Impact of High Concentration Solutions on Hydraulic Properties of Geosynthetic Clay Liner Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
| Parameters | Na-bentonite |
|---|---|
| Specific gravity | 2.76 a |
| Montmorillonite content | 76.3% b |
| Mass per unit area | 4.91 kg/m2 |
| Void ratio | 4.2 |
| Dry density | 0.62 g/cm3 |
2.2. Experimental Method
| Working condition | Soak for 5 days | Soak for 10 days | Soak for 20 days | |||||||
| 50 mM | 100 mM | 200 mM | 50 mM | 100 mM | 200 mM | 50 mM | 100 mM | 200 mM | ||
| solutions | CaCl2 | 50-5 | 100-5 | 200-5 | 50-10 | 100-10 | 200-10 | 50-20 | 100-20 | 200-20 |
| MgCl2 | 50-5 | 100-5 | 200-5 | 50-10 | 100-10 | 200-10 | 50-20 | 100-20 | 200-20 | |
| NaCl | 50-5 | 100-5 | 200-5 | 50-10 | 100-10 | 200-10 | 50-20 | 100-20 | 200-20 | |
| KCl | 50-5 | 100-5 | 200-5 | 50-10 | 100-10 | 200-10 | 50-20 | 100-20 | 200-20 | |
3. Results and Discussion
3.1. GCL Hydraulic Conductivity and Thickness Variation Results under Water Soaking and Permeation

