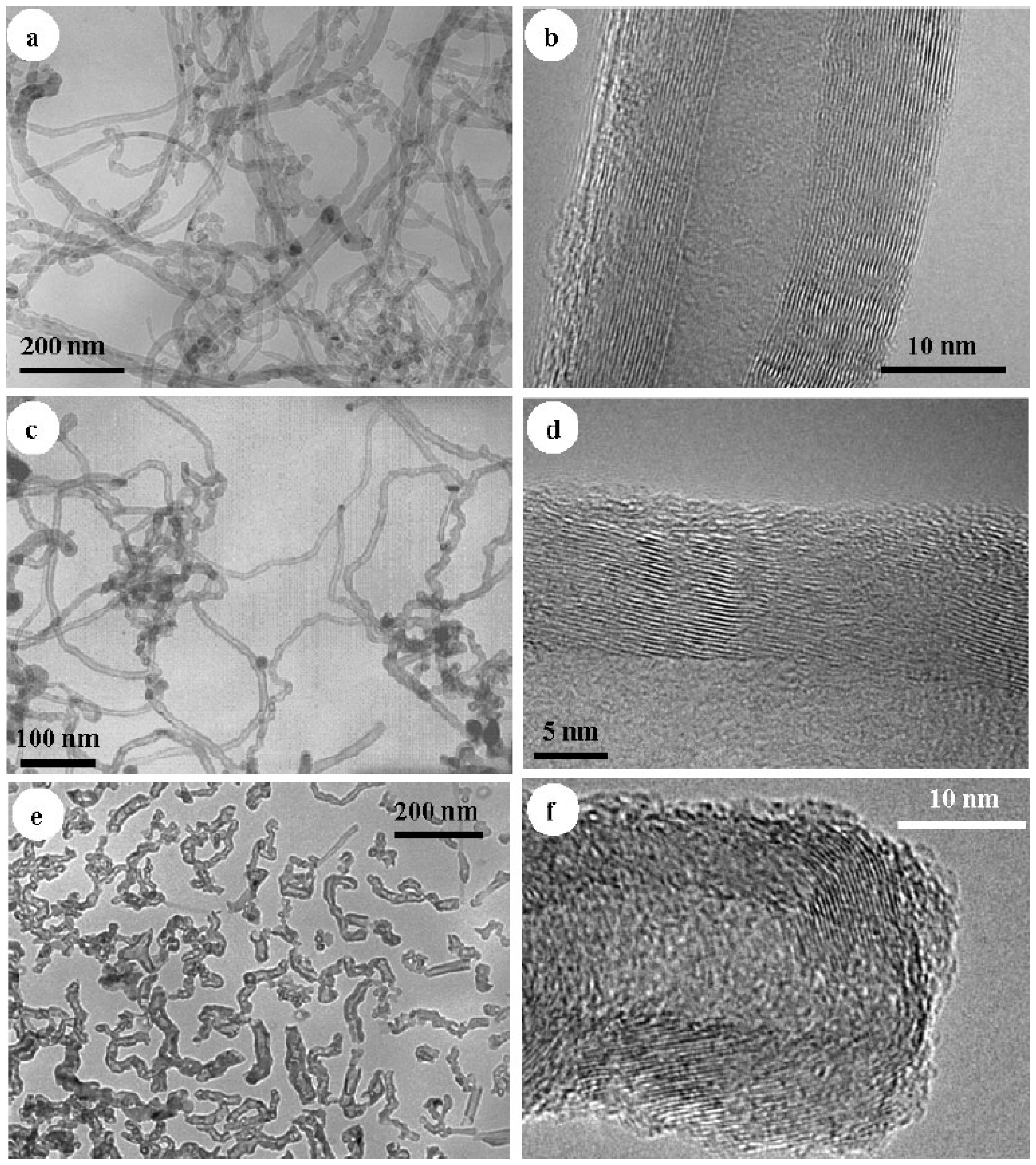
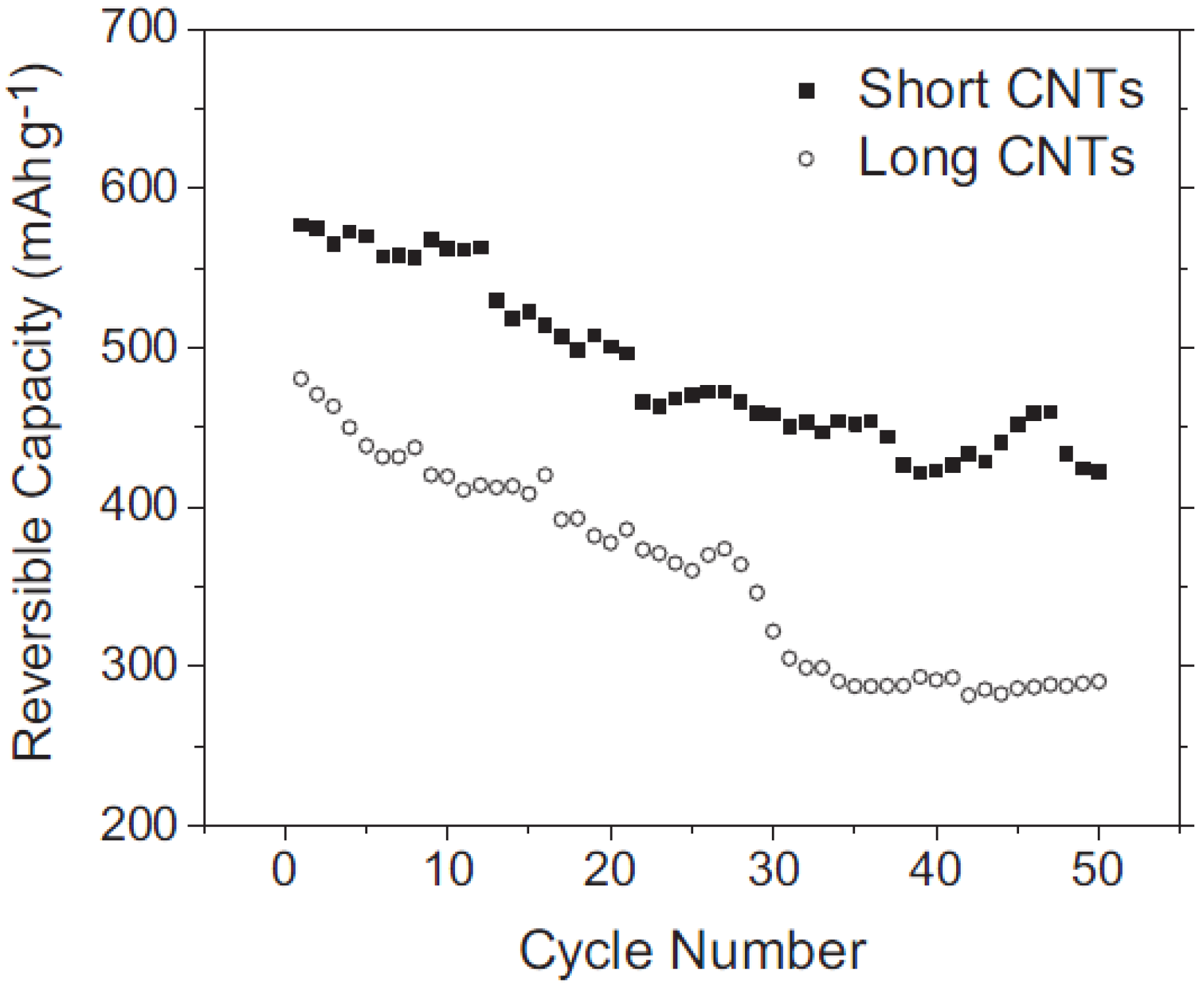
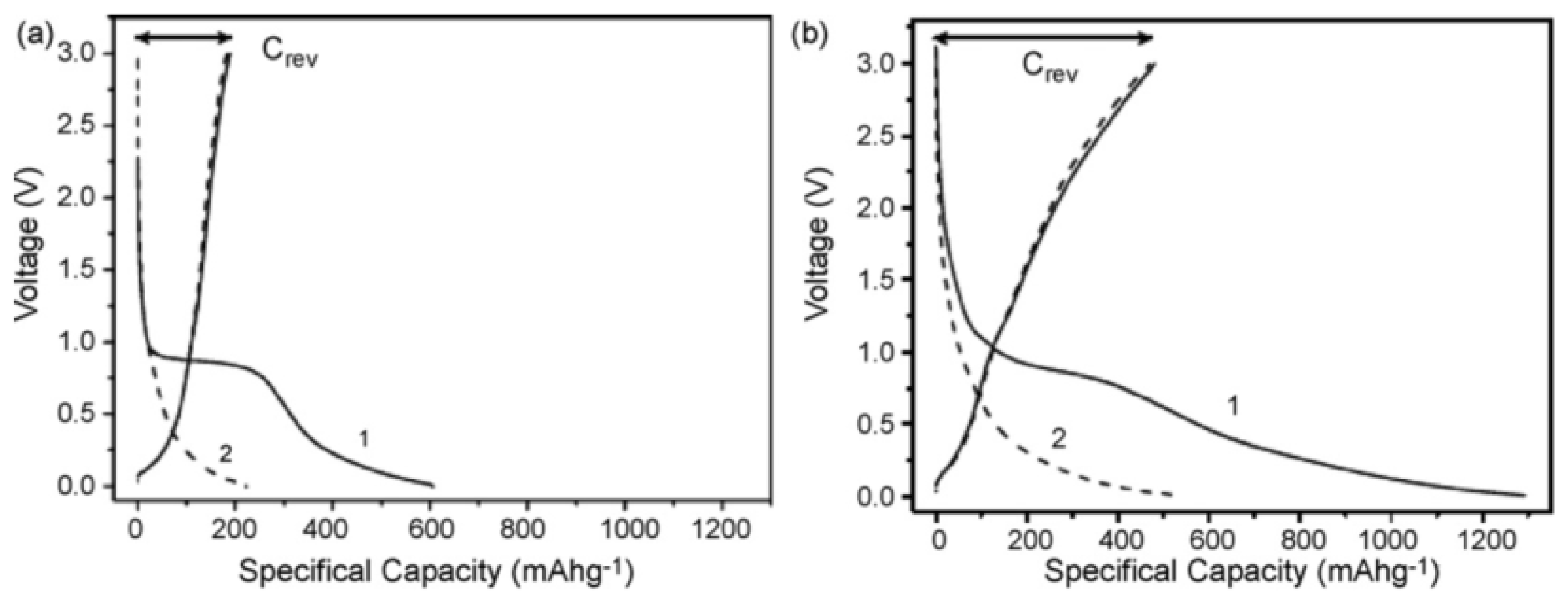
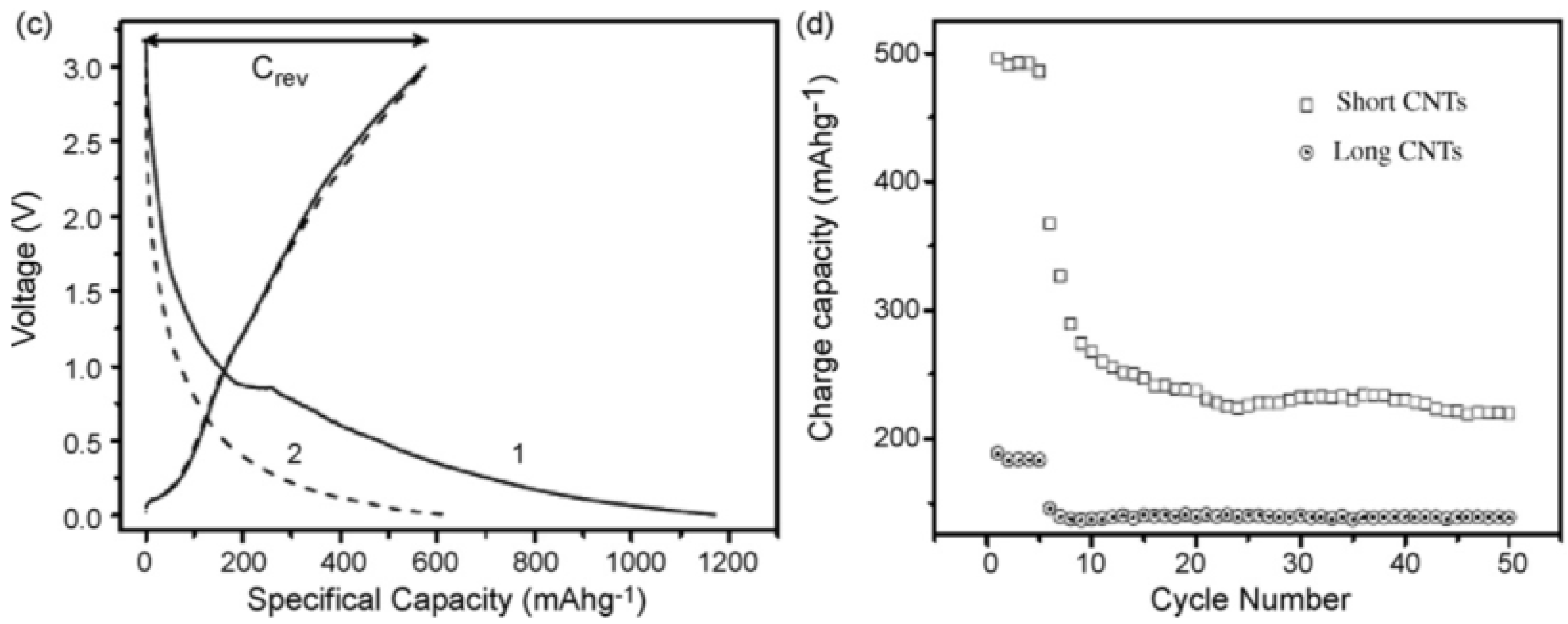
4.4. Methods of Modifying the Morphology of CNTs
5. Free-Standing CNT “Papers” for LIB Anodes
6. Conclusion and Future Prospects
Acknowledgments
References
- Yang, Z.-G.; Zhang, J.-L.; Kintner-Meyer, M.C.W.; Lu, X.-C.; Choi, D.-W.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Kaskhedikar, N.A.; Maier, J. Lithium storage in carbon Nanostructures. Adv. Mater. 2009, 21, 2664–2680. [Google Scholar] [CrossRef]
- Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.; Petit, P.; Robert, J.; Xu, C.; Lee, Y.H.; Kim, S.G.; Rinzler, A.G.; et al. Crystalline ropes of metallic carbon nanotubes. Science 1996, 273, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Zhao, X.; Shimoyama, H.; Sakai, G.; Kaneto, K. Physical properties of multiwalled carbon nanotubes. Int. J. Inorg. Mater. 1999, 1, 77–82. [Google Scholar] [CrossRef]
- Yu, M.-F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef] [PubMed]
- De las Casas, C.; Li, W.-Z. A review of application of carbon nanotubes for lithium ion battery anode material. J. Power Sources 2012, 208, 74–85. [Google Scholar]
- Yang, Z.-H.; Wu, H.-Q. Electrochemical intercalation of lithium into raw carbon nanotubes. Mater. Chem. Phys. 2001, 71, 7–11. [Google Scholar] [CrossRef]
- Zhao, J.-J.; Buldum, A.; Han, J.; Lu, J.-P. First-principles study of Li-intercalated carbon nanotube ropes. Phys. Rev. Lett. 2000, 85, 1706–1709. [Google Scholar] [CrossRef] [PubMed]
- Senami, M.; Lkeda, Y.; Fukushima, A.; Tachibana, A. Theoretical study of adsorption of lithium atom on carbon nanotube. AIP Adv. 2011, 1, 042106:1–042116:2. [Google Scholar] [CrossRef]
- Shimoda, H.; Gao, B.; Tang, X.P.; Kleinhammes, A.; Fleming, L.; Wu, Y.; Zhou, O. Lithium intercalation into opened single-wall carbon nanotubes: Storage capacity and electronic properties. Phys. Rev. Lett. 2002, 88, 015502:1–015502:4. [Google Scholar]
- Song, B.; Yang, J.; Zhao, J.; Fang, H. Intercalation and diffusion of lithium ions in a carbon nanotube bundle by ab initio molecular dynamics simulations. Energy Environ. Sci. 2011, 4, 1379–1384. [Google Scholar] [CrossRef]
