Recent Advances in the Photorefraction of Doped Lithium Niobate Crystals
Abstract
:1. Introduction
2. The Optical Damage Resistance of Tetravalent Ions Doped LiNbO3
| Crystals Properties | LN:Mg | LN:Hf | LN:Zr | LN:Sn |
|---|---|---|---|---|
| Optical damage resistance (W/cm2, 514.5 nm) | 5.0 × 105 | 5.0 × 105 | >2.0 × 107 | 4.8 × 105 |
| Saturation refractive index change (514.5 nm) | 7.8 × 10−6 | 8.4 × 10−6 | 7.1 × 10−7 | 7.6 × 10−6 |
| Doping threshold (mol% in melt) | 4.6 | 2.0~2.5 | 2.0 | 2.5 |
| Distribution coefficient | 1.2 | 0.93 | 0.97 | 0.98 |
| Optical damage resistance (W/cm2, 351 nm) | – | 1.8 × 104 | 1.6 × 105 | – |
| References | [19,31] | [31,33,56] | [38,55] | [43] |
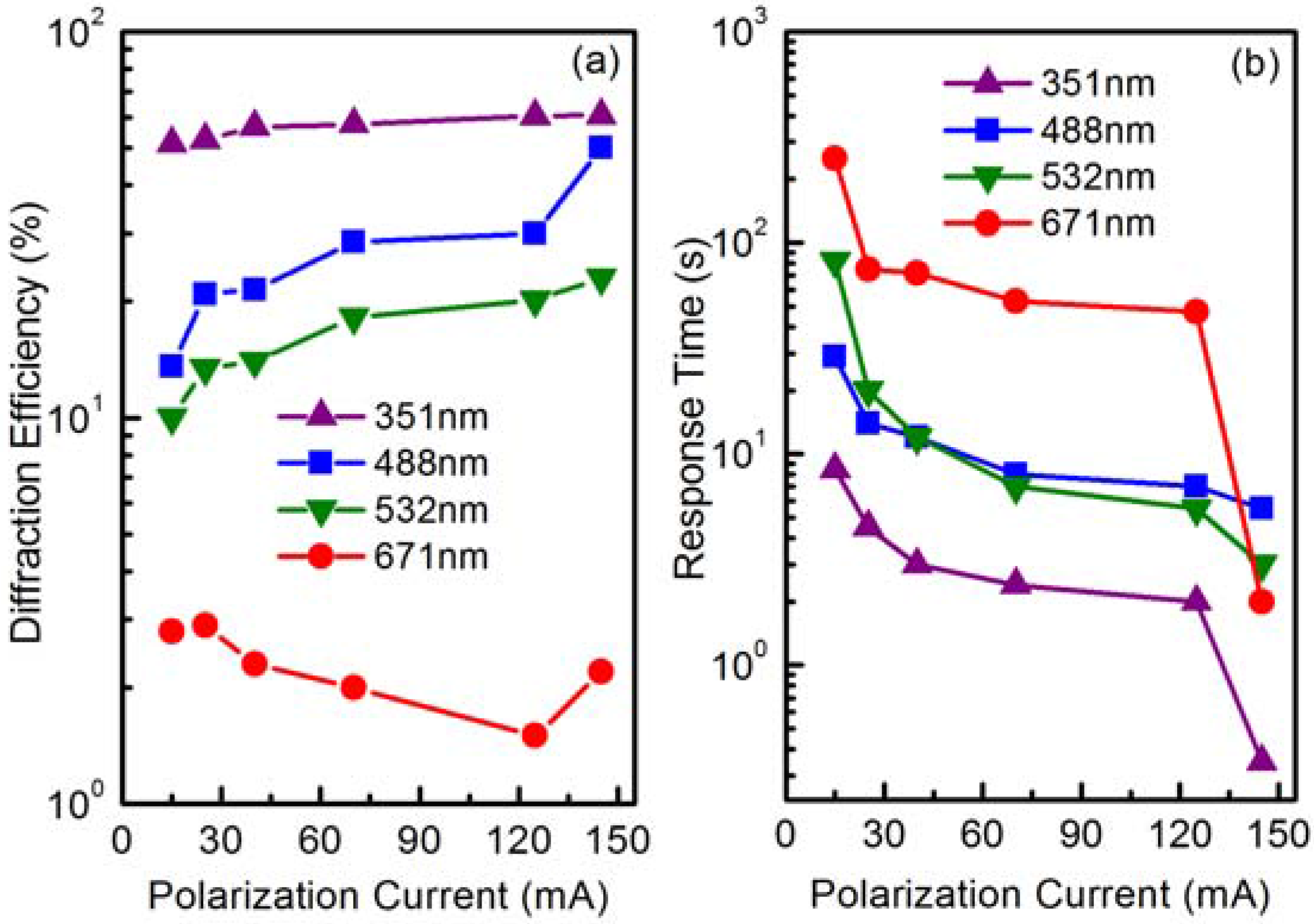
3. The Ultraviolet Photorefraction of Tetravalent Ions Doped LiNbO3


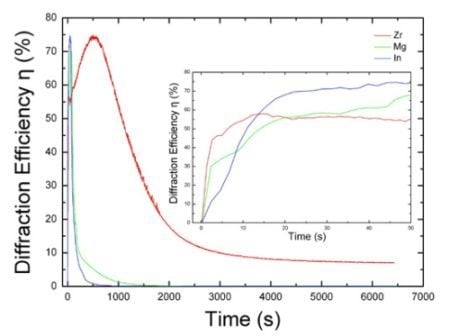
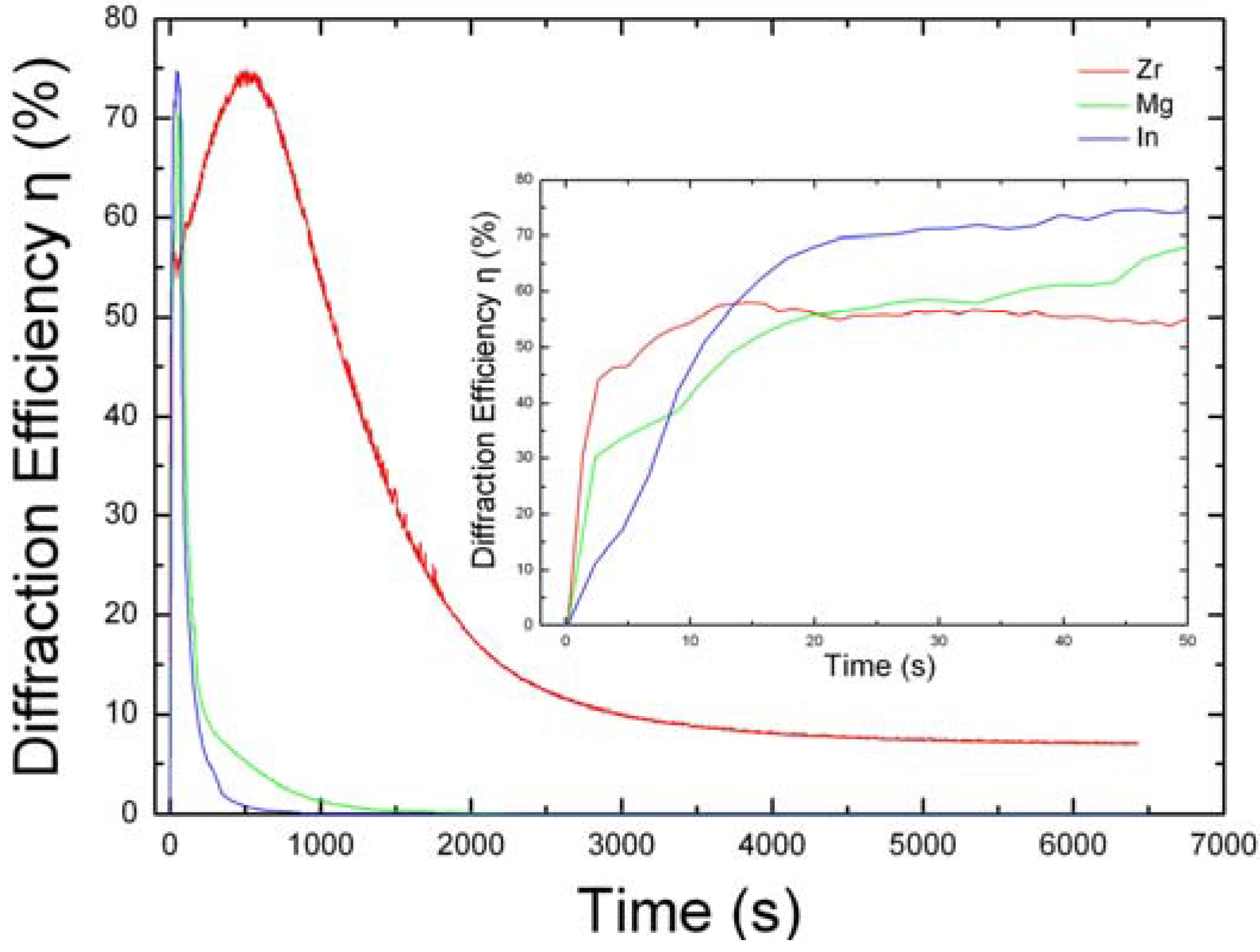
4. The Photorefraction of Tetravalent Ions Co-Doped LiNbO3

| Crystal | Doping concentration | Photorefractive properties | Reference | |||||||
| Fe2O3 (wt%) | MgO (mol%) | HfO2 (mol%) | ZrO2 (mol%) | ηsat (%) | τr (s) | S (cm/J) | S’ (cm/J) | |||
| LN:Fe | 0.01 | 70 | 160 | [23] | ||||||
| LN:Mg,Fe | 0.01 | 6 | 15 | 15 | [23] | |||||
| LN:Hf,Fe | 0.03 | 5 | 55.4 | 10.7 | 5.23 | [59] | ||||
| LN:Zr,Fe | 0.03 | 2 | 32.0 | 1.8 | 12.87 | [68] | ||||
| LN:Zr,Fe,Mn | 0.075 | 0.01 wt% MnO | 2 | 56.4 | 0.95 | 3.47 | 1.31 | [72] | ||
| LN:Zr,Cu,Ce | 0.011 wt% CuO | 0.085 wt% Ce2O3 | 2 | 62.4 | 120 | 0.312 | 0.099 | [78] | ||
| LN:Zr,Ru,Fe | 0.15 wt% Fe2O3 | 0.15 wt% RuO2 | 2 | 60.5 | 9.1 | 0.92 | [85] | |||
