Hydrothermal Synthesis of Nanostructured Vanadium Oxides
Abstract
:1. Introduction
2. Vanadium (V) Species in Aqueous Solutions
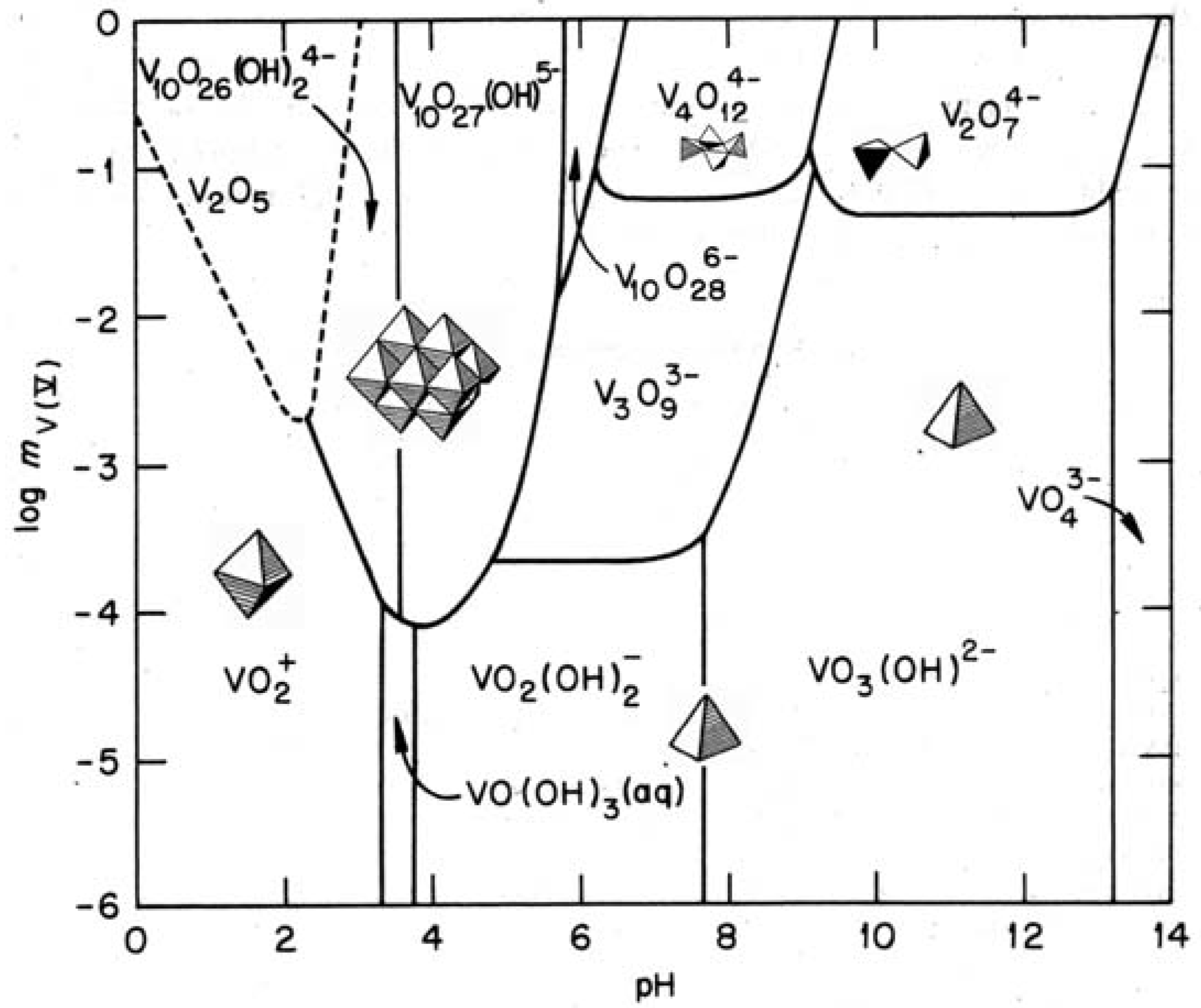
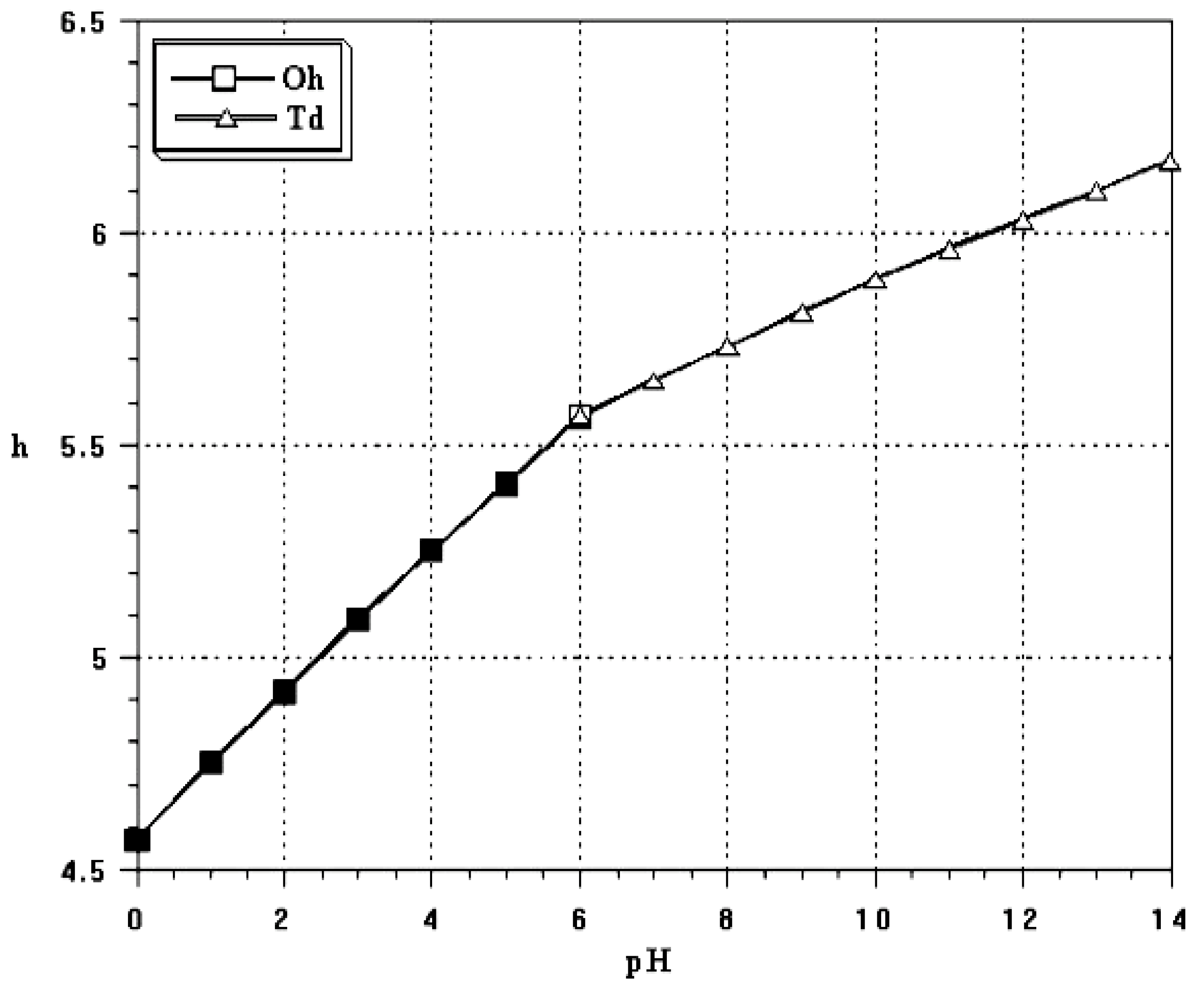
2.1. Hydrolysis
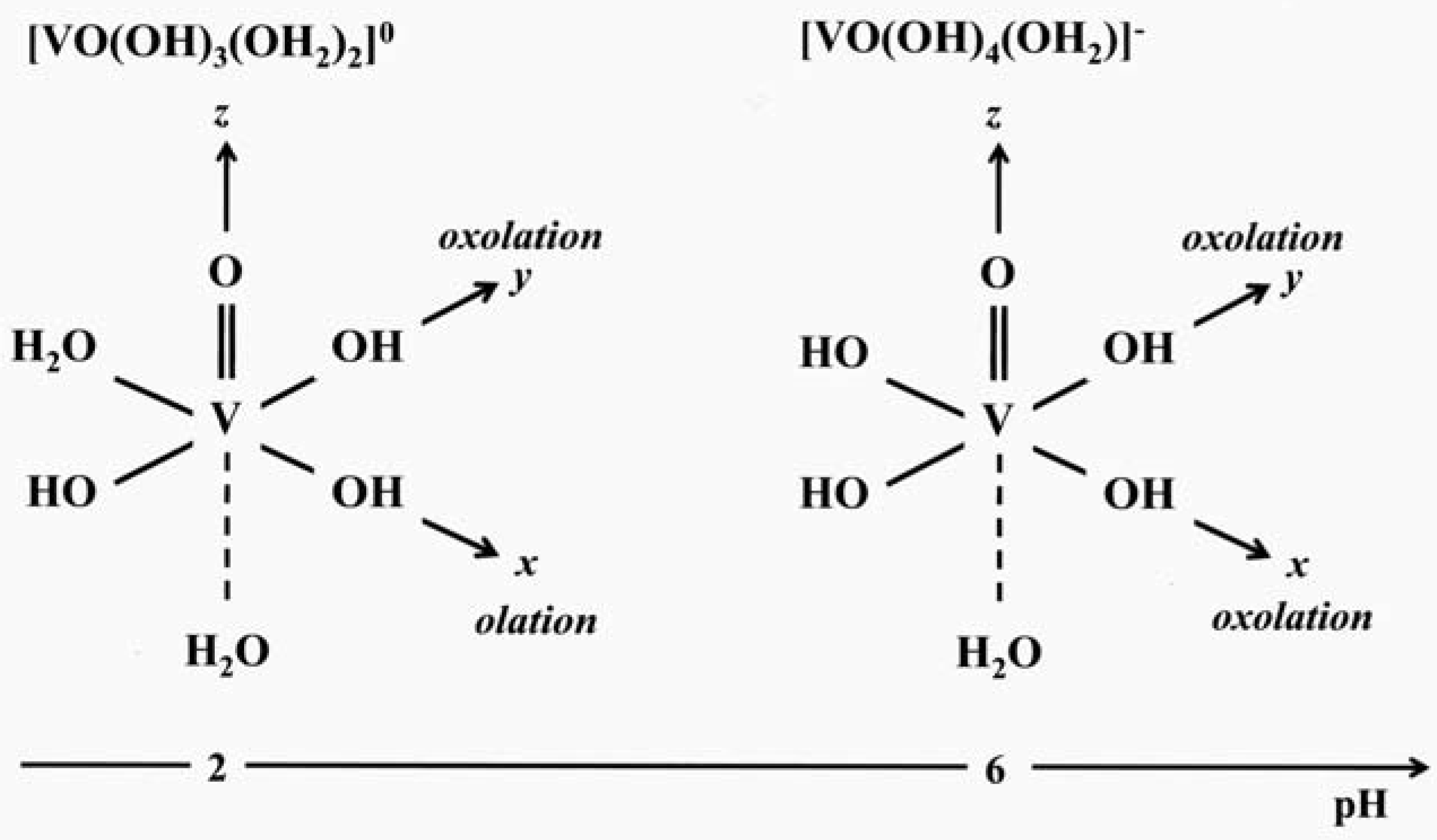
2.2. Condensation
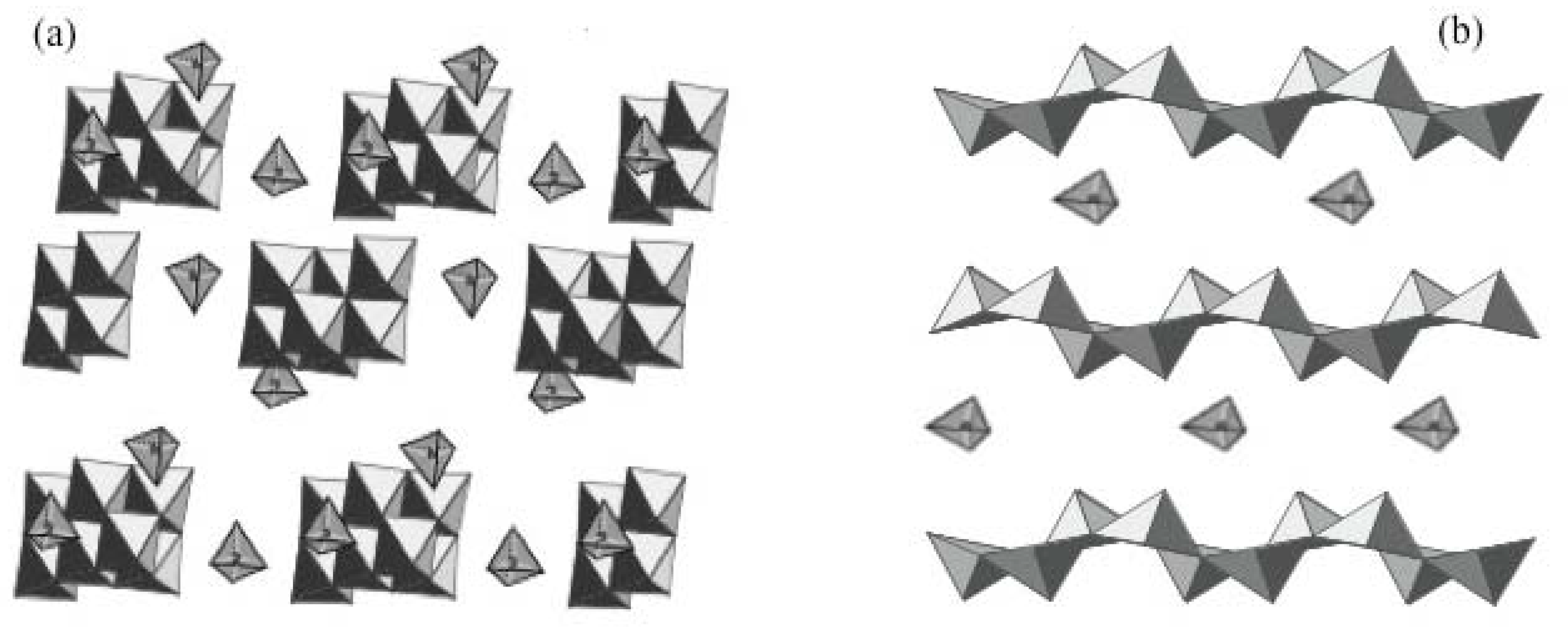
2.3. Evolution of molecular precursors upon heating
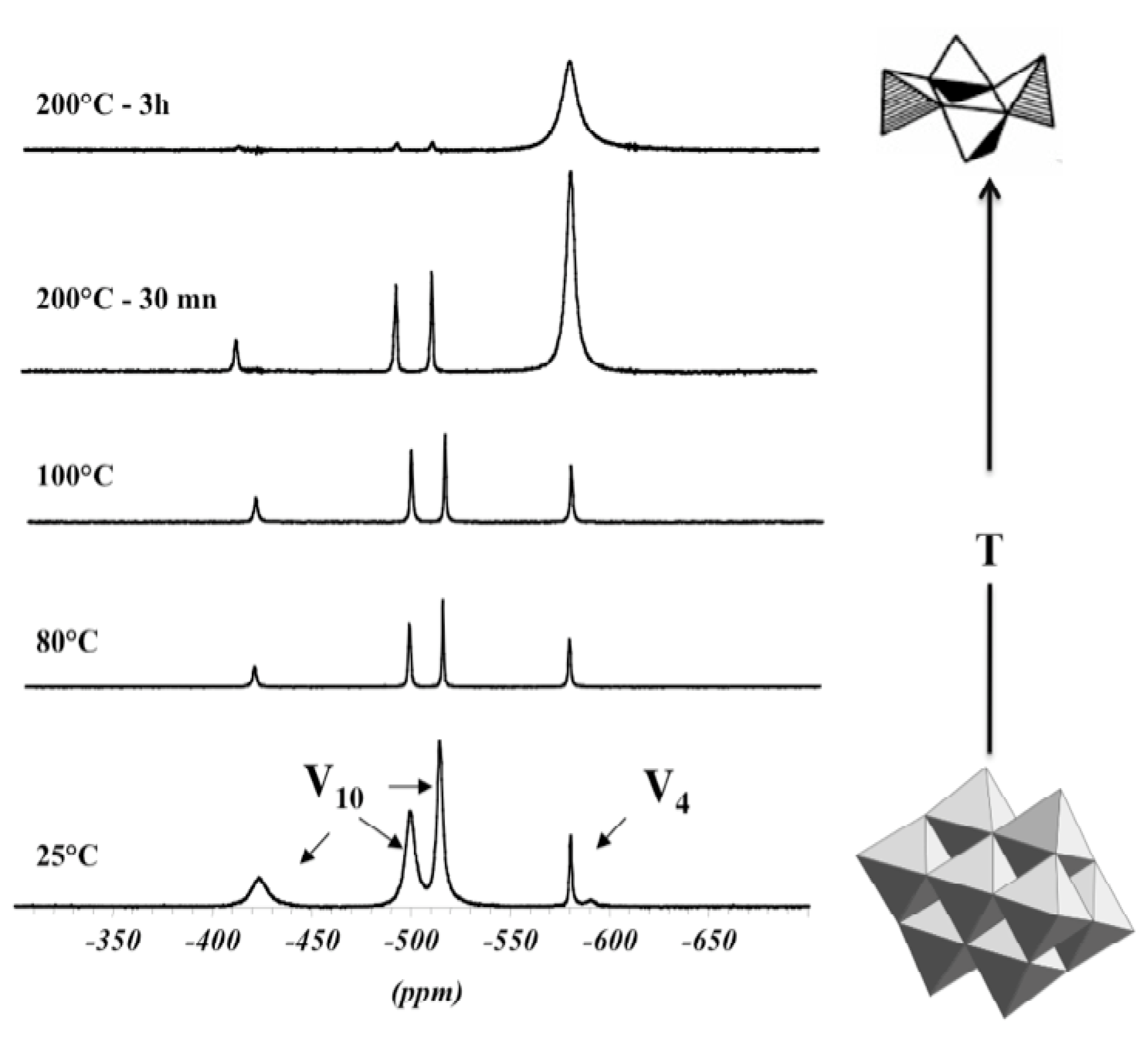
3. Mixed Valence Polyoxovanadate Molecular Clusters

4. Nanostructured Vanadium Oxides
4.1. From 1D to 2D oxides
4.2. Vanadium oxide nanotubes
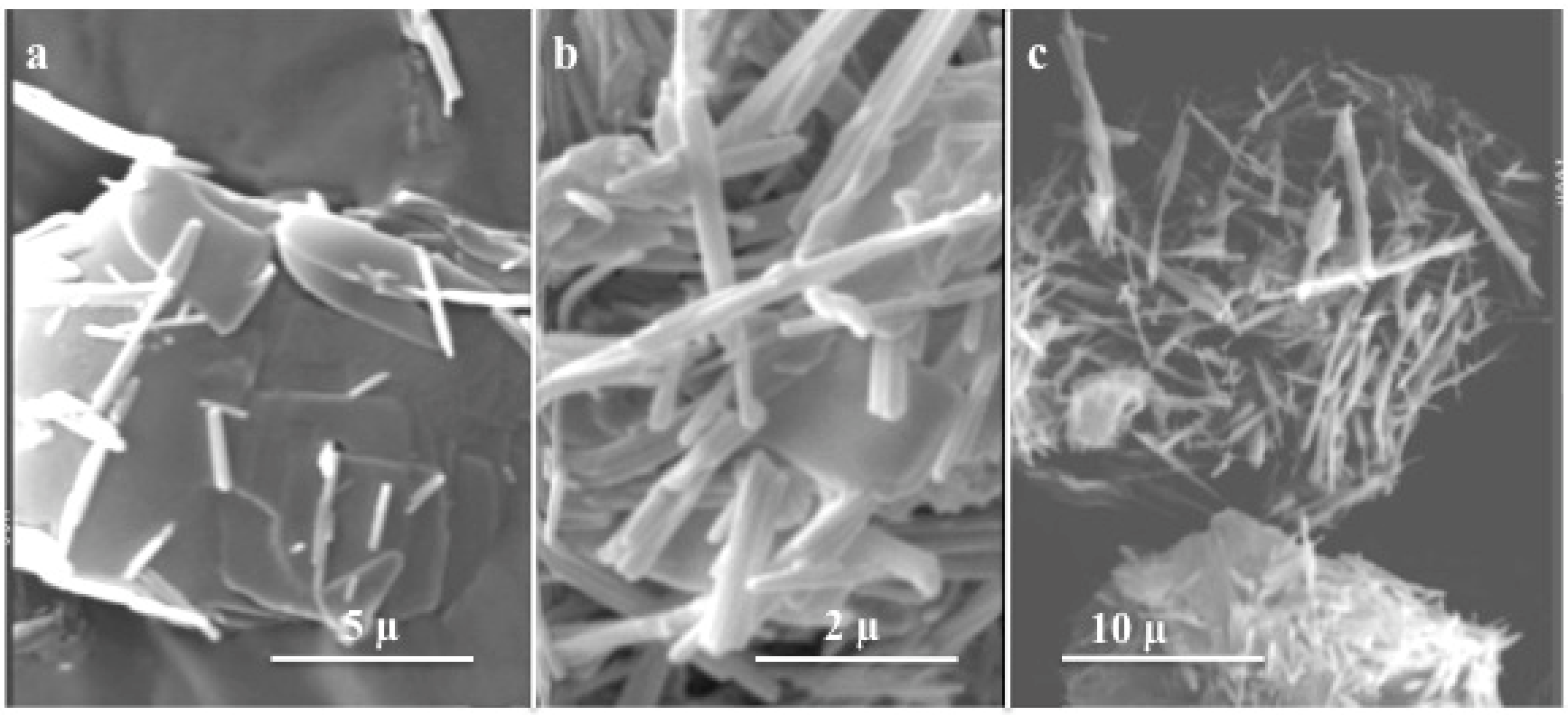
4.3. Nanostructured oxides

5. Conclusion
References
- Wang, Z.L. Nanobelts, nanowires and nanodiskettes of semiconducting oxides - from materials to nanodevices. Adv. Mater. 2003, 15, 432–436. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-dimensional nanostructures: synthesis, characterization and applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Bazito, F.C.; Torresi, R.M. Cathodes for lithium ion batteries: the benefit of using nanostructured materials. J. Braz. Chem. Soc. 2006, 17, 627–642. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, G. Developments in nanostructured cathode materials for high-performance lithium-ion batteries. Adv. Mater. 2008, 20, 2251–2269. [Google Scholar] [CrossRef]
- Spahr, M.E.; Bitterli, P.; Nesper, R.; Muller, M.; Krumeich, F.; Nissen, H.U. Redox active nanotubes of vanadium oxide. Angew. Chem. Int. Ed. 1998, 37, 1263–1265. [Google Scholar] [CrossRef]
- Nesper, R.; Muhr, H.J. Nanotubes—an outstanding set of nano particles. Chimia 1998, 52, 571–578. [Google Scholar]
- Schoiswohl, J.; Surnev, S.; Netzer, F.P.; Kresse, G. Vanadium oxide nanostructure: from zero to three-dimensional. J. Phys.-Condens. Matter. 2006, 18, R1–R14. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, G. Synthesis and enhanced intercalation properties of nanostructured vanadium oxides. Chem. Mater. 2006, 18, 2787–2804. [Google Scholar] [CrossRef]
- Sun, D.; Kwon, C.W.; Baure, G.; Rivhman, E.; MacLean, J.; Dunn, B.; Tolbert, S.H. The relationship between nanoscale structure and electrochemical properties of vanadium oxide nanorolls. Adv. Funct. Mater. 2004, 14, 1197–1204. [Google Scholar] [CrossRef]
- Lee, K.; Wang, Y.; Cao, G. Dependence of electrochemical properties of vanadium oxide films on their nano- and microstructures. J. Phys. Chem. B 2005, 109, 16700–16704. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Takahashi, K.; Lee, K.; Cao, G. Nanostructured vanadium oxide electrodes for enhanced lithium-ion intercalation. Adv. Funct. Mater. 2006, 16, 1133–1144. [Google Scholar] [CrossRef]
- Shimizu, A.; Watanabe, T.; Inagaki, M. Single-crystal study of topotactic changes between NH4VO3 and V2O5. J. Mater. Chem. 1994, 4, 1475–1478. [Google Scholar] [CrossRef]
- Zavalij, P.Y.; Whittingham, M.S. Structural chemistry of vanadium oxides with open frameworks. Acta Cryst. 1999, B55, 627–663. [Google Scholar] [CrossRef]
- Hagrman, P.J.; Finn, R.C.; Zubieta, J. Molecular manipulation of solid state structure: influence of organic components on vanadium oxide architectures. Solid State Sci. 2001, 3, 745–774. [Google Scholar] [CrossRef]
- Chirayil, T.; Zavalij, P.Y.; Whittingham, M.S. Hydrothermal synthesis of vanadium oxides. Chem. Mater. 1998, 10, 2629–2640. [Google Scholar] [CrossRef]
- Baess, C.F.; Mesmer, R.E. Hydrolysis of cations; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Livage, J.; Henry, M.; Sanchez, C. Sol-gel chemistry of transition metal oxides. Prog. Solid State Chem. 1988, 18, 259–341. [Google Scholar] [CrossRef]