- Khantha, M.; Cordero, N.A.; Alonso, J.A.; Cawkwell, M.; Girifalco, L.A. Interaction and concerted diffusion of lithium in a (5,5) carbon nanotube. Phys. Rev. B 2008, 78, 115430:1–115430:9. [Google Scholar] [CrossRef]
- Zhao, M.; Xia, Y.; Liu, X.; Tan, Z.; Huang, B.; Li, F.; Ji, Y.; Song, C. Curvature-induced condensation of lithium confined inside single-walled carbon nanotubes: First-principles calculations. Phys. Lett. A 2005, 340, 434–439. [Google Scholar] [CrossRef]
- Liu, Y.; Yukawa, H.; Morinaga, M. First-principles study on lithium absorption in carbon nanotubes. Comp. Mater. Sci. 2004, 30, 50–56. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.J.; Chan, C.T. Theoretical study of alkali-atom insertion into small-radius carbon nanotubes to form single-atom chains. Phys. Rev. B 2001, 64, 085420:1–085420:5. [Google Scholar]
- Meunier, V.; Kephart, J.; Roland, C.; Bernholc, J. Ab initio investigations of lithium diffusion in carbon nanotube systems. Phys. Rev. Lett. 2002, 88, 075506:1–075506:4. [Google Scholar] [CrossRef]
- Dubot, P.; Cenedese, P. Modeling of molecular hydrogen and lithium adsorption on single-wall carbon nanotubes. Phys. Rev. B 2001, 63, 241402:1–241402:4. [Google Scholar] [CrossRef]
- Garau, C.; Frontera, A.; Quiñonero, D.; Costa, A.; Ballester, P.; Deya, P.M. Ab initio investigations of lithium diffusion in single-walled carbon nanotubes. Chem. Phys. 2004, 297, 85–91. [Google Scholar] [CrossRef]
- Garau, C.; Frontera, A.; Quinonero, D.; Costa, A.; Ballester, P.; Deya, P.M. Lithium diffusion in single-walled carbon nanotubes: a theoretical study. Chem. Phys. Lett. 2003, 374, 548–555. [Google Scholar] [CrossRef]
- Ishidate, K.N.; Hasegawa, M. Energetics of lithium ion adsorption on defective carbon nanotubes. Phys. Rev. B 2005, 71, 245418:1–245418:6. [Google Scholar]
- Ishidate, K.N.; Sasaki, K.; Oikawa, Y.; Baba, M.; Hasegawa, M. Density functional electronic structure calculations of lithium ion adsorption on defective carbon nanotubes. J. Surf. Sci. Nanotech. 2005, 3, 358–361. [Google Scholar] [CrossRef]
- Fagan, S.B.; Guerini, S.; Mendes Filho, J.; Lemos, V. Lithium intercalation into single-wall carbon nanotube bundles. Microelectron. J. 2005, 36, 499–501. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Raffaelle, R.P.; Landi, B.J.; Harris, J.D.; Bailey, S.G.; Hepp, A.F. Carbon nanotubes for power applications. Mater. Sci. Eng. B 2005, 116, 233–243. [Google Scholar] [CrossRef]