5. The Photorefraction of Pentavalent and Hexavalent Ions Doped LiNbO3

6. Summary
Acknowledgments
References
- Psaltis, D.; Mok, F. Holographic memories. Sci. Am. 1995, 273, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Haw, M. The light fantastic. Nature 2003, 422, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Dhar, L.; Curtis, K.; Fäcke, T. Holographic data storage: Coming of age. Nature Photonics 2008, 2, 403–405. [Google Scholar] [CrossRef]
- Hellemans, A. Holograms can store terabytes, but where? Science 1999, 286, 1502–1504. [Google Scholar] [CrossRef]
- Askin, A.; Boyd, G.D.; Dziedzic, J.M.; Smith, R.G.; Ballman, A.A.; Levinstein, J.J.; Nassau, K. Optically-induced refractive index inhomogeneities in LiNbO3 and LiTaO3. Appl. Phys. Lett. 1966, 9, 72–74. [Google Scholar] [CrossRef]
- Chen, F.S.; LaMacchia, J.T.; Fraser, D.B. Holographic storage in lithium niobate. Appl. Phys. Lett. 1968, 13, 223–225. [Google Scholar] [CrossRef]
- Phillips, W.; Amodei, J.J.; Staebler, D.L. Optical and holographic storage properties of transition metal doped lithium niobate. RCA Rev. 1972, 33, 94–109. [Google Scholar]
- McMillen, D.K.; Hudson, T.D.; Wagner, J.; Singleton, J. Holographic recording in specially doped lithium niobate crystals. Opt. Express 1998, 2, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Amodei, J.J.; Staebler, D.L. Holographic pattern fixing in electro-optic crystals. Appl. Phys. Lett. 1971, 18, 540–542. [Google Scholar] [CrossRef]
- Micheron, F.; Bismuth, G. Electrical control of fixation and erasure of holographic patterns in ferroelectric materials. Appl. Phys. Lett. 1972, 20, 79–81. [Google Scholar] [CrossRef]