3.2. GCL Hydraulic Conductivity and Thickness Variation Results under Solution Soaking and Permeation
3.2.1. Impact of Solution Categories on GCL Hydraulic Conductivity
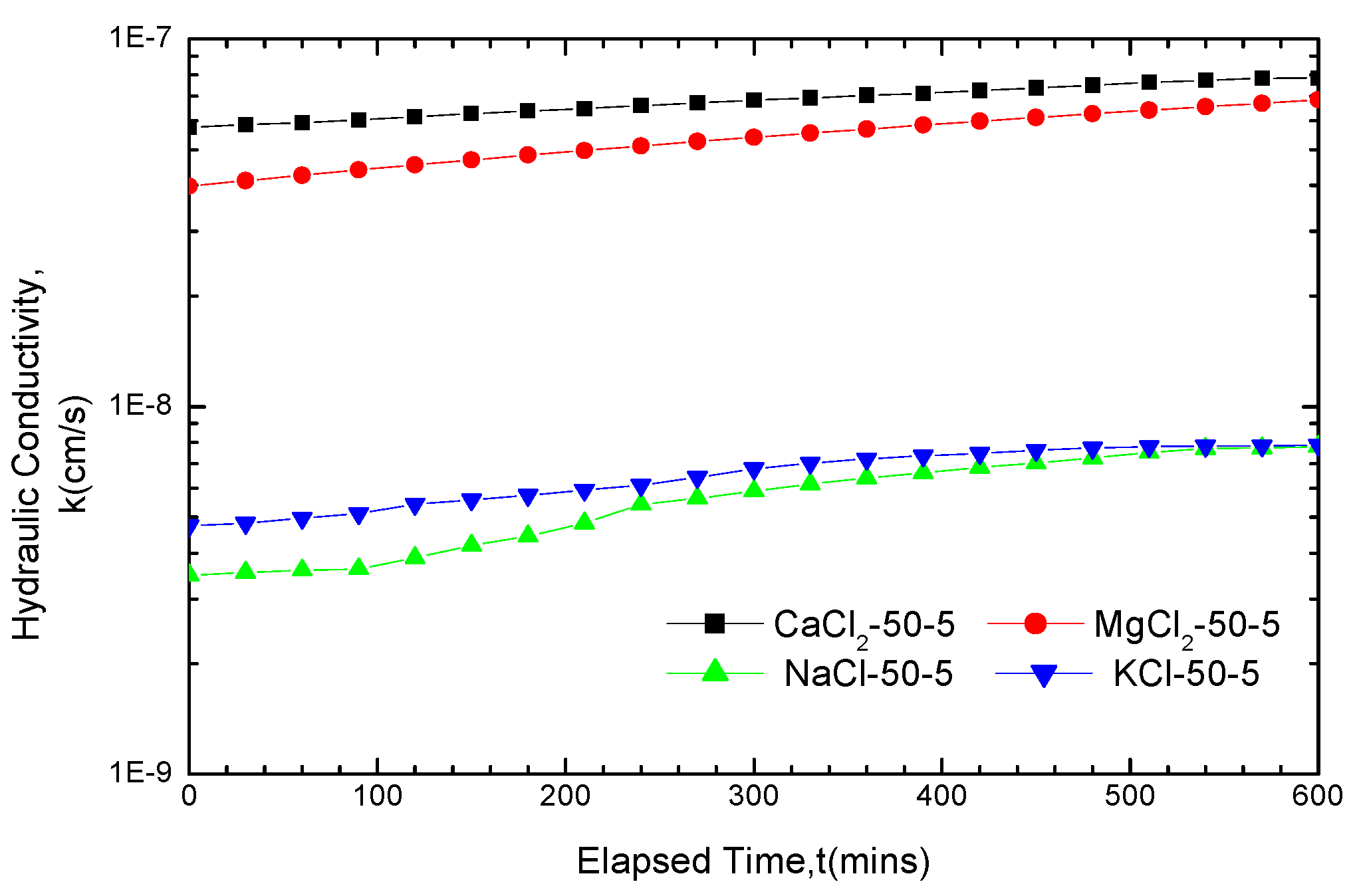
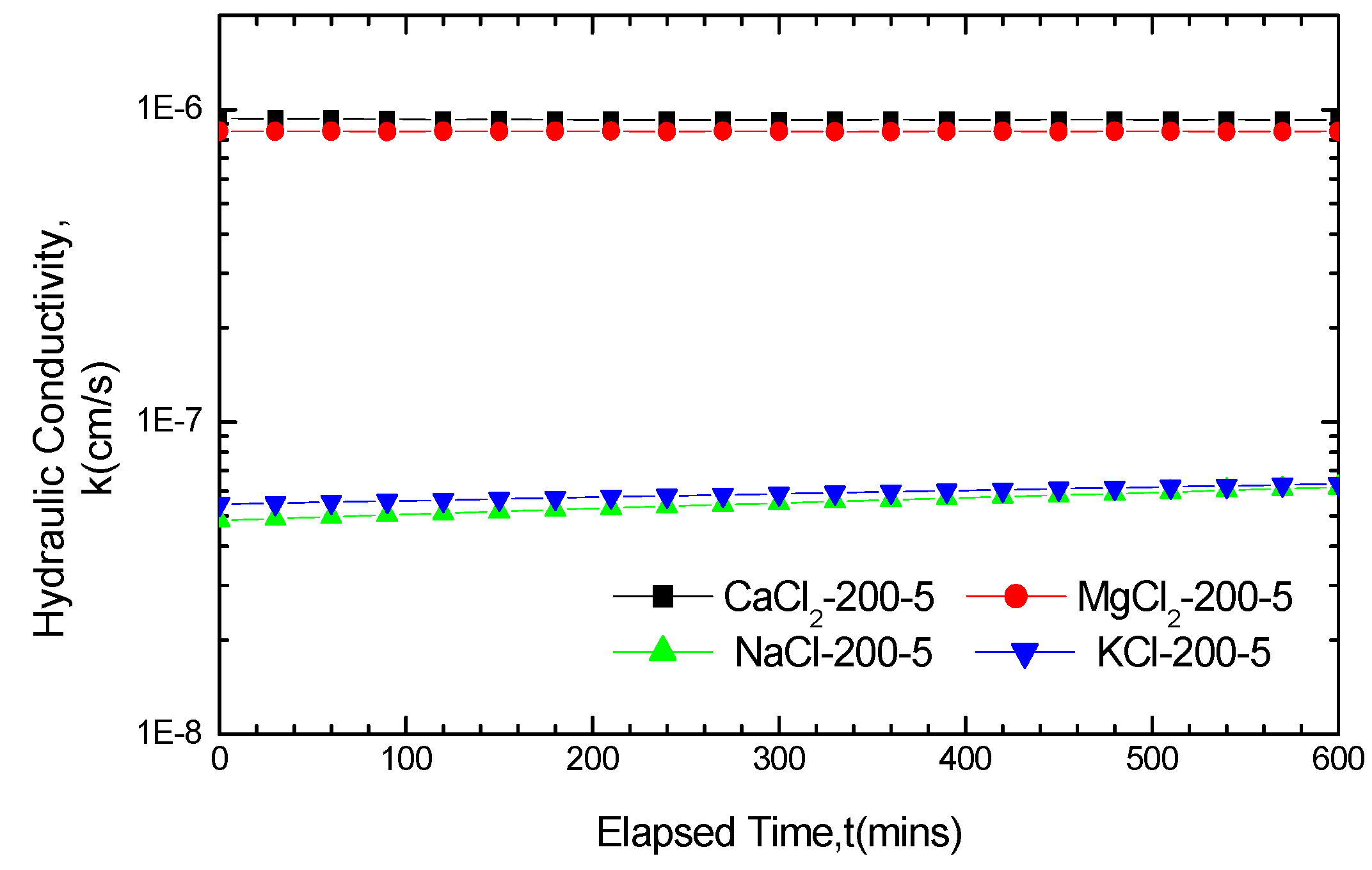
| Hydraulic conductivity k/10−7cm/s | 200-5 | 200-10 | 200-20 | 100-5 | 100-10 | 100-20 | 50-5 | 50-10 | 50-20 | |
|---|---|---|---|---|---|---|---|---|---|---|
| solutions | CaCl2 | 9.25 | 8.99 | 8.96 | 6.85 | 7.73 | 6.82 | 0.784 | 0.912 | 1.06 |
| MgCl2 | 8.52 | 8.17 | 7.84 | 4.57 | 5.65 | 6.31 | 0.684 | 0.771 | 0.835 | |
| NaCl | 0.617 | 0.643 | 0.532 | 0.0988 | 0.217 | 0.272 | 0.0779 | 0.115 | 0.171 | |
| KCl | 0.633 | 0.715 | 0.656 | 0.124 | 0.287 | 0.323 | 0.0786 | 0.147 | 0.205 | |
3.2.2. Impact of Solution Concentrations on GCL Hydraulic Conductivity
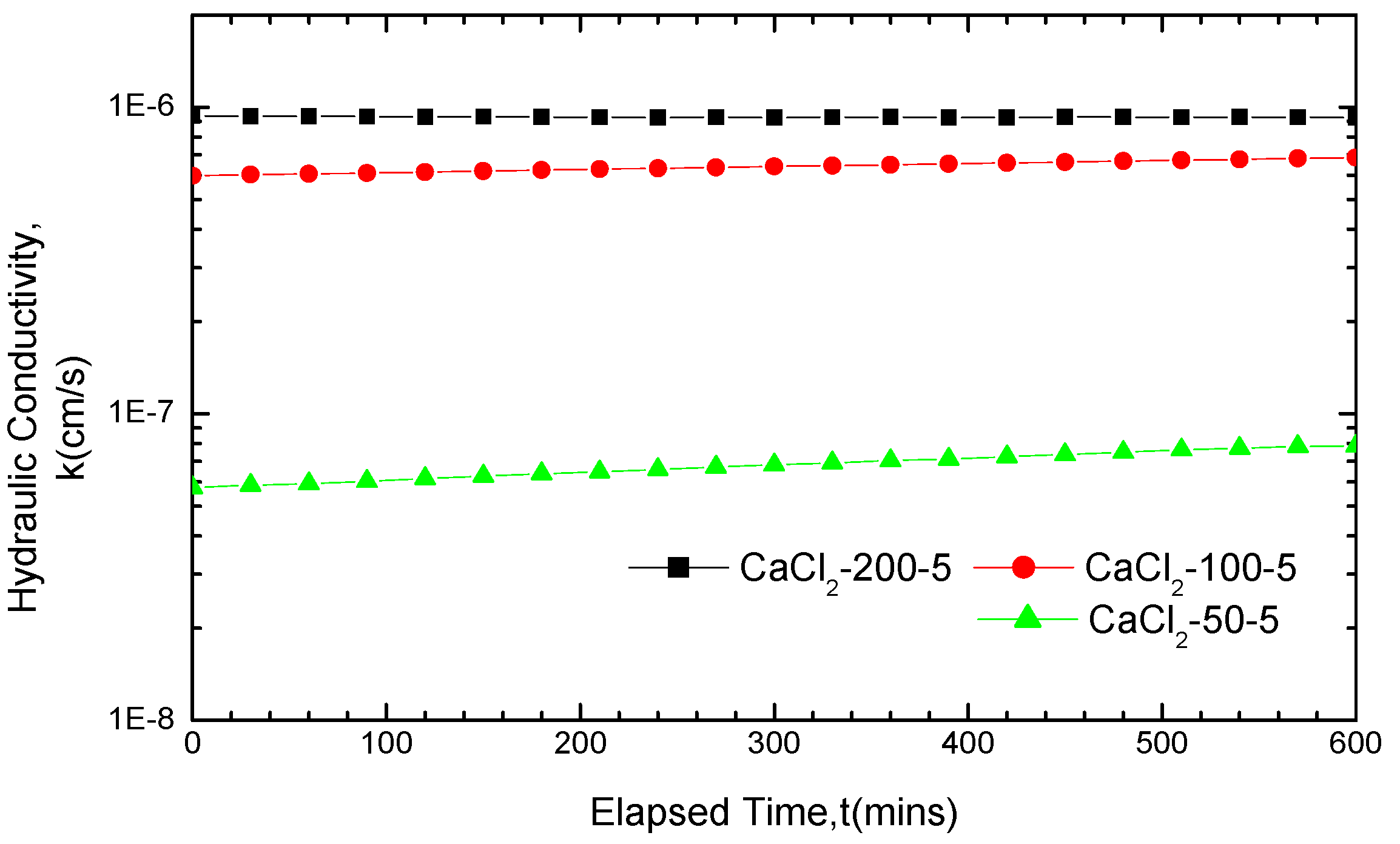
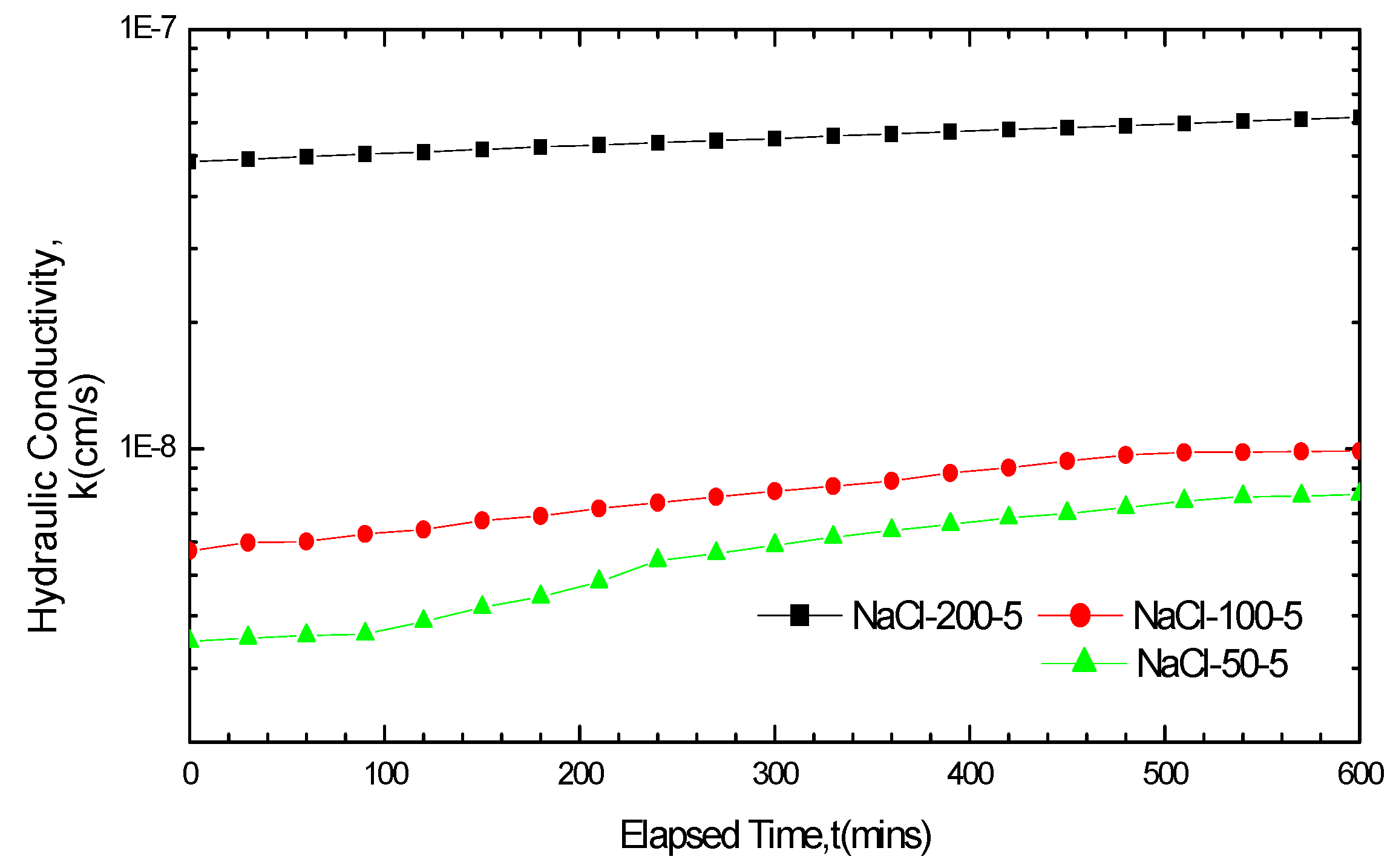
3.2.3. Impact of Soak Times on GCL Hydraulic Conductivity