- Qian, D.; Wagner, G.J.; Liu, W.K.; Yu, M.F.; Ruoff, R.S. Mechanics of carbon nanotubes. Appl. Mech. Rev. 2002, 55, 495–533. [Google Scholar] [CrossRef]
- Dai, H. Carbon nanotubes: Opportunities and challenges. Surf. Sci. 2002, 500, 218–241. [Google Scholar] [CrossRef]
- Huang, S.; Woodson, M.; Smalley, R.; Liu, J. Growth mechanism of oriented long single walled carbon nanotubes using “fast-heating” chemical vapor deposition process. Nano Lett. 2004, 4, 1025–1028. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C. Science of Fullerenes and Carbon Nanotubes; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Qin, L.-C. Determination of the chiral indices (n, m) of carbon nanotubes by electron diffraction. Phys. Chem. Chem. Phys. 2007, 9, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.A.; Bayazit, M.K.; Coleman, K.S.; Shaffer, M.S.P. Unweaving the rainbow: A review of the relationship between single-walled carbon nanotube molecular structures and their chemical reactivity. Chem. Soc. Rev. 2012, 41, 4409–4429. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, S.; Hara, T.; Iwai, Y.; Suzuki, Y. Metallic and semiconducting single-walled carbon nanotubes as the anode material of Li ion secondary battery. Mater. Lett. 2008, 62, 2917–2920. [Google Scholar] [CrossRef]
- Udomvech, A.; Kerdcharoen, T. Theoretical investigation of lithium-atom insertion into ultra-small diameter carbon nanotubes. J. Korean Phys. Soc. 2008, 52, 1350–1354. [Google Scholar] [CrossRef]
- Liu, H.; Nishide, D.; Tanaka, T.; Kataura, H. Large-scale single-chirality separation of single-wall carbon nanotubes by simple gel Chromatography. Nat. Commun. 2011, 2, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Krupke, R.; Hennrich, F.; Lohneysen, H.V.; Kappes, M.M. Separation of metallic from semiconducting single-walled carbon nanotubes. Science 2003, 301, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-C.; Liu, M.; Sadanadan, B.; Rao, A.M. Electrochemical insertion of lithium into multi-walled carbon nanotubes prepared by catalytic decomposition. J. Power Sources 2002, 112, 216–221. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, Y.; Goodenough, J.B.; Lu, Y.; Chen, C.; Wang, L.; Liu, J. Exfoliation from carbon nanotubes versus tube size on lithium insertion. Electrochem. Commun. 2011, 13, 125–128. [Google Scholar] [CrossRef]