- Heanue, J.F.; Bashaw, M.C.; Daiber, A.J.; Snyder, R.; Hesselink, L. Digital holographic storage system incorporating thermal fixing in lithium niobate. Opt. Lett. 1996, 21, 1615–1617. [Google Scholar] [CrossRef] [PubMed]
- Lande, D.; Orlov, S.S.; Akella, A.; Hesselink, L.; Neurgaonkar, R.R. Digital holographic storage system incorporating optical fixing. Opt. Lett. 1997, 22, 1722–1724. [Google Scholar] [CrossRef] [PubMed]
- Buse, K.; Adibi, A.; Psaltis, D. Non-volatile holographic storage in doubly doped lithium niobate crystals. Nature 1998, 393, 665–668. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Zhou, C.; Xu, L. Nonvolatile photorefractive holograms in LiNbO3:Cu:Ce crystals. Opt. Lett. 2000, 25, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Vitova, T.; Hormes, J.; Peithmann, K.; Woike, T. X-ray absorption spectroscopy study of valence and site occupation of copper in LiNbO3:Cu. Phys. Rev. B 2008, 77, 144103:1–144103:10. [Google Scholar] [CrossRef]
- Darwish, A.; Aggarwal, M.D.; Mortis, J.; Choi, J.; Wang, J.C.; Venkateswarlu, P.; Willimas, A.; Banerjee, P.P.; McMillen, D.; Hudson, T.D. Investigations of the charge transfer and the photosensitivity in single and double doped LiNbO3 single crystals: an optical-electron paramagnetic resonance study: I. Proc. SPIE 1997, 3137, 63–74. [Google Scholar]
