Release of Ciprofloxacin-HCl and Dexamethasone Phosphate by Hyaluronic Acid Containing Silicone Polymers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ciprofloxacin-HCl Release from HA Materials
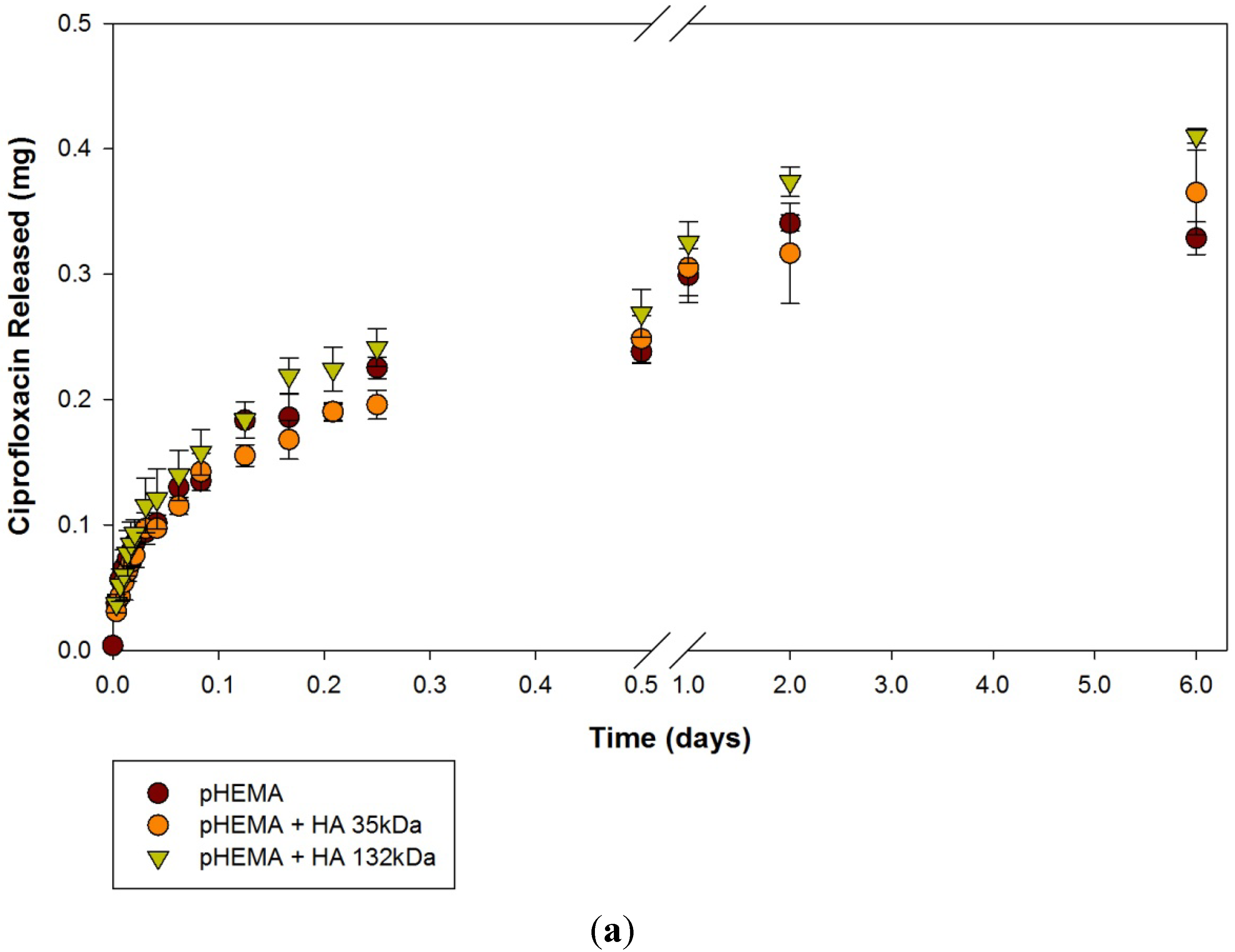
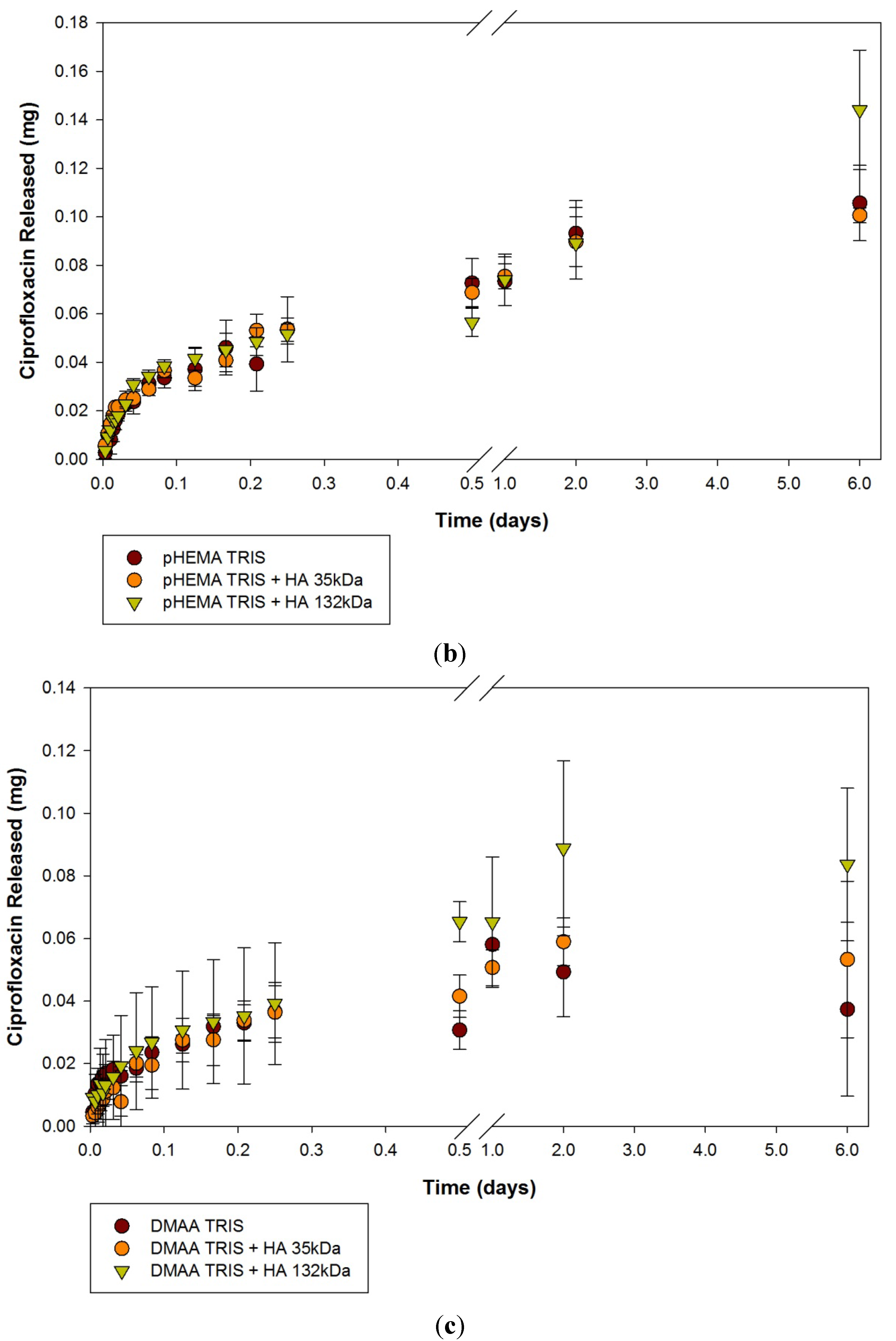
| Modification | None (control) | HA 35 | HA 132 | |||
|---|---|---|---|---|---|---|
| Material | mean | SD | mean | SD | mean | SD |
| pHEMA | 328 | 13 | 337 | 33 | 361 | 5 |
| pHEMA TRIS | 99 | 12 | 85 | 0.4 | 133 | 22 |
| DMAA TRIS | 48 | 3 | 68 | 5 | 86 | 22 |

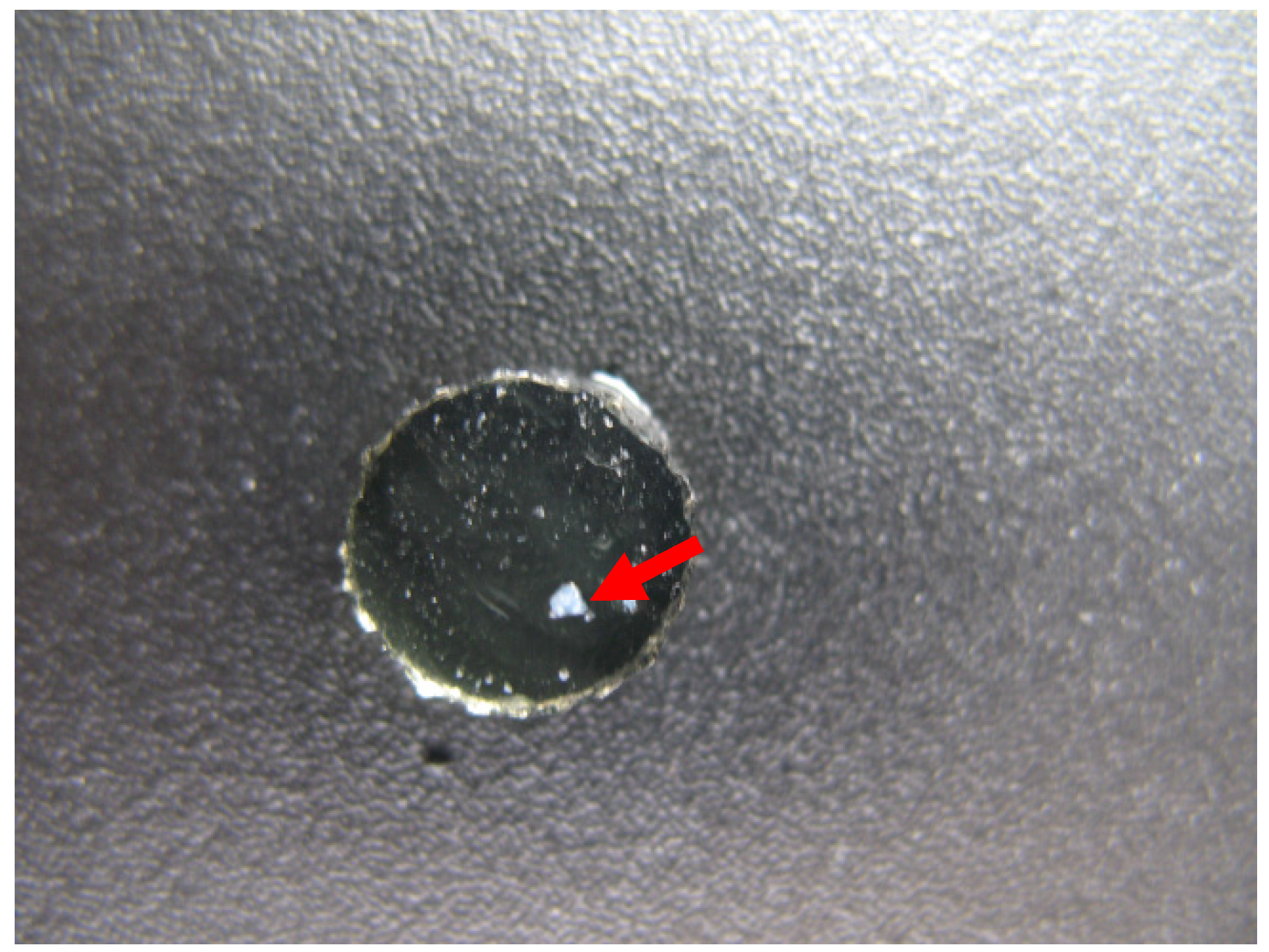
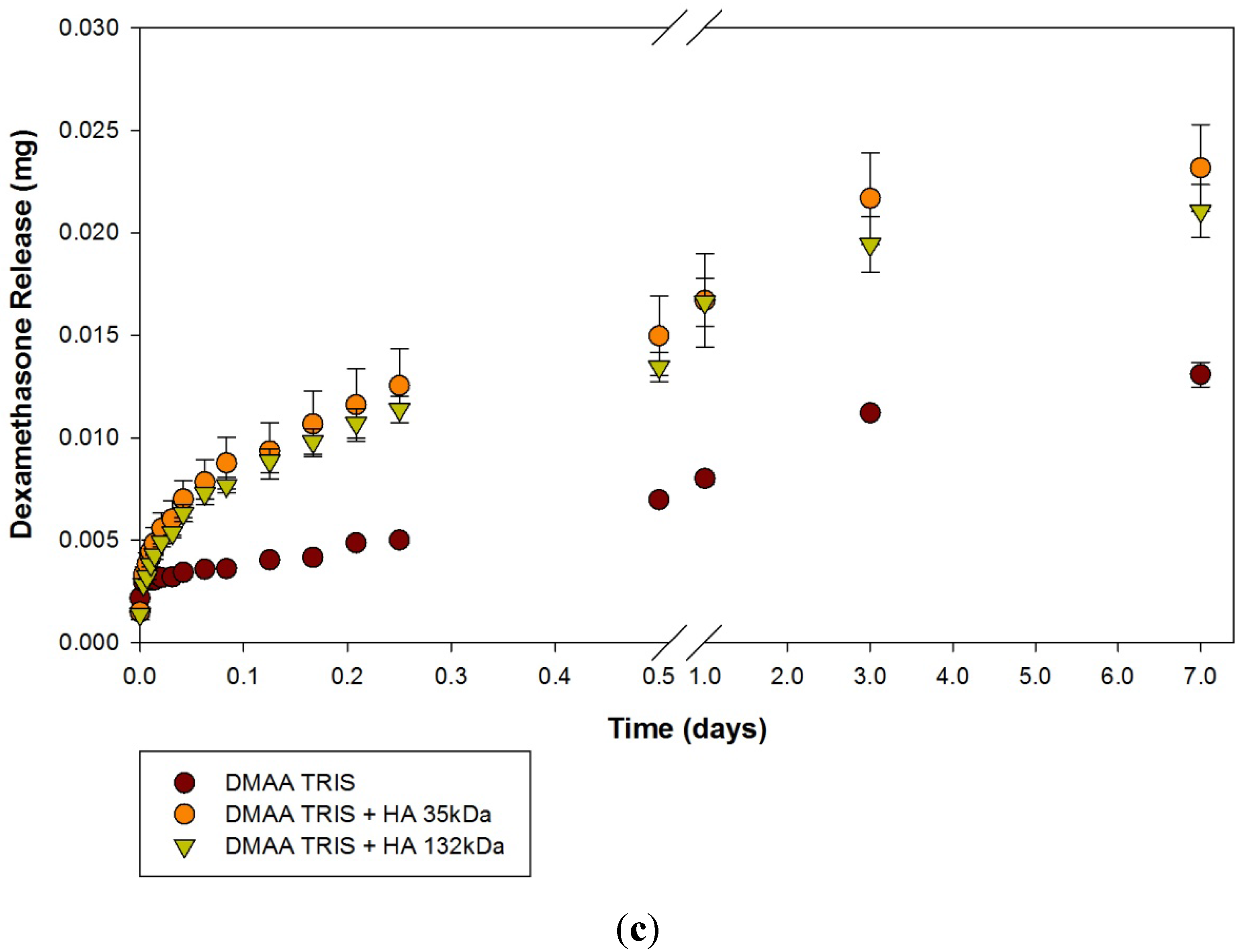
2.2. Dexamethasone Release from HA Materials
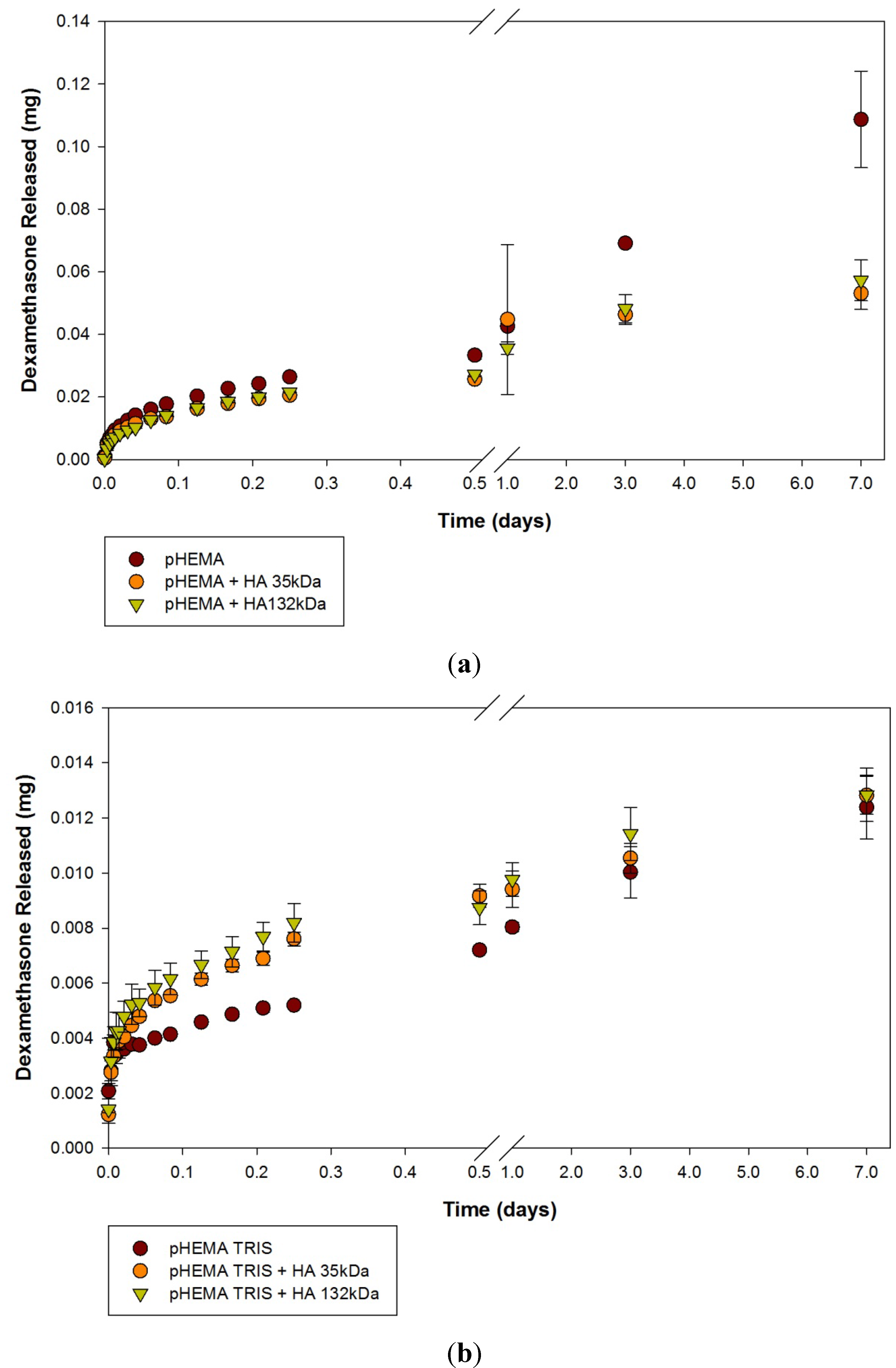
| Modification | None (control) | HA 35 | HA 132 | |||
|---|---|---|---|---|---|---|
| Material | mean | SD | Mean | SD | mean | SD |
| pHEMA | 109 | 15 | 53 | 5 | 57 | 6 |
| pHEMA TRIS | 12 | 1 | 13 | 0.7 | 13 | 0.9 |
| DMAA TRIS | 13 | 0.6 | 23 | 2 | 21 | 1 |
3. Experimental Section
3.1. Solution Preparation
3.2. Model Materials
3.3. Drug Loading
3.4. Release Kinetics
3.5. Analysis
3.6. Photographs
4. Conclusions
Acknowledgments
References
- Le Bourlais, C.; Acar, L.; Zia, H.; Sado, P.A.; Needham, T.; Leverge, R. Ophthalmic drug delivery systems—Recent advances. Prog. Retin. Eye Res. 1998, 17, 33–58. [Google Scholar] [CrossRef] [PubMed]