- Kang, C.; Lahiri, I.; Baskaran, R.; Kim, W.-G.; Sun, Y.-K.; Choi, W. 3-dimensional carbon nanotube for Li-ion battery anode. J. Power Sources 2012, 219, 364–370. [Google Scholar] [CrossRef]
- Welna, D.T.; Qu, L.; Taylor, B.E.; Dai, L.; Durstock, M.F. Vertically aligned carbon nanotube electrodes for lithium-ion batteries. J. Power Sources 2011, 196, 1455–1460. [Google Scholar] [CrossRef]
- Varzi, A.; Täubert, C.; Wohlfahrt-Mehrens, M.; Kreis, M.; Schütz, W. Study of multi-walled carbon nanotubes for lithium-ion battery electrodes. J. Power Sources 2011, 196, 3303–3309. [Google Scholar] [CrossRef]
- Chew, S.-Y.; Ng, S.-H.; Wang, J.; Novak, P.; Krumeich, F.; Chou, S.-L.; Chen, J.; Liu, H.-K. Flexible free-standing carbon nanotube films for model lithium-ion batteries. Carbon 2009, 47, 2976–2983. [Google Scholar] [CrossRef]
- Frackowiak, E.; Gautier, S.; Gaucher, H.; Bonnamy, S.; Beguin, F. Electtrochemical storage of lithium multiwalled carbon nanotubes. Carbon 1999, 37, 61–69. [Google Scholar] [CrossRef]
- Sheem, K.; Lee, Y.H.; Lim, H.S. High-density positive electrodes containing carbon nanotubes for use in Li-ion cells. J. Power Sources 2006, 158, 1425–1430. [Google Scholar] [CrossRef]
- Guo, Z.P.; Zhao, Z.W.; Liu, H.K.; Dou, S.X. Electrochemical lithiation and de-lithiation of MWNT–Sn/SnNi nanocomposites. Carbon 2005, 43, 1392–1399. [Google Scholar] [CrossRef]
- Lin, K.; Xu, Y.; He, G.; Wang, X. The kinetic and thermodynamic analysis of Li ion in multi-walled carbon nanotubes. Mater. Chem. Phys. 2006, 99, 190–196. [Google Scholar] [CrossRef]
- Maurin, G.; Bousquet, Ch.; Henn, F.; Bernier, P.; Almairac, R.; Simon, B. Electrochemical intercalation of lithium into multiwall carbon nanotubes. Chem. Phys. Lett. 1999, 312, 14–18. [Google Scholar] [CrossRef]
- Mukhopadhyay, I.; Hoshino, N.; Kawasaki, S.; Okino, F.; Hsu, W.K.; Touhara, H. Electrochemical Li insertion in B-doped multiwall carbon nanotubes. J. Electrochem. Soc. 2002, 149, A39–A44. [Google Scholar] [CrossRef]