- Li, X.; Kong, Y.; Wang, Y.; Wang, L.; Liu, F.; Liu, H.; An, Y.; Chen, S.; Xu, J. Nonvolatile holographic storage of near-stoichiometric LiNbO3:Cu:Ce with green light. Appl. Opt. 2007, 46, 7620–7624. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xie, X.; Kong, Y.; Yan, W.; Li, X.; Shi, L.; Xu, J.; Zhang, G. Photorefractive properties of near-stoichiometric lithium niobate crystals doped with iron. Opt. Mater. 2006, 28, 212–215. [Google Scholar] [CrossRef]
- Zhong, G.; Jin, J.; Wu, Z. Measurement of optically induced refractive index damage in lithium niobate doped with different concentrations of MgO. J. Opt. Soc. Am. 1980, 70, 631. [Google Scholar]
- Volk, T.R.; Pryalkin, V.J.; Rubinina, M.M. Optical-damage-resistant LiNbO3:Zn crystal. Opt. Lett. 1990, 15, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.K.; Kitamura, K.; Iyi, N.; Kimura, S.; Furukawa, Y.; Sato, M. Increased optical damage resistance in Sc2O3 doped LiNbO3. Appl. Phys. Lett. 1992, 61, 2156–2158. [Google Scholar] [CrossRef]
- Kong, Y.; Wen, J.; Wang, H. New doped lithium niobate crystal with high resistance to photorefraction − LiNbO3:In. Appl. Phys. Lett. 1995, 66, 280–281. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, J.; Liu, S.; Sun, Q.; Zhang, G.; Fang, Q.; Ma, C. Study of resistance against photorefractive light-induced scattering in LiNbO3:Fe,Mg crystals. Proc. SPIE 1995, 2529, 14–17. [Google Scholar]
- Chen, S.; Liu, H.; Kong, Y.; Huang, Z.; Xu, J. The high photorefractive sensitivity and fast response time of near-stoichiometric and MgO doped LiNbO3:Fe crystal. Crys. Res. Technol. 2006, 41, 790–794. [Google Scholar] [CrossRef]
- Günter, P.; Huignard, J.P. (Eds.) Photorefractive Materials and Their Applications 2: Materials; Springer: New York, NY, USA, 2007; pp. 83–204.