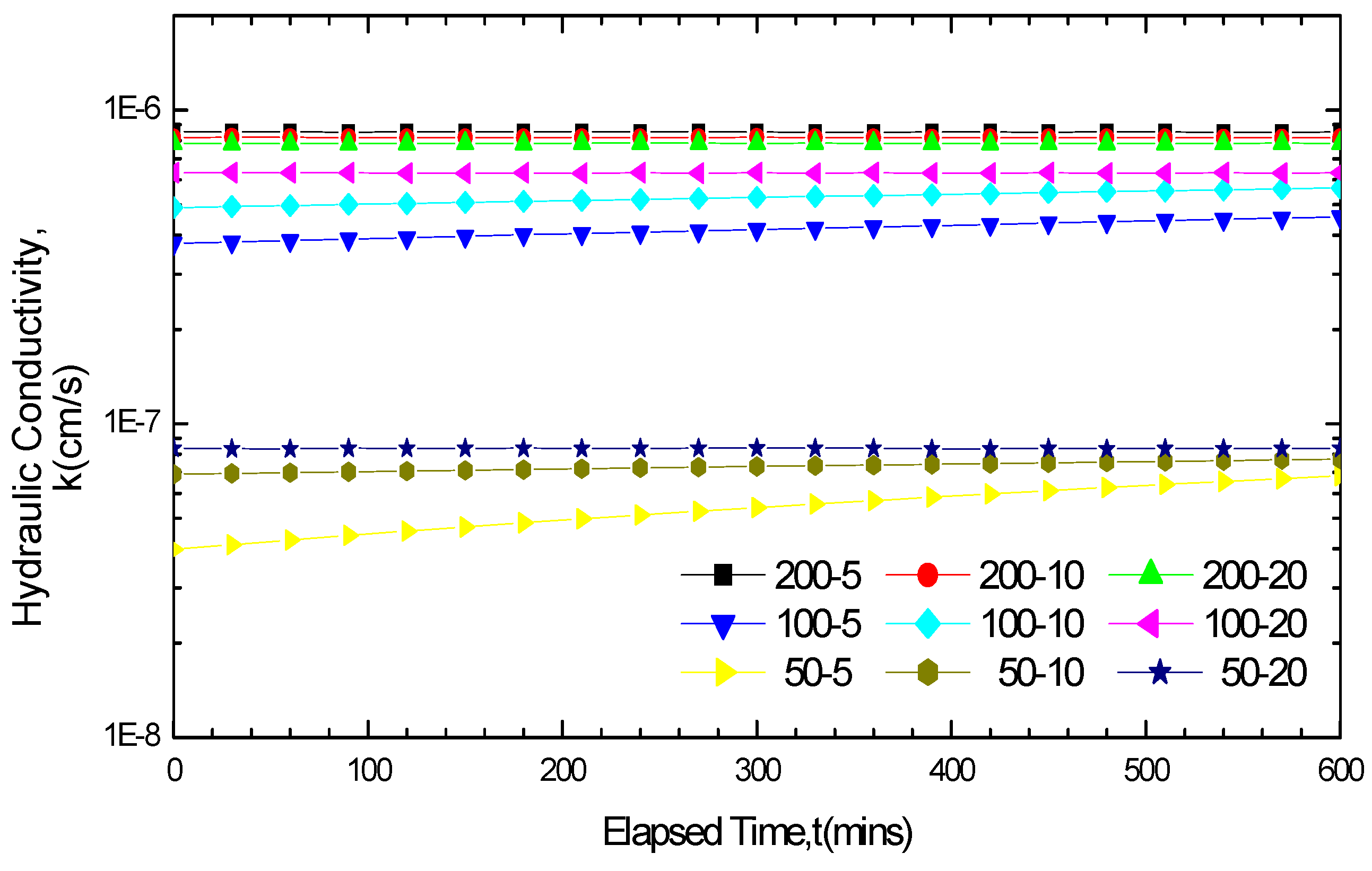
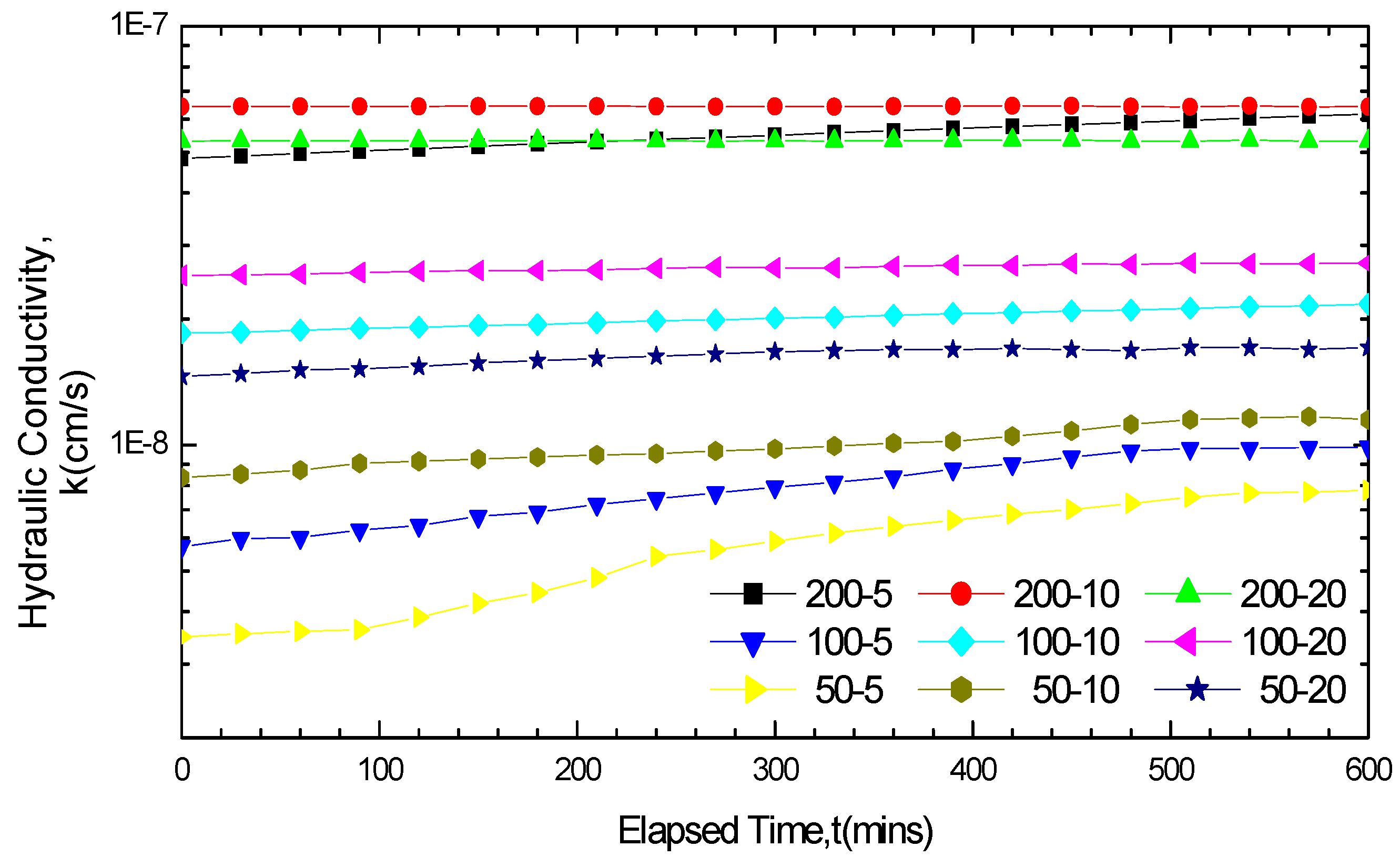
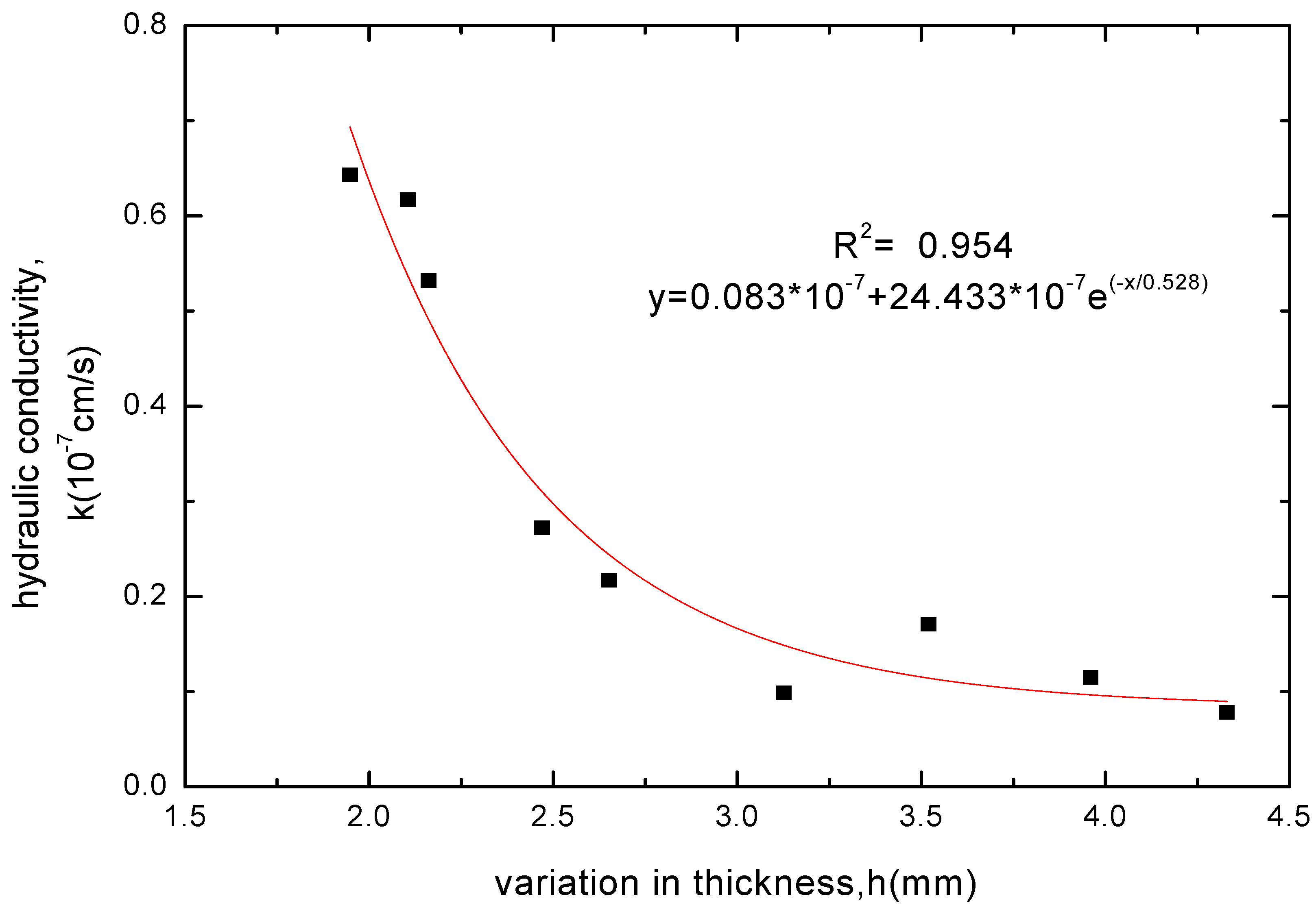
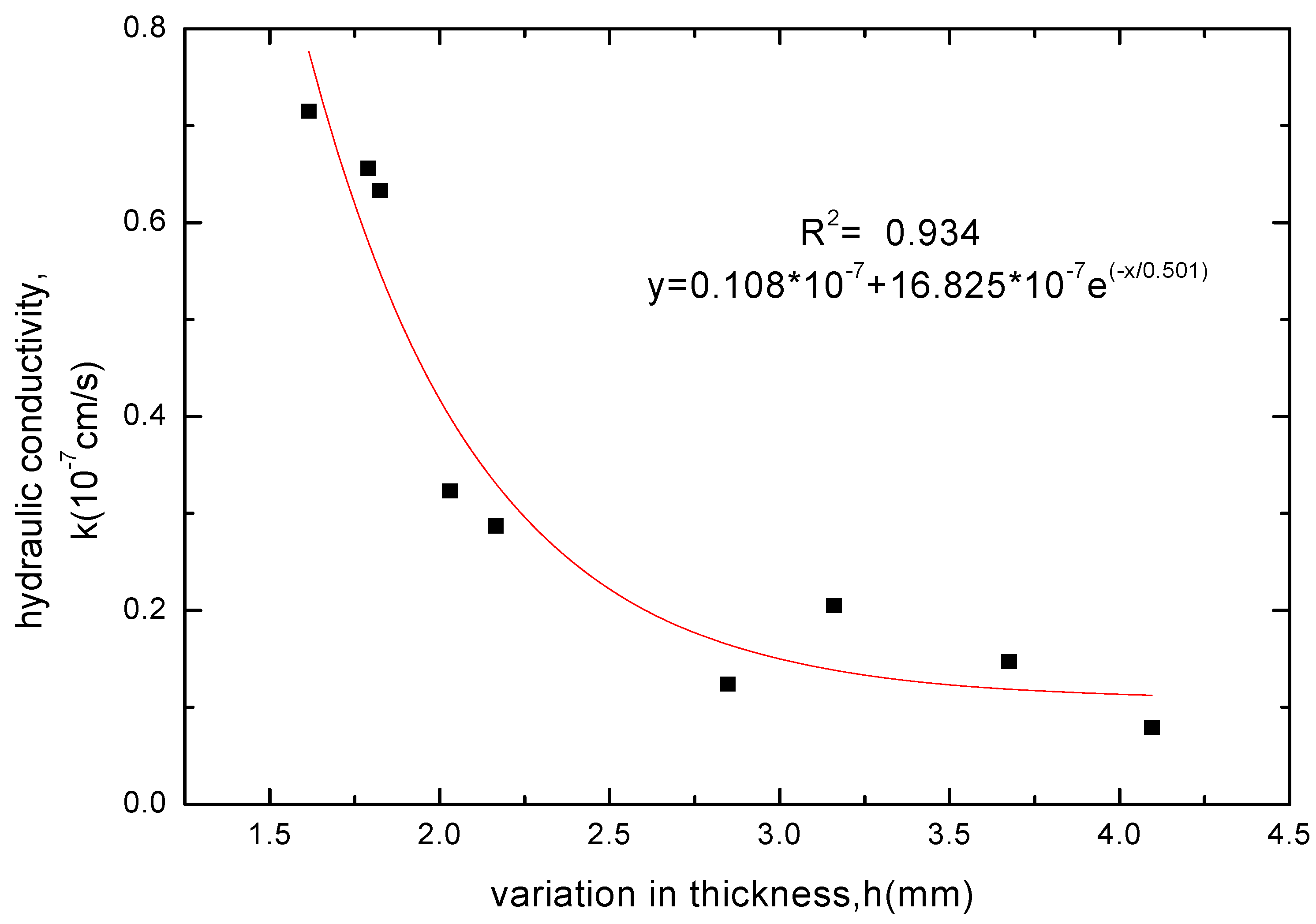
3.2.4. Variation Law of GCL Thickness before and after Soaking
| variations in thickness Δh /mm | 200-5 | 200-10 | 200-20 | 100-5 | 100-10 | 100-20 | 50-5 | 50-10 | 50-20 | |
| solutions | CaCl2 | 0.11 | 0.21 | 0.215 | 0.645 | 0.53 | 0.685 | 2.28 | 2.075 | 2.015 |
| MgCl2 | 0.38 | 0.41 | 0.49 | 1.36 | 1.02 | 1.01 | 2.589 | 2.501 | 2.43 | |
| NaCl | 2.105 | 1.948 | 2.162 | 3.126 | 2.651 | 2.47 | 4.33 | 3.96 | 3.52 | |
| KCl | 1.824 | 1.615 | 1.79 | 2.847 | 2.165 | 2.03 | 4.095 | 3.675 | 3.16 | |
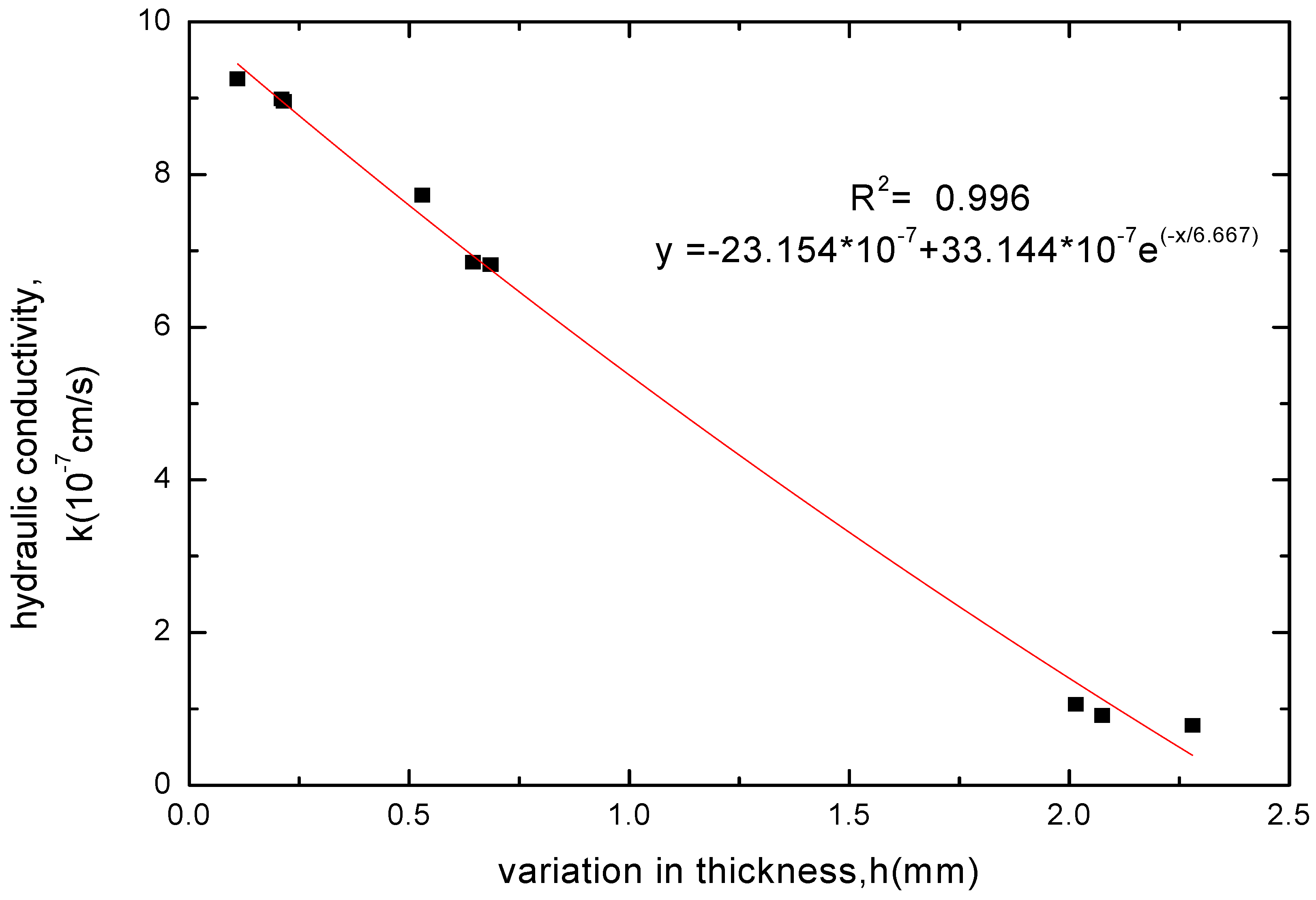
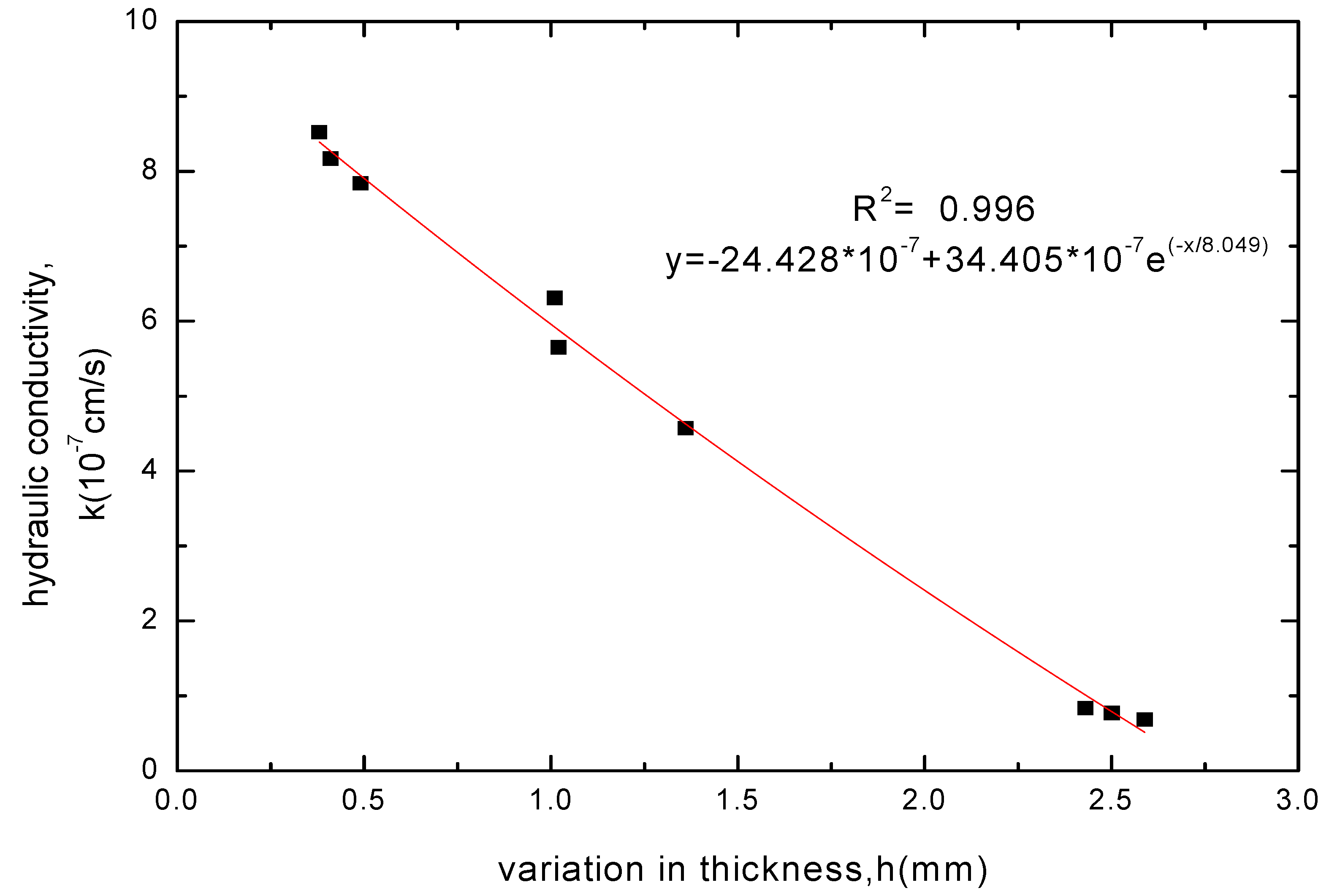
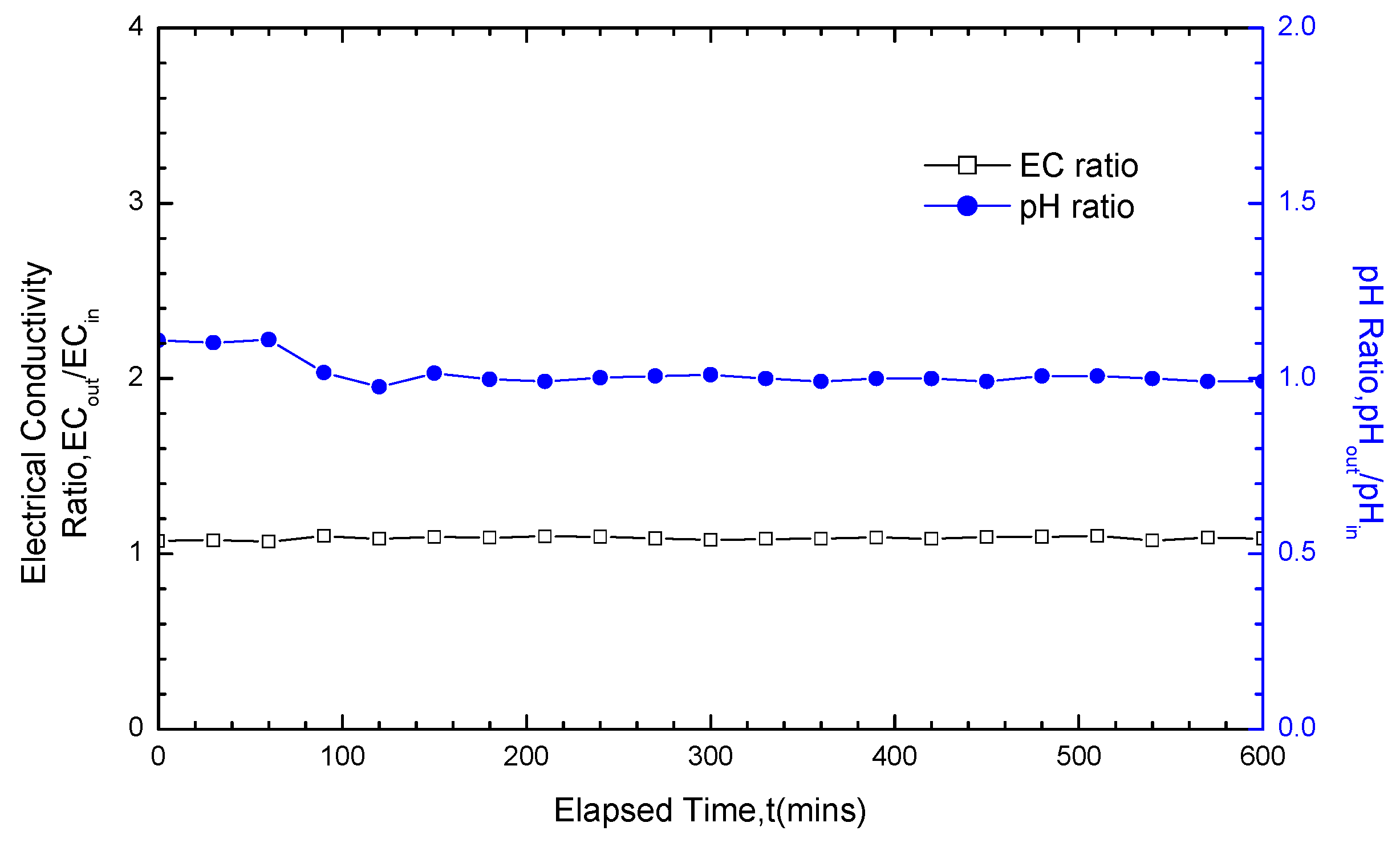
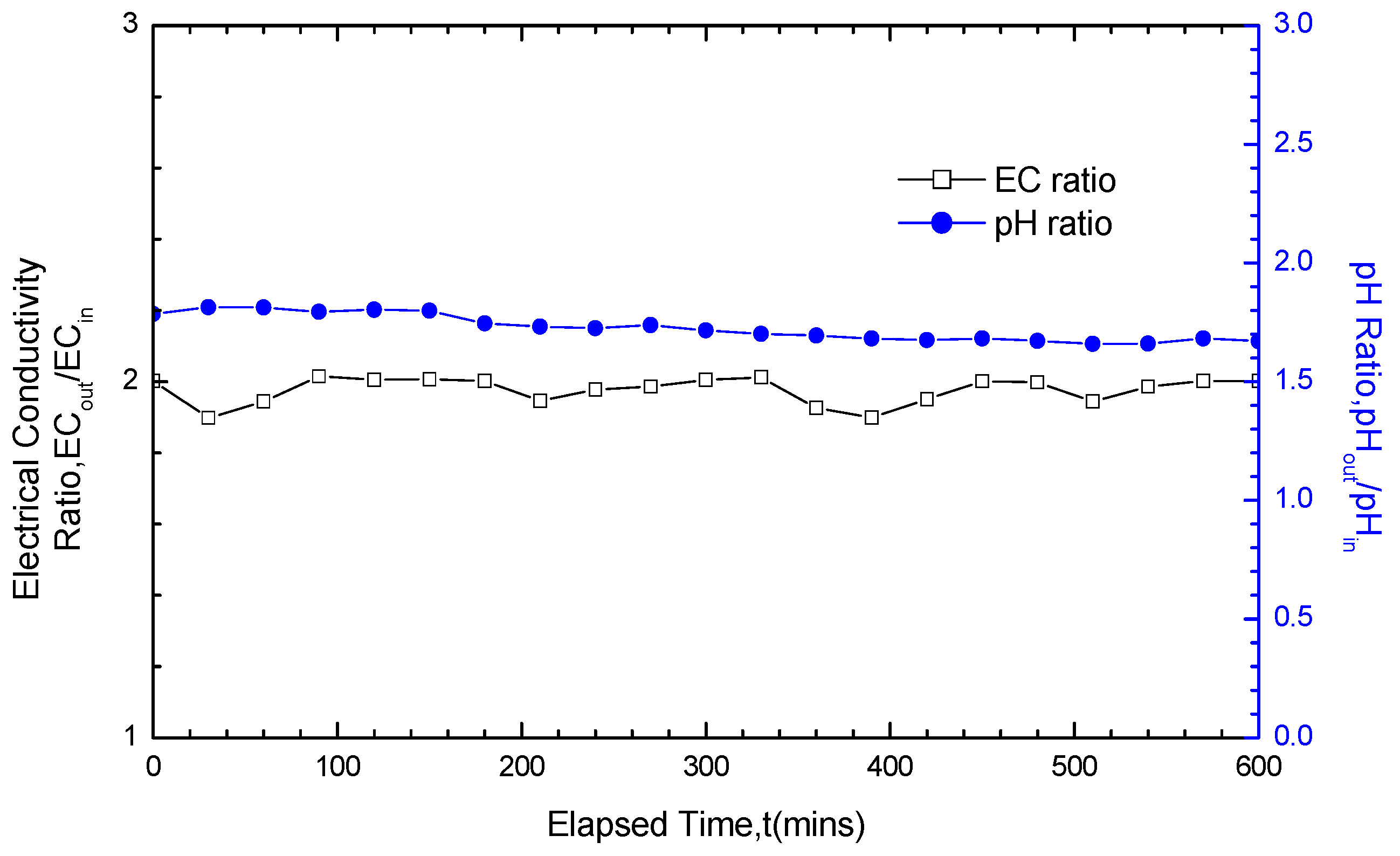
3.3. Analysis of pH and EC Test Results
3.4. Analysis and Discussion of the Test Results
4. Conclusions
Acknowledgements
References