- Yang, S.; Song, H.; Chen, X.; Okotrub, A.V.; Bulusheva, L.G. Electrochemical performance of arc-produced carbon nanotubes as anode material for lithium-ion batteries. Electrochim. Acta 2007, 52, 5286–5293. [Google Scholar] [CrossRef]
- Yang, Z.H.; Zhou, Y.H.; Sang, S.B.; Feng, Y.; Wu, H.Q. Lithium insertion into multi-walled raw carbon nanotubes pre-doped with lithium. Mater. Chem. Phys. 2005, 89, 295–299. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, Q.Y.; Gu, C.; Yang, Y. Preparation of multi-walled carbon nanotube array electrodes and its electrochemical intercalation behavior of Li ions. Chem. Phys. Lett. 2002, 358, 77–82. [Google Scholar] [CrossRef]
- Maurin, G.; Bousquet, Ch.; Henna, F.; Bernier, P.; Almairac, R.; Simon, B. Electrochemical lithium intercalation into multiwall carbon nanotubes: A micro-Raman study. Solid State Ionics 2000, 136–137, 1295–1299. [Google Scholar] [CrossRef]
- Leroux, F.; Méténier, K.; Gautier, S.; Frackowiak, E.; Bonnamy, S.; Béguin, F. Electrochemical insertion of lithium in catalytic multi-walled carbon nanotubes. J. Power Sources 1999, 81–82, 317–322. [Google Scholar] [CrossRef]
- Yang, Z.; Sang, S.; Huang, K.; Wu, H.-Q. Lithium insertion into the raw multi-walled carbon nanotubes pre-doped with lithium—An electrochemical impedance study. Diam. Relat. Mater. 2004, 13, 99–105. [Google Scholar] [CrossRef]
- Oktaviano, H.S.; Yamada, K.; Waki, K. Nano-drilled multiwalled carbon nanotubes: Characterizations and application for LIB anode materials. J. Mater. Chem. 2012, 22, 25167:1–25167:7. [Google Scholar] [CrossRef]
- Eom, J.Y.; Kwon, H.S.; Liu, J.; Zhou, O. Lithium insertion into purified and etched multi-walled carbon nanotubes synthesized on supported catalysts by thermal CVD. Carbon 2004, 42, 2589–2596. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Wu, H.-Q. Electrochemical intercalation of lithium into carbon nanotubes. Solid State Ionics 2001, 143, 173–180. [Google Scholar] [CrossRef]
- Scott Morris, R.; Dixon, B.G.; Gennett, T.; Raffaelle, R.; Heben, M.J. High-energy, rechargeable Li-ion battery based on carbon nanotube technology. J. Power Sources 2004, 138, 277–280. [Google Scholar] [CrossRef]