- Henry, M.; Jolivet, J.P.; Livage, J. Aqueous chemistry of metal cations: hydrolyis, condensation and complexation. Struct. Bond. 1992, 77, 154–206. [Google Scholar]
- Livage, J. Synthesis of polyoxovanadates via chimie douce. Coord. Chem. Reviews 1998, 178-180, 999–1018. [Google Scholar] [CrossRef]
- Sadoc, A.; Messaoudi, S.; Furet, E.; Gautier, R.; Le Fur, E.; Le Pollès, L.; Pivan, J.Y. Structure and stability of VO2+ in aqueous solution: a car-parrinello and static ab initio study. Inorg. Chem. 2007, 46, 4835–4843. [Google Scholar] [CrossRef] [PubMed]
- Fratzky, D.; Götze, T.; Worzala, H.; Meisel, M. The formation of vanadyl phosphate hydrates from aqueous phase: a systematic study. Mater. Res. Bull. 1998, 33, 635–643. [Google Scholar] [CrossRef]
- Livage, J. Vanadium pentoxide gels. Chem. Mater. 1991, 3, 578–593. [Google Scholar] [CrossRef]
- Giorgetti, M.; Passerini, S.; WSmyrl, W.H. Evidence of bilayer structure in V2O5 xerogel. Inorg. Chem. 2000, 39, 1514–1517. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.; Trikalitis, P.N.; Bozin, E.S.; Billinge, S.J.; Vogt, T.; Kanatzidis, M.G. Structure of V2O5.nH2O xerogel solved by the atomic pair distribution function technique. J. Am. Chem. Soc. 2002, 124, 10157–10162. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, C.J.; Wiench, J.W.; Schrader, G.L.; Pruski, M. 17O MAS and 3QMAS NMR investigation of crystalline V2O5 and layered V2O5.nH2O gels. J. Am. Chem. Soc. 2002, 124, 8435–8444. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.T. The molecular structure of the isopoly complex ion, decavanadate (V10O286-). Inorg. Chem. 1966, 5, 967–977. [Google Scholar] [CrossRef]
- Heath, E.; Howard, O.W. Vanadium-51 and oxygen-17 nuclear magnetic resonance study of vanadate (V) equilibria and kinetics. J. Chem. Soc. Dalton 1981, 1105–1110. [Google Scholar] [CrossRef]
- Wery, A.S.; Gutierez-Zorrilla, J.M.; Luque, A.; Ugalde, M.; Roman, P. Phase transition in metavanadates. Polymerization of tetrakis(tert-butylammonium)-cyclo-tetrametavanadate. Chem. Mater. 1996, 8, 408–413. [Google Scholar] [CrossRef]
- Bouhedja, L.; Steunou, N.; Maquet, J.; Livage, J. Synthesis of polyoxovanadates from aqueous solutions. J. Solid State Chem. 2001, 162, 315–321. [Google Scholar] [CrossRef]
- Riou, D.; Roubeau, O.; Ferey, G. Evidence for the solid state structural transformation of the network type decavanadate into a lamellar topology. Z. Anorg. Allg. Chem. 1998, 624, 1021–1025. [Google Scholar] [CrossRef]
- Drezen, T.; Joubert, O.; Ganne, M.; Brohan, L. Synthesis and structure determination of a novel centered tricosahedral cluster compound related to the Müller-type structure. J. Solid State Chem. 1998, 136, 298–304. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, X.; Peng, J.; Shi, Z.; Yu, H.; Wang, E.; Hu, N. The first discrete mixed-valence hexadecavanadate host shell cluster anions: hydrothermal synthesis, structure and characterization of [V16O38Cl]8-. Inorg. Chem. Comm. 2004, 7, 705–707. [Google Scholar] [CrossRef]
- Klemperer, W.G.; Marquaet, T.A.; Yaghi, O.M. New directions in polyvanadate chemistry: from cages and clusters to baskets, belts, bowls and barrels. Angew. Chem. Int. Ed. 1992, 31, 49–51. [Google Scholar] [CrossRef]
- Müller, A.; Krickemeyer, E.; Penk, M.; Rohlfing, R.; Armatage, A.; Bögge, H. Template-controlled formation of cluster shells or a type of molecular recognition: synthesis of [HV22O54(ClO4)]6- and [H2V18O44(N3)]5. Angew. Chem. Int. Ed. 1991, 30, 1674–1677. [Google Scholar] [CrossRef]
