Large-Scale Synthesis of Carbon Nanomaterials by Catalytic Chemical Vapor Deposition: A Review of the Effects of Synthesis Parameters and Magnetic Properties
Abstract
:1. Introduction
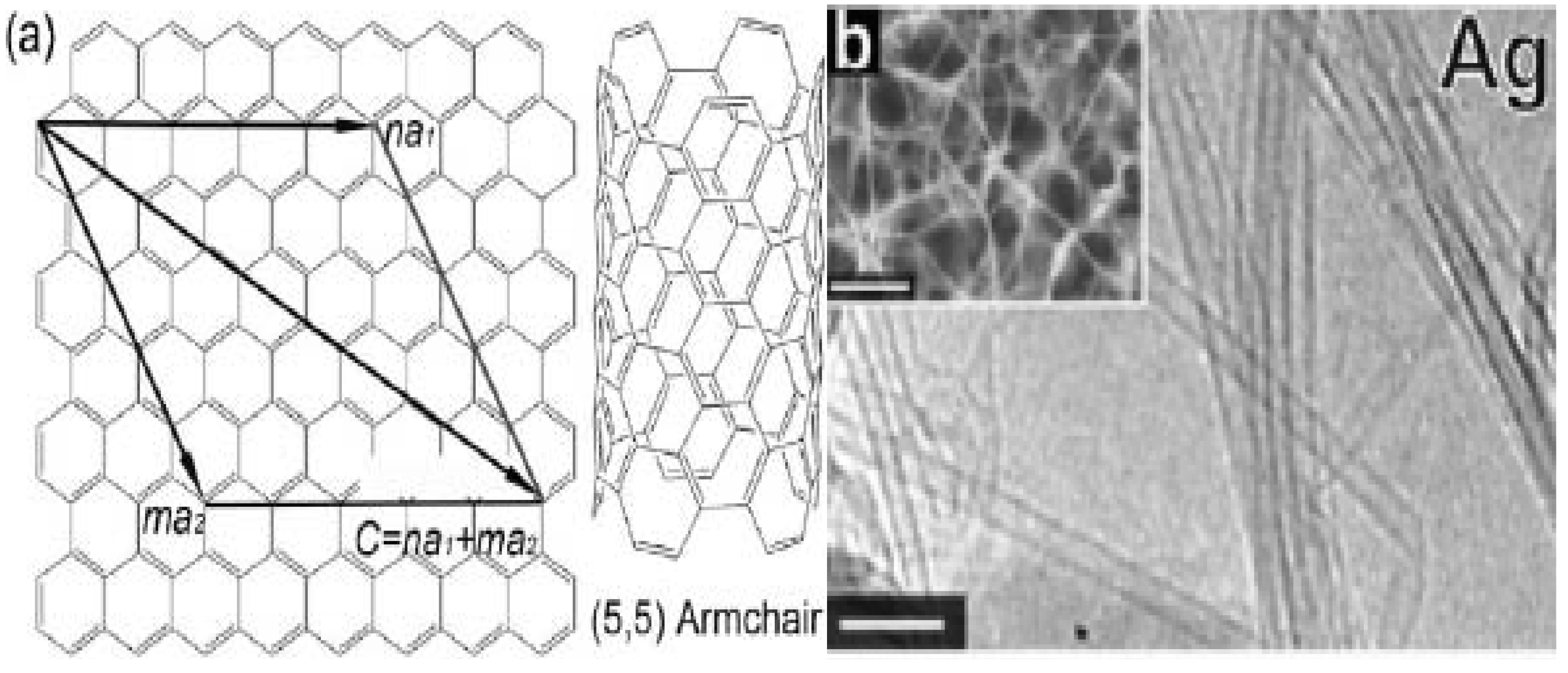
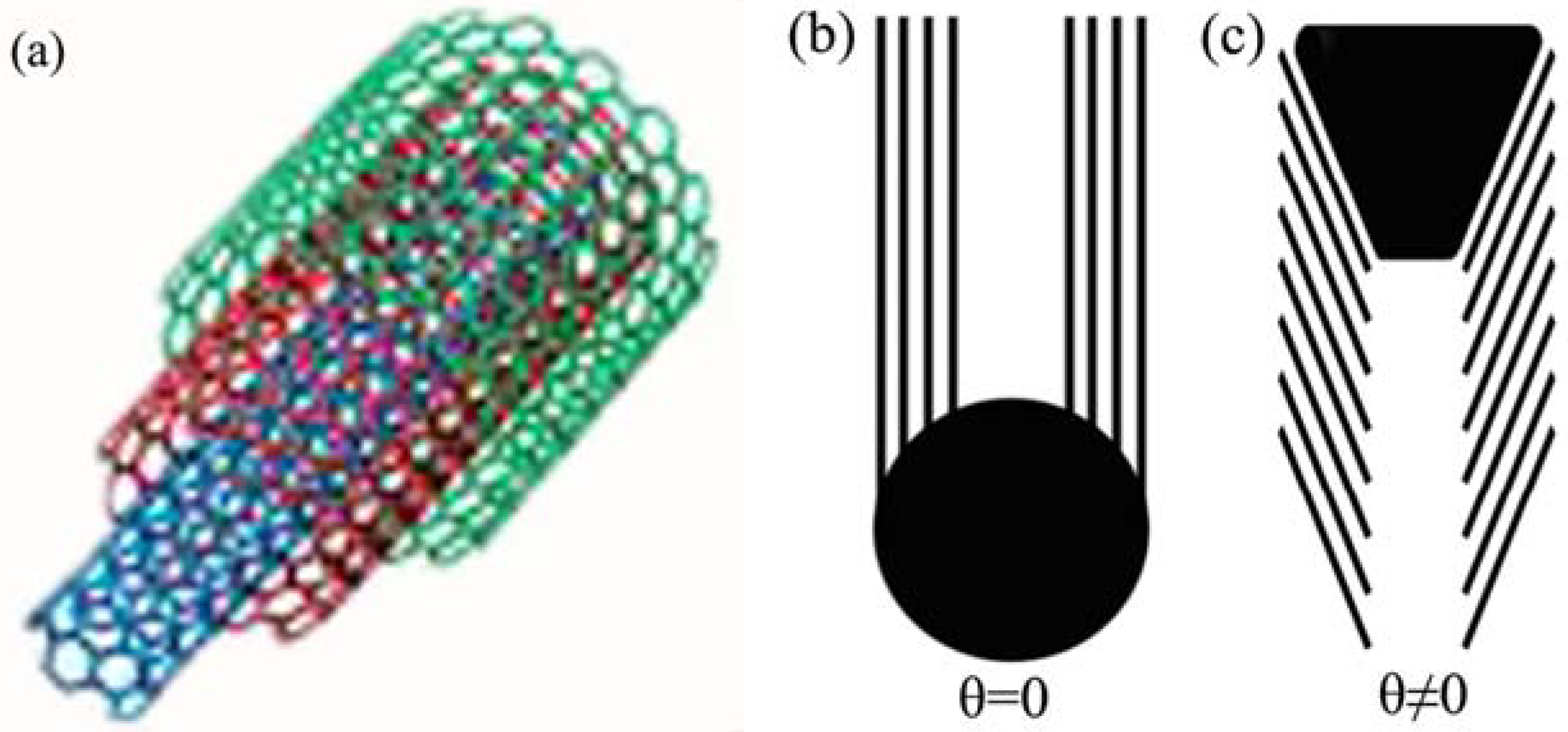