- Klink, S.; Ventosa, E.; Xia, W.; La Mantia, F.; Muhler, M.; Schuhmann, W. Tailoring of CNT surface oxygen groups by gas-phase oxidation and its implications for lithium ion batteries. Electrochem. Commun. 2012, 15, 10–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, T.; Wang, J.; Min, G.; Pan, L.; Song, Z.; Sun, Z.; Zhou, W.; Zhang, J. The study of multi-walled carbon nanotubes with different diameter as anodes for lithium-ion batteries. Appl. Surf. Sci. 2012, 258, 4729–4732. [Google Scholar] [CrossRef]
- Udomvech, A.; Kerdcharoen, T.; Osotchan, T. First principles study of Li and Li+ adsorbed on carbon nanotube: Variation of tubule diameter and length. Chem. Phys. Lett. 2005, 406, 161–166. [Google Scholar] [CrossRef]
- Eom, J.-Y.; Kwon, H.-S. Improved lithium insertion/extraction properties of single-walled carbon nanotubes by high-energy ball milling. J. Mater. Res. 2008, 23, 2458–2466. [Google Scholar] [CrossRef]
- Pierard, N.; Fonseca, A.; Konya, Z.; Willems, I.; van Tendeloo, G.; Nagy, J.B. Production of short carbon nanotubes with open tips by ball milling. Chem. Phys. Lett. 2001, 335, 1–8. [Google Scholar] [CrossRef]
- Gao, B.; Bower, C.; Lorentzen, J.D.; Fleming, L.; Kleinhammes, A.; Tang, X.P.; McNeil, L.E.; Wu, Y.; Zhou, O. Enhanced saturation lithium composition in ball-milled single-walled carbon nanotubes. Chem. Phys. Lett. 2000, 327, 69–75. [Google Scholar] [CrossRef]
- Yang, S.; Huo, J.; Song, H.; Chen, X. A comparative study of electrochemical properties of two kinds of carbon nanotubes as anode materials for lithium ion batteries. Electrochim. Acta 2008, 53, 2238–2244. [Google Scholar] [CrossRef]
- Yang, S.; Song, H.; Chen, X. Electrochemical performance o expanded mesocarbon microbeads as anode material for lithium-ion batteries. Electrochem. Commun. 2006, 8, 137–142. [Google Scholar] [CrossRef]
- Ren, Z.F.; Huang, Z.P.; Xu, J.W.; Wang, J.H.; Bush, P.; Siegal, M.P.; Provencio, P.N. Synthesis of large arrays of well-aligned carbon nanotubes on glass. Science 1998, 282, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-X.; Wang, J.-N.; Chang, H.; Zhang, Y.-F. Preparation of short carbon nanotubes and application as an electrode material in li-ion batteries. Adv. Func. Mater. 2007, 17, 3613–3618. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Su, L. Preparation and electrochemical performance of ultra-short carbon nanotubes. J. Power Sources 2009, 186, 194–200. [Google Scholar] [CrossRef]