- Volk, T.; Wohlecke, M. Lithium Niobate: Defects, Photorefraction and Ferroelectric Switching; Springer: Heidelberg, Germany, 2008. [Google Scholar]
- Kösters, M.; Sturman, B.; Werheit, P.; Haertle, D.; Buse, K. Optical cleaning of congruent lithium niobate crystals. Nature Photonics 2009, 3, 510–513. [Google Scholar] [CrossRef]
- Kokanyan, E.P.; Babajanyan, V.G.; Demirkhanyan, G.G.; Gruber, J.B.; Erdei, S. Periodically poled structures in doped lithium niobate crystals. J. Appl. Phys. 2002, 92, 1544–1547. [Google Scholar] [CrossRef]
- Kokanyan, E.P.; Razzari, L.; Cristiani, I.; Degiorgio, V.; Gruber, J.B. Reduced photorefraction in hafnium-doped single-domain and periodically poled lithium niobate crystals. Appl. Phys. Lett. 2004, 84, 1880–1882. [Google Scholar] [CrossRef]
- Razzari, L.; Minzioni, P.; Cristiani, I.; Degiorgio, V.; Kokanyan, E.P. Photorefractivity of Hafnium-doped congruent lithium–niobate crystals. Appl. Phy. Lett. 2005, 86, 131914:1–131914:3. [Google Scholar]
- Li, S.; Liu, S.; Kong, Y.; Deng, D.; Gao, G.; Li, Y.; Gao, H.; Zhang, L.; Hang, Z.; Chen, S.; Xu, J. The optical damage resistance and absorption spectra of LiNbO3:Hf crystals. J. Phys. Condens. Matter 2006, 18, 3527–3534. [Google Scholar] [CrossRef]
- Minzioni, P.; Cristiani, I.; Degiorgio, V.; Kokanyan, E.P. Strongly sublinear growth of the photorefractive effect for increasing pump intensities in doped lithium-niobate crystals. J. Appl. Phys. 2007, 101, 116105:1–116105:3. [Google Scholar] [CrossRef]
- Minzioni, P.; Cristiani, I.; Yu, J.; Parravicini, J.; Kokanyan, E.P.; Degiorgio, V. Linear and nonlinear optical properties of Hafnium-doped lithium-niobate crystals. Opt. Express 2007, 15, 14171–14176. [Google Scholar] [CrossRef] [PubMed]
- Hammoum, R.; Fontana, M.D.; Gilliot, M.; Bourson, P.; Kokanyan, E.P. Site spectroscopy of Hf doping in Hf-doped crystals. Solid State Commun. 2009, 149, 1967–1970. [Google Scholar] [CrossRef]
- Galinetto, P.; Rossella, F.; Cristiani, I.; Minzioni, P.; Degiorgio, V.; Kokanyan, E.P. Structural and optical properties of hafnium-doped lithium-niobate crystals. Phys. Status Solidi C 2007, 4, 1372–1375. [Google Scholar] [CrossRef]
- Abarkan, M.; Aillerie, M.; Salvestrini, J. P.; Fontana, M D.; Kokanyan, E.P. Electro-optic and dielectric properties of Hafnium-doped congruent lithium–niobate crystals. Appl. Phys. B 2008, 92, 603–608. [Google Scholar] [CrossRef]
- Rossella, F.; Grando, D.; Galinetto, P.; Degiorgio, V.; Kokanyan, E. Photoconductive and electro-optical properties of Hf doped lithium niobate crystals. Ferroelectrics 2007, 352, 143–147. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, S.; Zhao, Y.; Liu, H.; Chen, S.; Xu, J. Highly optical damage resistant crystal: Zirconium-oxide-doped lithium niobate. Appl. Phys. Lett. 2007, 91, 081908:1–081908:3. [Google Scholar]
- Sun, L.; Guo, F.; Lv, Q.; Yu, H.; Li, H.; Cai, W.; Xu, Y.; Zhao, L. Increased optical damage resistance of Zr:LiNbO3 crystals. Cryst. Res. Technol. 2007, 42, 1117–1122. [Google Scholar] [CrossRef]
- Argiolas, N.; Bazzan, M.; Ciampolillo, M.V.; Pozzobon, P.; Sada, C.; Saoner, L.; Zaltron, A.M.; Bacci, L.; Minzioni, P.; Nava, G.; Parravicini, J.; Yan, W.; Cristiani, I.; Degiorgio, V. Structural and optical properties of zirconium doped lithium niobate crystals. J. Appl. Phys. 2010, 108, 093508:1–093508:5. [Google Scholar] [CrossRef]