- Xinming, L.; Yingde, C.; Lloyd, A.W.; Mikhalovsky, S.V.; Sandeman, S.R.; Howel, C.A.; Liewen, L. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: A review. Cont. Lens Anterior Eye 2008, 31, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, J.B.; Dohlman, C.H.; Kohane, D.S. Contact lenses for drug delivery. Semin. Ophthalmol. 2009, 24, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, K.; Järvinen, T.; Urtii, A. Ocular absorption following topical delivery. Adv. Drug Deliv. Rev. 1995, 16, 3–19. [Google Scholar] [CrossRef]
- Ali, M.; Horikawa, S.; Venkatesh, S.; Saha, J.; Hong, J.W.; Byrne, M.E. Zero-order therapeutic release from imprinted hydrogel contact lenses within in vitro physiological ocular tear flow. J. Control. Release 2007, 124, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Sedlacek, J. Possibility of the application of ophthalmic drugs with the use of gel contact lenses. Ceska a Slov. Oftalmol. 1965, 21, 509–512. [Google Scholar]
- Waltman, S.R.; Kaufman, H.E. Use of hydrophilic contact lenses to increase ocular penetration of topical drugs. Investig. Ophthalmol. Vis. Sci. 1970, 9, 250–255. [Google Scholar]
- Hehl, E.-M.; Beck, R.; Luthard, K.; Guthoff, R.; Drewelow, B. Improved penetration of aminoglycosides and fluoroquinolones into the aqueous humour of patients by means of Acuvue contact lenses. Eur. J. Clin. Pharmacol. 1999, 55, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.R. Drug delivery through soft contact lenses. Br. J. Ophthalmol. 