- Spinney, P.S.; Howitt, D.G.; Collins, S.D.; Smith, R.L. Electron beam stimulated oxidation of Carbon. Nanotechnology 2009, 20, 465301:1–465301:6. [Google Scholar] [CrossRef]
- Mikó, C.; Milas, M.; Seo, J.W.; Couteau, E.; Barišić, R.G.; Forró, L. Effect of electron irradiation on the electrical properties of fibers of aligned single-walled carbon nanotubes. Appl. Phys. Lett. 2003, 83, 4622–4624. [Google Scholar] [CrossRef]
- Mikó, C.; Seo, J.W.; Gaál, R.; Kulik, A.; Forró, L. Effect of electron and ultraviolet irradiation on aligned carbon nanotube fibers. Phys. Stat. Sol. B 2006, 243, 3351–3354. [Google Scholar] [CrossRef]
- Beuneu, F.; Huillier, C. Modification of multiwall carbon nanotubes by electron irradiation: An ESR study. Phys. Rev. B 1999, 59, 5945–5949. [Google Scholar] [CrossRef]
- Skakaloval, V.; Kaiser, A.B.; Osvath, Z.; Vertesy, G.; Biro, L.P.; Roth, V. Ion-irradiation effects on conduction in single-wall carbon nanotube networks. Appl. Phys. A 2008, 90, 597–602. [Google Scholar] [CrossRef]
- Prem Kumar, T.; Manuel Stephan, A.; Thayananth, P.; Subramanian, V.; Gopukumar, S.; Renganathan, N.G.; Raghavan, M.; Muniyandi, N. Thermally oxidized graphites as anodes for lithum-ion cells. J. Power Sources 2001, 97–98, 118–121. [Google Scholar] [CrossRef]
- Cui, L.-F.; Hu, L.; Choi, J.-W.; Cui, Y. Light-weight free-standing carbon nanotube-silicon films for anodes of lithium ion batteries. ACS Nano 2010, 4, 3671–3678. [Google Scholar] [CrossRef] [PubMed]
- Gwon, H.; Kim, H.-S.; Lee, K.-U.; Seo, D.-H.; Park, Y.-C.; Lee, Y.-S.; Ahn, B.-T.; Kang, K. Flexible energy storage devices based on graphene papar. Energy Environ. Sci. 2011, 4, 1277–1283. [Google Scholar] [CrossRef]
- Ng, S.H.; Wang, J.; Guo, Z.P.; Chen, J.; Wang, G.X.; Liu, H.K. Single wall carbon nanotube paper as anode for lithium-ion battery. Electrochim. Acta 2005, 51, 23–28. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Hu, Y.; Wang, J.; Li, Y.; Cai, M.; Li, R.; Sun, X. Novel approach toward a binder-free and current collector-free anode configuration: Highly flexible nanoporous carbon nanotube electrodes with strong mechanical strength harvesting improved lithium storage. J. Mater. Chem. 2012, 22, 18847–18853. [Google Scholar] [CrossRef]