- Müller, A.; Penk, M.; Rohlfing, R.; Krickemeyer, E.; Döring, J. Formation of a cluster sheath around a central cluster by a 'self-organization process': the mixed valence polyoxovanadate [V34O82]10-. Angew. Chem. Int. Ed. 1991, 30, 588–590. [Google Scholar] [CrossRef]
- Müller, A.; Sessoli, R.; Krickemeyer, E.; Bögge, H.; Meyer, J.; Gatteschi, D.; Pardi, L.; Westphal, J.; Hovemeier, K.; Rohlfing, R.; Döring, J.; Hellweg, F.; Beugholt, C.; Schmidtmann, M. Polyoxovanadates: high-nuclearity spin clusters with interesting host-guest systems and different electron populations. Synthesis, spin organization, magnetochemistry, and spectroscopic studies. Inorg. Chem. 1997, 36, 5239–5250. [Google Scholar] [CrossRef]
- Müller, A. Induced molecule self-organization. Nature 1991, 352, 115. [Google Scholar] [CrossRef] [PubMed]
- Steunou, N.; Bouhedja, L.; Castro-Garcia, S.; Livage, J. Chemically controlled hydrothermal syntheses of vanadium oxides. High Pressure Res. 2001, 20, 55–62. [Google Scholar] [CrossRef]
- Rohmer, M.M.; Deveny, J.; Wiest, R.; Benard, M. Ab initio modeling of the endohedral reactivity of polyoxometallates: host-guest interactions in [RCN(V12O324-] (R=H, CH3, C6H5). J. Am. Chem. Soc. 1996, 118, 13007–13014. [Google Scholar] [CrossRef]
- Zavalij, P.Y.; Whittingham, M.S.; Boylan, E.A.; Pecharsky, V.K.; Jacobson, R.A. New structure TMAV4O10. Z. Krist. 1996, 211, 464. [Google Scholar] [CrossRef]
- Chirayil, T.G.; Boylan, E.A.; Mammak, M.; Zavalij, P.Y.; Whittingham, M.S. NMe4V3O7: critical role of pH in hydrothermal synthesis of vanadium oxides. Chem. Commun. 1997, 33–34. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, X.; Yuan, C.; Li, L.; Xu, H. Low-temperature hydrothermal synthesis of orthorhombic vanadium pentoxide nanowires. Chem. Lett. 2007, 36, 310. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, X.; Yuan, C.; Li, L. Vanadium pentoxide nanowires: hydrothermal synthesis, formation, mechanism and phase control parameters. Cryst. Growth Des. 2008, 8, 723–727. [Google Scholar]
- Gao, S.; Chen, Y.; Luo, H.; Jiang, L.; Ye, B.; Wei, M.; Wei, K. Single-crystal vanadium pentoxide nanowires. J. Nanosci. Nanotechnol. 2008, 8, 3500–3503. [Google Scholar] [CrossRef] [PubMed]
- Muster, J.; Kim, G.T.; Krstic, V.; Park, J.G.; Park, Y.W.; Roth, S.; Burghard, M. Electrical transport through individual vanadium pentoxide nanowires. Adv. Mater. 2000, 12, 420–424. [Google Scholar] [CrossRef]
- Kim, G.T.; Muster, J.; Krstic, V.; Park, J.G.; Park, Y.W.; Roth, S.; Burghard, M. Field-effect transistor made of individual V2O5 nanofibers. Appl. Phys. Lett. 2000, 76, 1875–1877. [Google Scholar] [CrossRef]
- Gu, G.; Schmid, M.; Chiu, P.W.; Minett, A.; Fraysse, J.; Kim, G.T.; Roth, S.; M. Kozlov, M.; Munoz, E.; Baughman, R.H. V2O5 nanofibre sheet actuators. Nature 2003, 2, 316–318. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Twesten, R.D.; Jarausch, K.; Zhang, F.; Cui, Y. Fast, completely reversible Li insertion in vanadium pentoxide nanoribbons. Nano Lett. 2007, 7, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Pinna, N.; Willinger, W.; Weiss, K.; Urban, J.; Schlögl, R. Local structure of nanoscopic materials: V2O5 nanorods and nanowires. Nano Lett. 2003, 3, 1131–1134. [Google Scholar] [CrossRef]
- Pinna, N.; Wild, U.; Urban, J.; Schlögl, R. Divanadium pentoxide nanorods. Adv. Mater. 2003, 15, 329–331. [Google Scholar] [CrossRef]