1988, 72, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Lesher, G.A.; Gunderson, G.G. Continuous drug delivery through the use of disposable contact lenses. Optom. Vis. Sci. 1993, 70, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Yañez, F.; Barreiro-Iglesias, R.; Concheiro, A. Imprinted soft contact lenses as norfloxacin delivery systems. J. Control. Release 2006, 113, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, J.B.; Hoare, T.R.; Iwata, N.G.; Behlau, I.; Dohlman, C.H.; Langer, R.; Kohane, D.S. A drug-eluting contact lens. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3346–3352. [Google Scholar] [CrossRef]
- Brown, M.B.; Jones, S.A. Hyaluronic acid: A unique topical vehicle for the localized delivery of drugs to the skin. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Byrne, M.E. Controlled release of high molecular weight hyaluronic acid from molecularly imprinted hydrogel contact lenses. Pharm. Res. 2009, 26, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, J.; Matou-Nasri, S.; Grau-Olivares, M.; Slevin, M. Therapeutic applications of hyaluronan. Mol. BioSyst. 2010, 6, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Mishra, P.; Agrawal, G.P. An insight on hyaluronic acid in drug targeting and drug delivery. J. Drug Target. 2008, 16, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-H.; Jones, S.A.; Forbes, B.; Martin, G.P.; Brown, M.B. Hyaluronan: Pharmaceutical characterization and drug delivery. Drug Deliv. 