- Landi, B.J.; Ganter, M.J.; Schauerman, C.M.; Cress, C.D.; Raffaelle, R.P. Lithium ion capacity of single wall carbon nanotube paper electrodes. J. Phys. Chem. C 2008, 112, 7509–7515. [Google Scholar] [CrossRef]
- DiLeo, R.A.; Frisco, S.; Ganter, M.J.; Rogers, R.E.; Raffaelle, R.P.; Landi, B.J. Hybrid germanium nanoparticle—Single-wall carbon nanotube free-standing anodes for lithium ion batteries. J. Phys. Chem. C 2011, 115, 22609–22614. [Google Scholar] [CrossRef]
- Lahiri, I.; Oh, S.-W.; Hwang, J.Y.; Cho, S.; Sun, Y.-K.; Banerjee, R.; Choi, W. High capacity and excellent stability of lithium ion battery anode using interface-controlled binder-free multiwall carbon nanotubes grown on copper. ACS Nano 2010, 4, 3440–3446. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Minett, A.; Lynam, C.; Wang, J.; Wallace, G.G. Flexible, aligned carbon nanotube/conducting polymer electrodes for a lithium-ion battery. Chem. Mater. 2007, 19, 3595–3601. [Google Scholar] [CrossRef]
- Landi, B.J.; Ganter, M.J.; Cress, C.D.; DiLeo, R.A.; Raffaelle, R.P. Carbon nanotubes for lithium ion batteries. Energy Environ. Sci. 2009, 2, 638–654. [Google Scholar] [CrossRef]
- Liu, X-M.; Huang, Z.; Oh, S.W.; Zhang, B.; Ma, P-M.; Yuen, M.M.F.; Kim, J-K. Carbon nanotube (CNT)-based composites as electrode material for rechargeable Li-ion batteries: A review. Compos. Sci. Technol. 2012, 72, 121–144. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xiong, Z.; Yun, Y.S.; Jin, H.-J. Applications of Carbon Nanotubes for Lithium Ion Battery Anodes. Materials 2013, 6, 1138-1158. https://doi.org/10.3390/ma6031138
Xiong Z, Yun YS, Jin H-J. Applications of Carbon Nanotubes for Lithium Ion Battery Anodes. Materials. 2013; 6(3):1138-1158. https://doi.org/10.3390/ma6031138
Chicago/Turabian StyleXiong, Zhili, Young Soo Yun, and Hyoung-Joon Jin. 2013. "Applications of Carbon Nanotubes for Lithium Ion Battery Anodes" Materials 6, no. 3: 1138-1158. https://doi.org/10.3390/ma6031138
APA StyleXiong, Z., Yun, Y. S., & Jin, H.-J. (2013). Applications of Carbon Nanotubes for Lithium Ion Battery Anodes. Materials, 6(3), 1138-1158. https://doi.org/10.3390/ma6031138