- Takahashi, K.; Limmer, S.J.; Wang, Y.; Cao, G. Synthesis and electrochemical properties of single-crystal V2O5 nanorods arrays by templated-based electrodeposition. J. Phys. Chem. B 2004, 108, 9795–9800. [Google Scholar] [CrossRef]
- Pavasupree, S.; Suzuki, Y.; Kitiyanan, A.; Pivsa-Art, S.; Yoshikawa, S. Synthesis and characterization of vanadium oxides nanorods. J. Solid State Chem. 2005, 18, 2152–2158. [Google Scholar] [CrossRef]
- Asim, N.; Radiman, S.; Yarmo, M.A.; Banaye Golriz, M.S. Vanadium pentoxide: synthesis and characterization of nanorod and nanoparticle V2O5 using CTAB micelle solution. Microporous Mesoporous Mat. 2009, 120, 397–401. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Peng, Q.; Li, Y. Vanadium pentoxide nanobelts: highly selective and stable ethanol sensor materials. Adv. Mater. 2005, 17, 764–767. [Google Scholar] [CrossRef]
- Li, G.; Pang, S.; Jiang, L.; Guo, Z.; Zhang, Z. Environmentally friendly chemical route to vanadium oxide single-crystalline nanobelts as a cathode material for lithium-ion batteries. J. Phys. Chem. B 2006, 110, 9383–9386. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, Y.; Rong, G.; Jing, M.; Xie, Y. Vanadium pentoxide nanobelts and nanorolls: from controllable synthesis to investigation of their electrochemical properties and photocatalytic activities. Nantechnology 2006, 17, 2560–2566. [Google Scholar] [CrossRef]
- Ren, X.; Jiang, Y.; Zhang, P.; Liu, J.; Zhang, Q. Preparation and electrochemical properties of V2O5 submicron-belts sythesized by a sol-gel H2O2 route. J. Sol-Gel Sci. Technol. 2009, 51, 133–138. [Google Scholar] [CrossRef]
- Lu, C.; Ding, Z.; Lipson, R.H. A new chimie douce approach to crystalline vanadium pentoxide nanobelts. J. Mater. Chem. 2009, 19, 6512–6515. [Google Scholar] [CrossRef]
- Yu, J.; Liu, S.; Cheng, B.; Xiong, J.; Yu, Y.; Wang, J. Polymer-directed large-scale synthesis of sigle-crystal vanadium oxide nanobelts. Mater. Chem. Phys. 2006, 95, 206–210. [Google Scholar] [CrossRef]
- Mai, L.Q.; Lao, C.S.; Hu, B.; Zhou, J.; Qi, Y.Y.; Chen, W.; Gu, E.D.; Wang, Z.L. Synthesis and electrical transport of single-crystal NH4V3O8 nanobelts. J. Phys. Chem. B 2006, 110, 18138–18141. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.V.; Wei, J.; Yao, Q.Z.; Rong, Z.D.; Wen, C.; Mho, S.I.; Kalluru, T.R. Cathodic performance of (V2O5+PEG) nanobelts for Li ion rechargeable battery. J. Power Sources 2007, 166, 244–249. [Google Scholar] [CrossRef]
- Subba Reddy, C.V.; Mho, S.I.; Kalluru, R.R.; Williams, Q.L. Hydrothermal synthesis of hydrated vanadium oxide nanobelts using poly(ethylene oxide) as a template. J. Power Sources 2008, 179, 854–857. [Google Scholar] [CrossRef]
- Pang, S.; Li, G.; Zhang, Z. Synthesis of polyaniline-vanadium oxide nanocomposite nanosheets. Macromol. Rapid Commun. 2005, 26, 1262–1265. [Google Scholar] [CrossRef]
- Li, G.C.; Pang, S.P.; Wang, Z.B.; Peng, H.R.; Zhang, Z.K. Synthesis of H2V3O8 single-crystal nanobelts. Eur. J. Inorg. Chem. 2005, 2060–2063. [Google Scholar] [CrossRef]
- Chang, K.H.; Hu, C.C. H2V3O8 single-crystal nanobelts: Hydrothermal preparation and formation mechanism. Acta Mater. 2007, 55, 6192–6197. [Google Scholar] [CrossRef]
- Qiao, H.; Zhu, X.; Zheng, Z.; Liu, L.; Zhang, L. Synthesis of V3O7.H2O nanobelts as cathode materials for lithium-ion batteries. Electrochem. Commun. 2006, 8, 21–26. [Google Scholar] [CrossRef]
- Gao, S.; Chen, Z.; Wei, M.; Wei, K.; Zhou, H. Single crystal nanobelts of V3O7.H2O: a lithium intercalation host with a large capacity. Electrochim. Acta 2009, 54, 1115–1118. [Google Scholar] [CrossRef]