2005, 12, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Drobník, J. Hyaluronan in drug delivery. Adv. Drug Deliv. Rev. 1991, 7, 295–308. [Google Scholar] [CrossRef]
- Lapčík, L., Jr.; Lapčík, L.; de Smedt, S.; Demeester, J.; Chabreček, P. Hyaluronan: Preparation, structure, properties, and applications. Chem. Rev. 1998, 98, 2663–2684. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Laurent, U.B.G.; Fraser, J.R.E. Functions of hyaluronan. Ann. Rheum. Dis. 1995, 54, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Grau, E.L.; Polack, F.M.; Balazs, E.A. The protective effect of Na-hyaluronate to corneal endothelium. Exp. Eye Res. 1980, 31, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Camber, O.; Edman, P. Sodium hyaluronate as an ophthalmic vehicle: Some factors governing its effect on the ocular absorption of pilocarpine. Curr. Eye Res. 1989, 8, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Camber, O.; Edman, P.; Gurny, R. Influence of sodium hyaluronate on the meiotic effect of pilocarpine in rabbits. Curr. Eye Res. 1987, 6, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Gurny, R.; Ibrahim, H.; Aebi, A.; Buri, P.; Wilson, C.G.; Washington, N.; Edman, P.; Camber, O. Design and evaluation of controlled release systems for the eye. J. Control. Release 1987, 6, 367–373. [Google Scholar] [CrossRef]