- Zhou, Z.; Wang, Z.; Wang, J. Influence of ion exchange on hydraulic conductivity of GCL. J. Yangtze River Sci. Res. Inst. 2002, 19, 37–40. [Google Scholar]
- Yang, H.; Lu, Y. Municipal Solid Waste Treatment and Disposal; China Environmental Science Press: Beijing, China, 2006. [Google Scholar]
- Gharabaghi, B.; Singh, M.K.; Inkratas, C.; Fleming, I.R.; McBean, E. Comparison of slope stability in two Brazilian municipal landfills. Waste Manage. 2008, 28, 1509–1517. [Google Scholar] [CrossRef]
- Zhu, B.; Hu, H.; Chen, Q.; Li, Y. Experimental study on deformation and failure modes of municipal solid waste slope under rainfall conditions. J. Eng. Geol. 2004, 12, 312–317. [Google Scholar]
- Guyonnet, D.; Touze-Foltz, N.; Norotte, V.; Pothier, C.; Didier, G.; Gailhanou, H.; Blanc, P.; Warmont, F. Performance-based indicators for controlling geosynthetic clay liners in landfill applications. Geotext. Geomembrane. 2009, 27, 321–331. [Google Scholar] [CrossRef]
- Dickinson, S; Brachman, R.W.I. Permeability and internal erosion of a GCL beneath coarse gravel. Geosynth. Int. 2010, 17, 112–123. [Google Scholar] [CrossRef]
- Hornsey, W.P.; Scheirs, J.; Gates, W.P.; Bouazza, A. The impact of mining solution/liquors on geosynthetics. Geotext. Geomembrane. 2010, 28, 191–198. [Google Scholar] [CrossRef]
- Lange, K.; Rowe, R.K.; Jamieson, H. The potential role of geosynthetic clay liners in mine water treatment systems. Geotext. Geomembrane. 2010, 28, 199–205. [Google Scholar] [CrossRef]
- Gates, W.; Bouazza, M. Bentonite transformations in strongly alkaline solutions. Geotext. Geomembrane. 2010, 28, 219–225. [Google Scholar] [CrossRef]
- Rayhani, M.T.; Rowe, R.K.; Brachman, R.W.I.; Take, W.A.; Siemens, G. Factors affecting GCL hydration under isothermal conditions. Geotext. Geomembrane. 2011, 29, 525–533. [Google Scholar] [CrossRef]
- Kang, J.B.; Shackelford, C.D. Consolidation enhanced membrane behavior of a geosynthetic clay liner. Geotext. Geomembrane. 2011, 29, 544–556. [Google Scholar] [CrossRef]