- Krumeich, F.; Muhr, H.J.; Niederberger, M.; Bieri, F.; Schnyder, B.; Nesper, R. Morphology and topochemical rections of novel vanadium oxide nanotubes. J. Am. Chem. Soc. 1999, 121, 8324–8328. [Google Scholar] [CrossRef]
- Avansi, W., Jr.; Ribeiro, C.; Leite, E.; V.R. Mastelaro, V.R. Vanadium pentoxide nanostructures: an effective control of morphology and crystal structure in hydrothermal conditions. Cryst. Growth Des. 2009, 9, 3626–3631. [Google Scholar] [CrossRef]
- Muhr, H.J.; Krumeich, F.; Schönholzer, U.P.; Bieri, F.; Niederberger, M.; L.J. Gauckler, L.J.; Nesper, R. Vanadium oxide nanotubes - a new flexible vanadate nanophase. Adv. Mater. 2000, 12, 231–234. [Google Scholar] [CrossRef]
- Ivanovskaya, V.V.; Enyashin, A.N.; Sofronov, A.A.; Makurin, Y.N.; Medvedeva, N.I.; Ivanovskii, A.L. Electronic properties of single-walled V2O5 nanotubes. Solid State Comm. 2003, 126, 489–493. [Google Scholar] [CrossRef]
- Enyashin, A.N.; Ivanovskzya, V.V.; Makurin, Y.N.; Ivanovskii, A.L. Electronic band structure of scroll-like divanadium pentoxide nanotubes. Phys. Letters A 2004, 326, 152–156. [Google Scholar] [CrossRef]
- Sipos, B.; Duchamp, M.; Magrez, A.; Forro, L.; Barisic, N.; A. Kis, A.; Seo, J.W.; F. Bien, F.; Krumeich, F.; Nesper, R.; Patzke, G.R. Mechanical and electronic properties of vanadium oxide nanotubes. J. Appl. Phys. 2009, 105, 074317:1–074317:5. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Musfeldt, J.L.; Mazumdar, S.; Chernova, N.A.; Whittingham, M.S. Pinned low-energy elctronic excitation in metal-exchange vanadium oxide nanoscrolls. Nano Letters 2007, 7, 2351–2355. [Google Scholar] [CrossRef]
- Liu, X.; Täschner, C.; Leonhardt, A.; Rümmeli, M.H.; Pichler, T.; Gemming, T.; B. Büchner, B.; Knupfer, M. Structural, optical and electronic properties of vanadium oxide nanotubes. Phys. Rev. B 2005, 72, 115407:1–115407:5. [Google Scholar]
- Webster, S.; Czerw, R.; Nesper, R.; DiMaio, J.; Xu, J.F.; Ballato, J.; Carroll, D.L. Optical properties of vanadium oxide nanotubes. J. Nanosci. Nanotech. 2004, 4, 260–264. [Google Scholar] [CrossRef]
- Vavilova, E.; Herllmann, I.; Kataev, V.; Täschner, C.; Büchner, B.; Klingeler, R. Magnetic properties of vanadium oxide nanotubes probed by static magnetization and 51V NMR. Phys. Rev. B 2006, 73, 1–7. [Google Scholar] [CrossRef]
- Spahr, M.E.; Stoschitzki-Bitterli, P.; Nesper, R.; Haas, O.; Novak, P. Vanadium oxide nanotubes, a new nanostructured redox-active material for the electrochemical insertion of lithium. J. Electrochem. Soc. 1999, 146, 2780–2783. [Google Scholar] [CrossRef]
- Sun, D.; Kwon, C.W.; Baure, G.; Richman, E.; MacLean, J.; Dunn, B.; Tolbert, S.H. The relationship between nanoscale structure and electrochemical properties of vanadium oxide nanorolls. Adv. Funct. Mater. 2004, 14, 1197–1204. [Google Scholar] [CrossRef]
- Li, H.X.; Jiao, L.F.; Yuan, H.T.; Zhang, M.; Guo, J.; Wang, L.Q.; Zhao, M.; Wang, Y.M. Factors affecting the electrochemical performance of vanadium oxide nanotube cathode materials. Electrochem. Comm. 2006, 8, 1693–1698. [Google Scholar] [CrossRef]
- Nordlinder, S.; Nyholm, L.; Gustafsson, T.; Edström, K. Lithium insertion into vanadium oxide nanotubes: electrochemical and structural aspects. Chem. Mater. 2006, 18, 495–503. [Google Scholar] [CrossRef]
- Mohan, V.M.; Hu, B.; Qiu, W.; Chen, W. Synthesis, stuctural and electrochemical performane of V2O5 nanotubes as cathode material for lithium battery. J. Appl. Electrochem. 2009, 39, 2001–2006. [Google Scholar] [CrossRef]