- Saettone, M.F.; Giannaccini, B.; Chetoni, P.; Torracca, M.T.; Monti, D. Evaluation of high- and low-molecular-weight fractions of sodium hyaluronate and an ionic complex as adjuvants for topical ophthalmic vehicles containing pilocarpine. Int. J. Pharm. 1991, 72, 131–139. [Google Scholar] [CrossRef]
- Weeks, A.; Luensmann, D.; Boone, A.; Jones, L.; Sheardown, H. Hyaluronic acid as an internal wetting agent in model DMAA/TRIS contact lenses. J. Biomater. Appl. 2011. [Google Scholar] [CrossRef]
- Weeks, A.; Subbaraman, L.N.; Jones, L.; Sheardown, H. The competing effects of hyaluronic and methacrylic acid in model contact lenses. J. Biomater. Sci. Polym. Ed. 2011, 23, 1021–1038. [Google Scholar] [CrossRef]
- Brook, M.A.; Holloway, A.C.; Ng, K.K.; Hrynyk, M.; Moore, C.; Lall, R. Using a drug to structure its release matrix and release profile. Int. J. Pharm. 2008, 358, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D.; Jaanus, S.D. Clinical Ocular Pharmacology, 5th ed.; Butterworth-Heinemann: St. Louis, MO, USA, 2008; pp. 194–196. [Google Scholar]
- Charoo, N.A.; Kohli, K.; Ali, A.; Anwer, A. Ophthalmic delivery of ciprofloxacin hydrochloride from different polymer formulations: In vitro and in vivo studies. Drug Dev. Ind. Pharm. 2003, 29, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chauhan, A. Dexamethasone transport and ocular delivery from poly(hydroxyethyl methacrylate) gels. Int. J. Pharm. 2008, 353, 205–222. [Google Scholar] [PubMed]