- Chevrier, B.; Cazaux, D.; Didier, G.; Gamet, M.; Guyonnet, D. Influence of subgrade, temperature and confining pressure on GCL hydration. Geotext. Geomembrane. 2012, 33, 1–6. [Google Scholar]
- Rosin-Paumier, S.; Touze-Foltz, N. Hydraulic and chemical evolution of GCLs during filter press and oedopermeametric tests performed with real leachate. Geotext. Geomembrane. 2012, 33, 15–24. [Google Scholar] [CrossRef]
- Lee, J.M.; Shackelford, C.D. Impact of bentonite quality on hydraulic conductivity of geosynthetic clay liners. J. Geotech. Geoenviron. Eng. 2005, 131, 64–77. [Google Scholar] [CrossRef]
- Shackelford, C.D.; Sevick, G.W.; Eykholt, G.R. Hydraulic conductivity of geosynthetic clay liners to tailings impoundment solutions. Geotext. Geomembrane. 2010, 28, 149–162. [Google Scholar] [CrossRef]
- Benson, C.H.; Oren, A.H.; Gates, W.P. Hydraulic conductivity of two geosynthetic clay liners permeated with a hyperalkaline solution. Geotext. Geomembrane. 2010, 28, 206–218. [Google Scholar] [CrossRef]