- Liu, H.; Liang, Q.; Zhu, M.; Li, W.; Liu, S.; Zhang, L.; Chen, S.; Kong, Y.; Xu, J. An excellent crystal for high resistance against optical damage in visible-UV range: Near-stoichiometric zirconium-doped lithium niobate. Opt. Express 2011, 19, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Nava, G.; Minzioni, P.; Yan, W.; Parravicini, J.; Grando, D.; Musso, E.; Cristiani, I.; Argiolas, N.; Bazzan, M.; Ciampolillo, M.V.; Zaltron, A.; Sada, C.; Degiorgio, V. Zirconium-doped lithium niobate: Photorefractive and electro-optical properties as a function of dopant concentration. Opt. Mater. Express 2011, 1, 270–277. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Kong, Y.; Chen, S.; Huang, Z.; Wu, L.; Rupp, R.; Xu, J. Increased optical damage resistance in Tin doped lithium niobate. Opt. Lett. 2010, 35, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Jungen, R.; Angelow, G.; Laeri, F.; Grabmaier, C. Efficient ultraviolet photorefraction in LiNbO3. Appl. Phys. A 1992, 55, 101–103. [Google Scholar] [CrossRef]
- Laeri, F.; Jungen, R.; Angelow, G.; Vietze, U.; Engel, T.; Würtz, M.; Hilgenberg, D. Photorefraction in the ultraviolet: Materials and effects. Appl. Phys. B 1995, 61, 351–360. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, G.; Li, F.; Zhang, X.; Sun, Q.; Liu, S.; Song, F.; Kong, Y.; Chen, X.; Qiao, H.; Yao, J.; Zhao, L. Enhancement of ultraviolet photorefraction in highly magnesium-doped lithium niobate crystals. Opt. Lett. 2000, 25, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Xu, J.; Zhang, G.; Zhang, X.; Sun, Q.; Zhang, G. Ultraviolet photorefractivity features in doped lithium niobate crystals. Phys. Rev. B 2004, 70, 094101:1–094101:11. [Google Scholar]
- Vikhnin, V.; Eglits, R.; Kapphan, S.; Borstel, G.; Kotomin, E. Polaronic-type excitons in ferroelectric oxides: Microscopic calculations and experimental manifestation. Phys. Rev. B 2002, 65, 104304:1–104304:11. [Google Scholar] [CrossRef]
- Lerner, P.; Legras, C.; Dumas, J.P. Stoechiometrie des monocristaux de metaniobate de lithium. J. Cryst. Growth 1968, 3–4, 231–235. [Google Scholar]
- Schirmer, O.F.; von der Linde, D. Two-photon- and X-ray-induced Nb4+ and O− small polarons in LiNbO3. Appl. Phys. Lett. 1978, 33, 35–38. [Google Scholar] [CrossRef]
- Harhira, A.; Guilbert, L.; Bourson, P.; Rinnert, H. Polaron luminescence in iron-doped lithium niobate. Appl. Phys. B 2008, 92, 555–561. [Google Scholar] [CrossRef]
- Kostritskii, S.M.; Aillerie, M.; Margueron, S.; Bourson, P. Two-photon luminescence of small polarons in reduced LiNbO3 crystals. IOP Conf. Ser. Mater. Sci. Eng. 2010, 15. [Google Scholar] [CrossRef]
- Kostritskii, S.M.; Sevostyanov, O.G.; Bourson, P.; Aillerie, M.; Fontana, M.D.; Kip, D. Comparative study of composition dependences of photorefractive and related effects in LiNbO3 and LiTaO3 crystals. Ferroelectrics 2007, 352, 61–71. [Google Scholar] [CrossRef]
- Hesselink, L.; Orlov, S.; Liu, A.; Akella, A.; Lande, D.; Neurgaonkar, R. Photorefractive materials for nonvolatile volume holographic data storage. Science 1998, 282, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Kong, Y.; Li, W.; Liu, H.; Liu, S.; Chen, S.; Zhang, X.; Rupp, R.; Xu, J. High resistance against ultraviolet photorefraction in zirconium-doped lithium niobate crystals. Opt. Lett. 2010, 35, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Shi, L.; Chen, H.; Zhang, X.; Kong, Y. Investigations on the UV photorefractivity of LiNbO3:Hf. Opt. Lett. 2010, 35, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Zhang, G.; Ge, X.; Liu, S.; Xuan, L.; Kong, Y.; Xu, J. Ultraviolet band edge photorefractivity in LiNbO3:Sn crystals. Opt. Lett. 2011, 36, 3163–3165. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Zhang, G.; Bo, F.; Sun, H.; Kong, Y.; Xu, J.; Volk, T.; Rubinina, N.M. Ultraviolet photorefraction at 325 nm in doped lithium niobate crystals. J. Appl. Phys. 2010, 107, 033113:1–033113:7. [Google Scholar]