- Cui, C.J.; Wu, G.M.; Shen, J.; Zhou, B.; Zhang, Z.H.; Yang, H.Y.; She, S.F. Synthesis and electrochemical performance of lithium vanadium oxide nanotubes as cathodes for rechargeable lithium-ion batteries. Electrochim. Acta 2010, 55, 2536–2541. [Google Scholar] [CrossRef]
- Patzke, G.R.; Krumeich, F.; R. Nesper, R. Oxidic nanotubes and nanorods - anisotropic modules for a future nanotechnology. Angew. Chem. Int. Ed. 2002, 41, 2446–2461. [Google Scholar] [CrossRef]
- Sharma, S.; Thomas, J.; Ramanan, A.; Panthöfer, M.; Jansen, M. Hydrothermal synthesis of vanadium oxide nanotubes from oxide precursors. J. Nanosci. Nanotechn. 2007, 6, 1985–1989. [Google Scholar] [CrossRef]
- Mai, L.; Chen, W.; Xu, Q.; Zhu, Q.; Han, C.; Peng, J. Cost-saving synthesis of vanadium oxide nanotubes. Solid State Comm. 2003, 126, 541–543. [Google Scholar] [CrossRef]
- Niedderberger, M.; Muhr, H.J.; Krumeich, F.; Bieri, F.; Günther, D.; Nesper, R. Low-cost synthesis of vanadium oxide nanotubes via two novel non-alkoxide routes. Chem. Mater. 2000, 12, 1995–2000. [Google Scholar] [CrossRef]
- Asim, N.; Radiman, S.; Yarmo, M.A.; Banaye Golriz, M.S. Vanadium pentoxide: synthesis and characterization of nanorod and nanoparticle V2O5 using CTAB micellle solution. Microporous Mesoporous Mat. 2009, 120, 397–401. [Google Scholar] [CrossRef]
- Chandrappa, G.T.; Steunou, N.; Cassaignon, S.; Bauvais, C.; Livage, J. Hydrothermal synthesis of vanadium oxide nanotubes from V2O5 gels. Catal. Today 2003, 78, 85–89. [Google Scholar] [CrossRef]
- Chen, W.; Peng, J.; Mai, L.; Zhu, Q.; Xu, Q. Synthesis of vanadium oxide nanotubes from V2O5 sols. Mater. Lett. 2004, 58, 2275–2278. [Google Scholar] [CrossRef]
- Petkov, V.; Zavalij, P.Y.; Lutta, S.; Whittingham, M.S.; Parvanov, V.; Shastri, S. Structure beyond Bragg: study of V2O5 nanotubes. Phys. Rev. B 2004, 69, 9085410:1–9085410:6. [Google Scholar] [CrossRef]
- Krumeich, F.; Muhr, H.J.; Niederberger, M.; Bieri, F.; Nesper, R. The cross-sectional structure of vanadium oxide nanotube studied by transmission electron microscopy and electron spectroscopic imaging. Z. Anorg. Allg. Chem. 2000, 626, 2208–2216. [Google Scholar] [CrossRef]
- Davidson, P. Vanadium pentoxide gels: from chimie douce to matière molle. C.R. Chim. 2010, 13, 142–153. [Google Scholar] [CrossRef]
- Chandrappa, G.T.; Steunou, N.; Cassaignon, S.; Bauvais, C.; Biswas, P.K.; Livage, J. Vanadium oxide: from gels to nanotubes. J. Sol-Gel Sci. Techn. 2003, 26, 593–596. [Google Scholar] [CrossRef]
- Muhr, H.J.; Schönholzer, U.P.; Bieri, F.; Niederberger, M.; Gauckler, L.J.; Nesper, R. vanadium oxide nanotubes—a new vanadate nanophase. Adv. Mater. 2000, 12, 231–234. [Google Scholar] [CrossRef]
- Kweon, H.; Lee, K.W.; Lee, C.E. Coexisting structural phases in a two-dimensional vanadium oxide/surfactant nanostructure. Curr. Appl. Phys. 2009, 9, 691–693. [Google Scholar] [CrossRef]
- Chen, W.; Mai, L.Q.; Peng, J.F.; Xu, Q.; Zhu, Q.Y. FTIR study of vanadium oxide nanotubes from lamellar structure. J. Mater. Sci. 2004, 39, 2625–2627. [Google Scholar] [CrossRef]
- Chen, X.; Sun, X.; Li, Y. Self-assembling vanadium oxide nanotubes by organic molecular templates. Inorg. Chem. 2002, 41, 4524–4530. [Google Scholar] [CrossRef] [PubMed]
- Sediri, F.; Gharbi, N. From crystalline V2O5 to nanostructured vanadium oxides using aromatic amines as templates. J. Phys. Chem. Solids 2007, 68, 1821–1829. [Google Scholar] [CrossRef]
- Grigorieva, A.V.; Goodilin, E.A.; Anikina, A.V.; Kolesnik, I.V.; Tretyakov, Y.D. Surfactants in the formation of vanadium oxide nanotubes. Mendeleev Commun. 2008, 18, 71–72. [Google Scholar] [CrossRef]