- Kong, X. Hydraulic Performance Experiment for GCL in Landfill Liner. Master’s Thesis, Hubei University of Technology, China, May 2011. [Google Scholar]
- He, J.; Xiao, H.; Kong, X. Test on the effect of simulated leachate and pressure on the permeability of compacted clay liners. J. Yangtze River Sci. Res. Inst. 2012, 29, 37–40. [Google Scholar]
- ASTM International. In ASTM D5084. Standard Test Methods for Measurement of Hydraulic Conductivity of Saturated Porous Materials Using a Flexible Wall Permeameter; ASTM: West Conshohocken, PA, USA, 2004.
- ASTM International. In ASTM D5887. Standard Test Method for Measurement of Index Flux through Saturated Geosynthetic Clay Liner Specimens Using a Flexible Wall Permeameter; ASTM: West Conshohocken, PA, USA, 2009.
- Jo, H.Y.; Katsumi, T.; Benson, C.H.; Edil, T.B. Hydraulic conductivity and swelling of non-prehydrated GCLs with single-species salt solutions. J. Geotech. Geoenviron. Eng. 2001, 127, 557–567. [Google Scholar] [CrossRef]
- Zhang, F.; Low, P.; Roth, C. Effects of monovalent exchangeable cations and electrolytes on the relation between swelling pressure and interlayer distance in montmorillonite. J. Colloid Interface Sci. 1995, 173, 34–41. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xue, Q.; Zhang, Q.; Liu, L. Impact of High Concentration Solutions on Hydraulic Properties of Geosynthetic Clay Liner Materials. Materials 2012, 5, 2326-2341. https://doi.org/10.3390/ma5112326
Xue Q, Zhang Q, Liu L. Impact of High Concentration Solutions on Hydraulic Properties of Geosynthetic Clay Liner Materials. Materials. 2012; 5(11):2326-2341. https://doi.org/10.3390/ma5112326
Chicago/Turabian StyleXue, Qiang, Qian Zhang, and Lei Liu. 2012. "Impact of High Concentration Solutions on Hydraulic Properties of Geosynthetic Clay Liner Materials" Materials 5, no. 11: 2326-2341. https://doi.org/10.3390/ma5112326