- Li, S.; Liu, S.; Kong, Y.; Xu, J.; Zhang, G. Enhanced photorefractive properties of LiNbO3:Fe crystals by HfO2 co-doping. Appl. Phys. Lett. 2006, 89, 101126:1–101126:3. [Google Scholar]
- Dai, L.; Wu, S.; Guo, J.; Xu, C.; Su, Y.; Xu, Y. Effect of Li/Nb ratio on growth and spectrometric characterization of Hf:Fe:LiNbO3 crystals. Mod. Phys. Lett. B 2009, 23, 1557–1565. [Google Scholar] [CrossRef]
- Shi, H.; Sun, X.; Luo, S.; Jiang, Y.; Meng, Q. Defect structure and optical damage resistance of Hf:Fe:LiNbO3 crystals. Opt. Laser Technol. 2010, 42, 1118–1121. [Google Scholar] [CrossRef]
- Shi, H.; Ren, C.; Luo, S.; Meng, Q.; Sun, X. Optical damage resistance of Hf:Fe:LiNbO3 crystals with various [Li]/[Nb] ratios. Cryst. Res. Technol. 2011, 46, 931–934. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Zhang, G. Microscopic mechanism of suppressing photorefraction in LiNbO3:Mg,Fe crystals. Solid State Commun. 1996, 98, 523–526. [Google Scholar] [CrossRef]
- Zhang, G.; Kong, Y.; Xu, J. Origin of the Enhancement of Resistance against Optical Damage in Mg-Doped nearly Stoichiometric Lithium Niobate Crystals. In Photorefractive Effects, Materials, and Devices; Salamo, G., Siahmakoun, A., Eds.; OSA Trends in Optics and Photonics: Delavan, WI, USA, 2001; Volume 62, pp. 166–170. [Google Scholar]
- Sun, X.; Shi, H.; Luo, S.; Meng, Q.; Jiang, Y. Improvement of photorefractive properties in Hf:Fe:LiNbO3 crystals with various [Li]/[Nb] ratios. Chinese Phys. B 2010, 19. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Bi, J.; Sun, L.; Xu, Y. Photorefractive features of non-stoichiometry codoped Hf:Fe:LiNbO3 single crystals. Cryst. Res. Technol. 2008, 43, 260–265. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, B.; Yuan, W.; Ling, F.; Ma, D.; Nie, Y. Photorefractive properties of double-doped Hf:Ce:LiNbO3 crystals. Microwave Opt. Technol. Lett. 2008, 50, 1693–1695. [Google Scholar] [CrossRef]
- Kong, Y.; Wu, S.; Liu, S.; Chen, S.; Xu, J. Fast photorefractive response and high sensitivity of Zr and Fe codoped LiNbO3 crystals. Appl. Phys. Lett. 2008, 92, 251107:1–251107:3. [Google Scholar]
- Fan, Y.; Xu, C.; Xia, S.; Guan, C.; Cao, L.; He, Q.; Jin, G. Growth and spectroscopic characterization of Zr:Fe:LiNbO3 crystals with various Li/Nb ratios. J Cryst. Growth 2010, 312, 1875–1878. [Google Scholar] [CrossRef]
- Fan, Y.; Yu, Z.; Li, W.; Xu, C.; Fu, K. Enhancement of blue holographic properties in Zr4+-doped near stoichiometric Fe:LiNbO3 crystals. Opt. Laser Technol. 2012, 44, 362–365. [Google Scholar] [CrossRef]
- Luo, S.; Wang, J.; Shi, H.; Sun, X. Photorefractive and optical scattering properties of Zr:Fe:LiNbO3 crystals. Opt. Laser Technol. 2012, 44, 2245–2248. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, F.; Tian, T.; Liu, S.; Chen, S.; Rupp, R.; Xu, J. Fast responsive nonvolatile holographic storage in LiNbO3 triply doped with Zr, Fe, and Mn. Opt. Lett. 2009, 34, 3896–3898. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, L.; Leng, X.; Xu, Y.; Yang, C. Defect structure and light-induced scattering of Zr-doped near-stoichiometric Mn:Fe:LiNbO3 crystals grown by TSSG method. Cryst. Res. Technol. 2012, 47, 19–24. [Google Scholar] [CrossRef]
- Xu, C.; Yang, C.; Dai, L.; Sun, L.; Xu, Y.; Cao, L. Influence of Li and Nb ratios on the defect structure and exposure energy in LiNbO3:Fe:Mn:Zr crystals. J. Alloys Compounds 2011, 509, 4167–4170. [Google Scholar] [CrossRef]