- Reinoso, J.M.; Muhr, H.J.; Krumeich, F.; Bieri, F.; Nesper, R. Controlled uptake and release of metal cations by vanadium oxide nanotube. Helv. Chim. Acta 2000, 83, 1724–1733. [Google Scholar] [CrossRef]
- Corr, S.A.; Grossman, M.; Furman, J.D.; Melot, B.C.; Cheetham, A.K.; Heier, K.R.; Seshadri, R. Controlled reduction of vanadium oxide nanoscrolls: crystal structure, morphology and electrical properties. Chem. Mater. 2008, 20, 6396–6404. [Google Scholar] [CrossRef]
- Vera-Robles, L.I.; Campero, A. A novel approach to vanadium oxide nanotubes by oxidation of V4+ species. J. Phys. Chem. C 2008, 112, 19930–19933. [Google Scholar] [CrossRef]
- Wörle, M.; Krumeich, F.; Bieri, F.; Muhr, H.J.; Nesper, R. Flexible V7O16 layers as the common structural element of vanadium oxide nanotubes and a new crystalline vanadate. Z. anorg. allg. Chem. 2002, 628, 2778–2784. [Google Scholar] [CrossRef]
- Sanchez, C.; Babonneau, F.; Morineau, R.; Livage, J.; Bullot, J. Semiconducting properties of V2O5 gels. Phil. Mag. B 1983, 47, 279–290. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, Z.; Lü, M.; Zhang, A.; Ma, Q. Preparation and characterization of V2O5 macro-plates. Mater. Lett. 2007, 61, 4073–4075. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Wong, Y.C.; Zainal, Z.; Hussein, M.Z. Synthesis of self-assembled nanorod vanadium oxide bundles by sonochemical treatment. J. Nat. Gas Chem. 2009, 18, 312–318. [Google Scholar] [CrossRef]
- Mao, C.J.; Pan, H.C.; Wu, X.C.; Zhu, J.J.; Chen, H.Y. Sonochemical route for self-assembled V2O5 bundles with spindle-like morphology and their novel application in serum albumin sensing. J. Phys. Chem. B 2006, 110, 14709–14713. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jiang, L.; Peng, H. A simple route to V2O5.xH2O bundle-like nanostructures. Mater. Lett. 2007, 61, 4070–4072. [Google Scholar] [CrossRef]
- Cao, A.M.; Hu, J.S.; Liang, H.P.; Wan, L.J. Self-assembled vanadium pentoxide (V2O5) hollow microspheres from nanorods and their application in lithium-ion batteries. Angew. Chem. Int. Ed. 2005, 44, 4391–4395. [Google Scholar] [CrossRef]
- Weeks, C.; Song, Y.; Suzuki, M.; Chernova, N.A.; Zavalij, P.Y.; Whittingham, M.S. The one dimensional chain structure of vanadyl glycolate and vanadyl acetate. J. Mater. Chem. 2003, 13, 1420–1423. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Y.; Herrivcks, T.; Xia, Y. Ethylene glycol-mediated synthesis of metal oxide nanowires. J. Mater. Chem. 2004, 14, 695–703. [Google Scholar] [CrossRef]
- Feng, C.Q.; Wang, S.Y.; Zeng, R.; Guo, Z.P.; Konstantinov, K.; Liu, H.K. Synthesis of spherical porous vanadium pentoxide and its electrochemical properties. J. Power Sources 2008, 184, 485–488. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Ye, W.L. Preparation and electrochemical properties of submicron spherical V2O5 as cathode material for lithium batteries. Scripta Mater. 2008, 59, 372–375. [Google Scholar] [CrossRef]
- Fei, H.L.; Zhou, H.J.; Wang, J.G.; Sun, P.C.; Ding, D.T.; Chen, T.H. Synthesis of hollow V2O5 microspheres and application to photocatalysis. Solid State Sci. 2008, 10, 1276–1284. [Google Scholar] [CrossRef]
- Lim, H.S.; Kwak, D.; Lee, D.Y.; Goo, S.; Cho, K. UV-driven reversible switching of a roselike vanadium oxide film between superhydrophobicity and superhydrophilicity. J. Am. Chem. Soc. 2007, 129, 4128–4129. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.L.; Zhou, H.J.; Wang, J.G.; Sun, P.C.; Ding, D.T.; Chen, T.H. Synthesis of V2O5 micro-architectures via in situ generation of single-crystalline nanoparticles. Solid State Sci. 2009, 11, 102–107. [Google Scholar] [CrossRef]