- Schwartz, B. The response of ocular pressure to corticosteroids. Int. Ophthalmol. Clin. 1966, 6, 929–989. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.O.; Borgmann, A.R.; Roberts, M.D.; Fox, L.G. Corneal wound healing. I. Inhibition of stromal healing by three dexamethasone derivatives. Investig. Ophthalmol. Vis. Sci. 1970, 9, 703–709. [Google Scholar]
- Hui, A.; Boone, A.; Jones, L. Uptake and release of ciprofloxacin-HCl from conventional and silicone hydrogel contact lens materials. Eye Contact Lens 2008, 34, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Karlgard, C.C.S.; Jones, L.W.; Moresoli, C. Ciprofloxacin interaction with silicon-based and conventional hydrogel contact lenses. Eye Contact Lens 2003, 29, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Karlgard, C.C.S.; Wong, N.S.; Jones, L.W.; Moresoli, C. In vitro uptake and release studies of ocular pharmaceutical agents by silicon-containing and p-HEMA hydrogel contact lens materials. Int. J. Pharm. 2003, 257, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.Y.; Chung, T.W.; Kim, B.C.; Kim, M.K.; Lee, J.H.; Wee, W.R.; Cho, C.S. Release of ciprofloxacin from poloxamer-graft-hyaluronic acid hydrogels in vitro. Int. J. Pharm. 2003, 260, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Boone, A.; Hui, A.; Jones, L. Uptake and release of dexamethasone phosphate from silicone hydrogel and group I, II, and IV hydrogel contact lenses. Eye Contact Lens 2009, 35, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.W.; Faragher, R.G.A.; Denyer, S.P. Ocular biomaterials and implants. Biomaterials 2001, 22, 769–785. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, M.; Weeks, A.; Jones, L.; Sheardown, H. Immobilized hyaluronic acid containing model silicone hydrogels reduce protein adsorption. J. Biomater. Sci. Polym. Ed. 2008, 19, 1425–1436. [Google Scholar]
- Van Beek, M.; Jones, L.; Sheardown, H. Hyaluronic acid containing hydrogels for the reduction of protein adsorption. Biomaterials 2008, 29, 780–789. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nguyen, D.; Hui, A.; Weeks, A.; Heynen, M.; Joyce, E.; Sheardown, H.; Jones, L. Release of Ciprofloxacin-HCl and Dexamethasone Phosphate by Hyaluronic Acid Containing Silicone Polymers. Materials 2012, 5, 684-698. https://doi.org/10.3390/ma5040684
Nguyen D, Hui A, Weeks A, Heynen M, Joyce E, Sheardown H, Jones L. Release of Ciprofloxacin-HCl and Dexamethasone Phosphate by Hyaluronic Acid Containing Silicone Polymers. Materials. 2012; 5(4):684-698. https://doi.org/10.3390/ma5040684
Chicago/Turabian StyleNguyen, Darrene, Alex Hui, Andrea Weeks, Miriam Heynen, Elizabeth Joyce, Heather Sheardown, and Lyndon Jones. 2012. "Release of Ciprofloxacin-HCl and Dexamethasone Phosphate by Hyaluronic Acid Containing Silicone Polymers" Materials 5, no. 4: 684-698. https://doi.org/10.3390/ma5040684