- Xu, C.; Yang, C.; Mo, Y.; Wang, Y.; Sun, L.; Cao, L.; Xu, Y. Improved blue photorefractive properties of near-stoichiometric LiNbO3:Mn:Fe:Zr crystal. Cryst. Res. Technol. 2010, 45, 1123–1126. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, B.; Lin, S.; Li, Y.; Wang, K. Defect structure and nonvolatile hologram storage properties in Hf:Fe:Mn:LiNbO3 crystals. Optik 2011, 122, 1179–1182. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Xu, L.; Zhou, C. Experimental study of non-volatile holographic storage in doubly- and triply-doped lithium niobate crystals. Opt. Commun. 2000, 181, 47–52. [Google Scholar] [CrossRef]
- Liu, F.; Kong, Y.; Ge, X.; Liu, H.; Liu, S.; Chen, S.; Rupp, R.; Xu, J. Improved sensitivity of nonvolatile holographic storage in triply doped LiNbO3:Zr,Cu,Ce. Opt. Express 2010, 18, 6333–6339. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Liu, L.; Liu, D.; Dai, C.; Ren, L. Effect of dopant composition ratio on nonvolatile holographic recording in LiNbO3:Cu:Ce crystals. Appl. Opt. 2004, 43, 5016–5022. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, C.; Leng, X.; Ben, Y.; Zhao, Y.; Xu, Y. Growth and nonvolatile holographic storage properties of Hf:Ce:Cu:LiNbO3 crystals. J. Cryst. Growth 2011, 318, 661–664. [Google Scholar] [CrossRef]
- Fujimura, R.; Shimura, T.; Kuroda, K. Nonvolatile Holographic Recording in Ru Doped LiNbO3 Crystals. In Photorefractive Effects, Materials, and Devices; Delaye, P., Denz, C., Mager, L., Montemezzani, G., Eds.; OSA Trends in Optics and Photonics: La Colle sur Loup, France, 2003; Volume 87, p. 660. [Google Scholar]
- Chiang, C.-H.; Chen, J.-C. Growth and properties of Ru-doped lithium niobate crystal. J. Cryst. Growth 2006, 294, 323–329. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Chen, J.-C.; Lee, Y.-C.; Lin, C.-H.; Chang, J.-Y. Photorefractive properties of Ru doped lithium niobate crystal. Opt. Mater. 2008, 31, 812–816. [Google Scholar] [CrossRef]
- Fujimura, R.; Shimura, T.; Kuroda, K. Two-color nonvolatile holographic recording and light-induced absorption in Ru and Fe codoped LiNbO3 crystals. Opt. Mater. 2009, 31, 1194–1199. [Google Scholar] [CrossRef]
- Xu, C.; Yang, C.; Zhu, C.; Sun, T.; Wang, R.; Xu, Y. Improved nonvolatile holographic storage properties in Zr:Ru:Fe:LiNbO3 crystal by blue light recording. Mater. Lett. 2012, 67, 320–322. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, S.; Li, W.; Kong, Y.; Chen, S.; Xu, J. Improved ultraviolet photorefractive properties of vanadium-doped lithium niobate crystals. Opt. Lett. 2011, 36, 1779–1781. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Liu, S.; Kong, Y.; Chen, S.; Rupp, R.; Xu, J. Fast photorefractive response of vanadium-doped lithium niobate in the visible region. Opt. Lett. 2012, 37, 1841–1843. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Kong, Y.; Liu, S.; Li, W.; Wu, L.; Chen, S.; Xu, J. The photorefraction of molybdenum-doped lithium niobate crystals. Opt. Lett. 2012, 37, 2679–2681. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kong, Y.; Liu, S.; Xu, J. Recent Advances in the Photorefraction of Doped Lithium Niobate Crystals. Materials 2012, 5, 1954-1971. https://doi.org/10.3390/ma5101954
Kong Y, Liu S, Xu J. Recent Advances in the Photorefraction of Doped Lithium Niobate Crystals. Materials. 2012; 5(10):1954-1971. https://doi.org/10.3390/ma5101954
Chicago/Turabian StyleKong, Yongfa, Shiguo Liu, and Jingjun Xu. 2012. "Recent Advances in the Photorefraction of Doped Lithium Niobate Crystals" Materials 5, no. 10: 1954-1971. https://doi.org/10.3390/ma5101954
APA StyleKong, Y., Liu, S., & Xu, J. (2012). Recent Advances in the Photorefraction of Doped Lithium Niobate Crystals. Materials, 5(10), 1954-1971. https://doi.org/10.3390/ma5101954