- Liu, X.; Huang, C.V.; Yi, S.; Xie, G.; Li, H.; Luo, Y. A new solvothermal method of preparing VO2 nanosheets and petaloid clusters. Solid State Comm. 2007, 144, 259–263. [Google Scholar] [CrossRef]
- Wu, C.; Xie, Y.; Lei, L.; Hu, S.; OuYang, C. Synthesis of new-phased VOOH 'dandelions' and their application in lithium-ion batteries. Adv. Mater. 2006, 18, 1727–1732. [Google Scholar] [CrossRef]
- O’Dwyer, C.; Navas, D.; Lavayen, V.; Benavente, E.; Santa Ana, M.A.; G. Gonzalez, G.; Newcomb, S.B.; Sotomayor Torres, C.M. Nano-urchin: the formation and structure of high-density spherical clusters of vanadium oxide nanotubes. Chem. Mater. 2006, 18, 3016–3022. [Google Scholar] [CrossRef]
- Lavayen, V.; O'Dwyer, C.; Santa Ana, M.A.; Newcomb, S.B.; Benavente, E.; Gonzalez, G.; Sotomayor Torres, C.M. Comparative structural-vibrational study of nano-urchin and nanorods of vanadium oxide. Phys. Stat. Sol. (b) 2006, 13, 3285–3289. [Google Scholar] [CrossRef]
- O'Dwyer, C.; Lavayen, V.; Newcomb, S.B.; Santa Ana, M.A.; E. Benavente, E.; Gonzàlez, G.; Sotomayor Torres, C.M. Vanadate conformation variations in vanadium pentoxide nanostructures. J. Electrochem. Soc. 2007, 154, K29–K35. [Google Scholar] [CrossRef]
- O'Dwyer, C.; Lavayen, V.; Tanner, D.A.; Newcomb, S.B.; Benavente, E.; Gonzales, G.; Clivia, C.; Sotomayor Torres, M. Reduced surfactant uptake in three dimensional assemblies of VOx nanotube improve reversible Li+ intercalation and charge capacity. Adv. Funct. Mater. 2009, 19, 1–10. [Google Scholar] [CrossRef]
- O'Dwyer, C.; Lavayen, V.; Fuenzalida, D.; Newcomb, S.B.; Sant Ana, M.A.; Benavente, E.; Gonzalez, G.; Sotomayor Torres, C.M. Six-fold rotationally symmetric vanadium oxide nanostructures by a morphotropic phase transition. Phys. Stat. Sol. (b) 2007, 244, 4157–4160. [Google Scholar] [CrossRef]
- Su, Q.; Huang, C.K.; Wang, Y.; Fan, Y.C.; Lu, B.A.; Lan, W.; Wang, Y.Y.; Liu, X.Q. Formation of vanadium oxides with various morphologies by chemical vapor deposition. J. Alloys Compounds 2009, 475, 518–523. [Google Scholar] [CrossRef]
- Hu, C.C.; Huang, C.M.; Chang, K.H. Anodic deposition of porous vanadium oxide network with high power characteristics for pseudocapacitors. J. Power Sources 2008, 185, 1594–1597. [Google Scholar] [CrossRef]
- Hu, Chi-Chang; Chang, Kuo-Hsin; Huang, Chao-Ming; Li, Jing-Mei. Anodic deposition of vanadium oxide for thermal-induced growth of vanadium oxide nanowires. J. Electrochem. Soc. 2009, 156, D485–D489. [Google Scholar]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Centi, G.; Perathoner, S. The role of nanostructure in improving the performance of electrodes for energy storage and conversion. Eur. J. Inorg. Chem. 2009, 3851–3878. [Google Scholar] [CrossRef]
- O'Dwyer, C.; Lavayen, V.; Santa, M.A.; Benavente, E.; Gonzàlez, G.; Torres, C.M.S. Anisotropic vanadium oxide nanostructured host matrices for lithium ion intercalation. Res. Lett. Phys. Chem. 2007, 32528–32533. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Livage, J. Hydrothermal Synthesis of Nanostructured Vanadium Oxides. Materials 2010, 3, 4175-4195. https://doi.org/10.3390/ma3084175
Livage J. Hydrothermal Synthesis of Nanostructured Vanadium Oxides. Materials. 2010; 3(8):4175-4195. https://doi.org/10.3390/ma3084175
Chicago/Turabian StyleLivage, Jacques. 2010. "Hydrothermal Synthesis of Nanostructured Vanadium Oxides" Materials 3, no. 8: 4175-4195. https://doi.org/10.3390/ma3084175