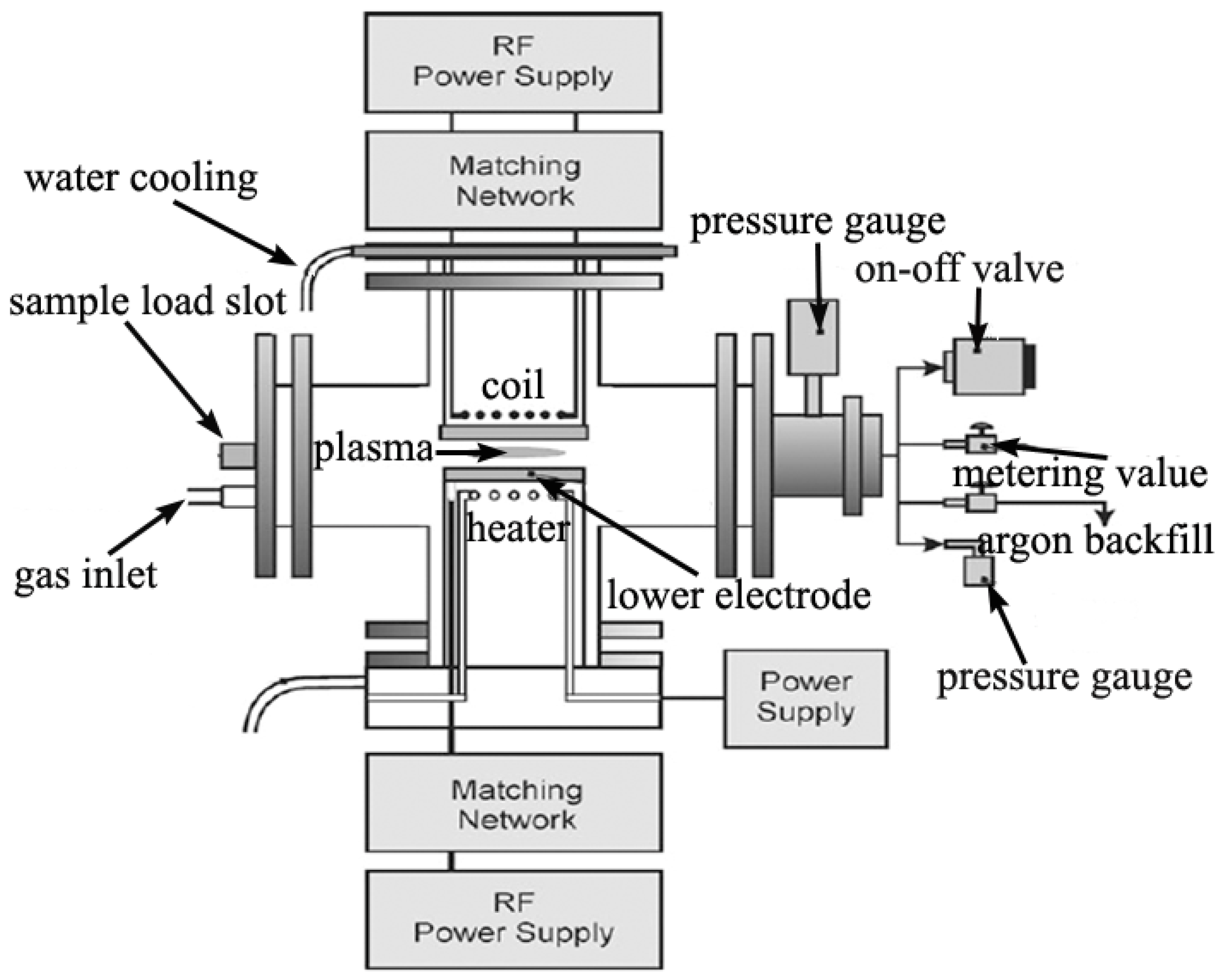
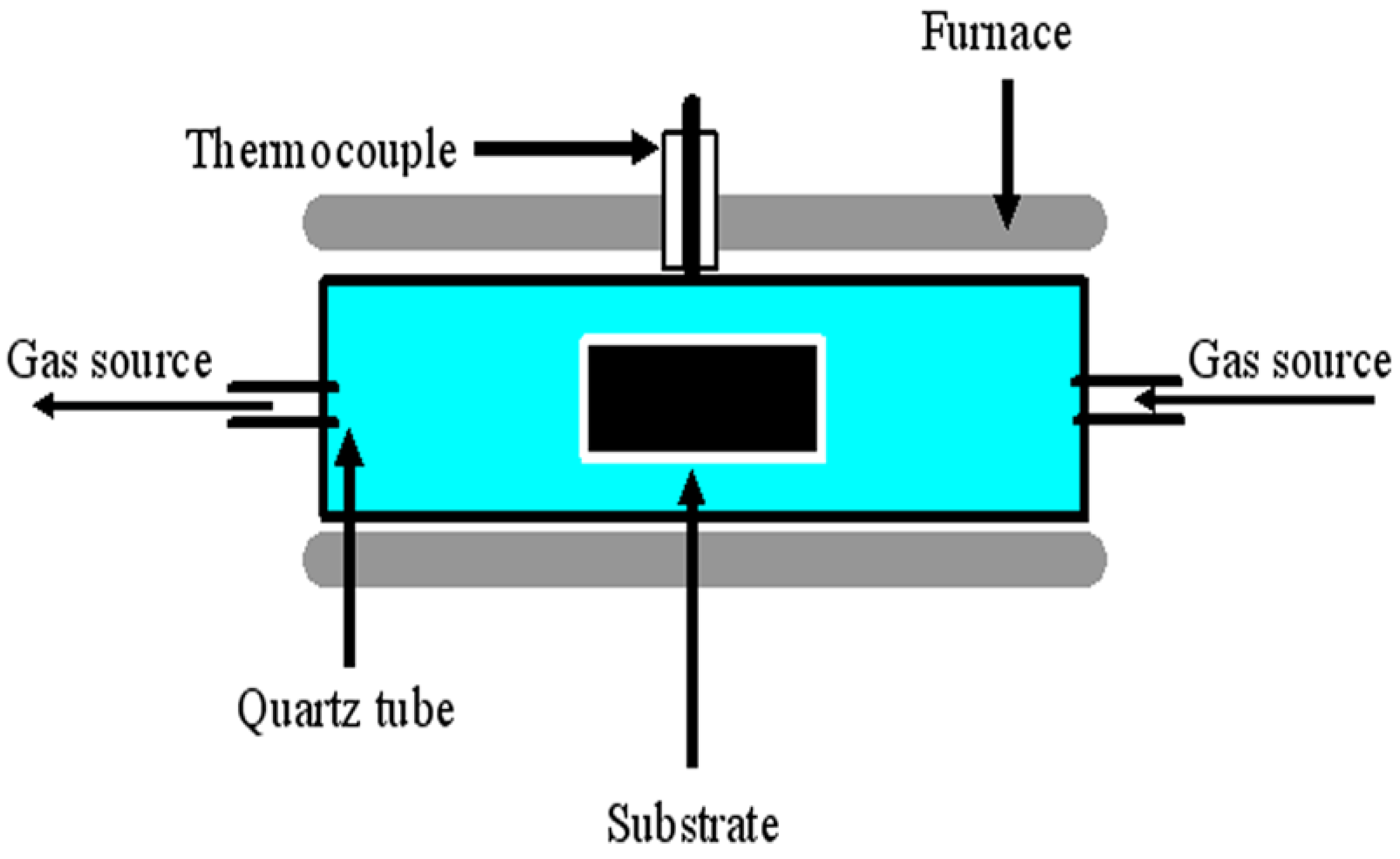
2. Results and Discussion
2.1. Effect of Reactor Setup

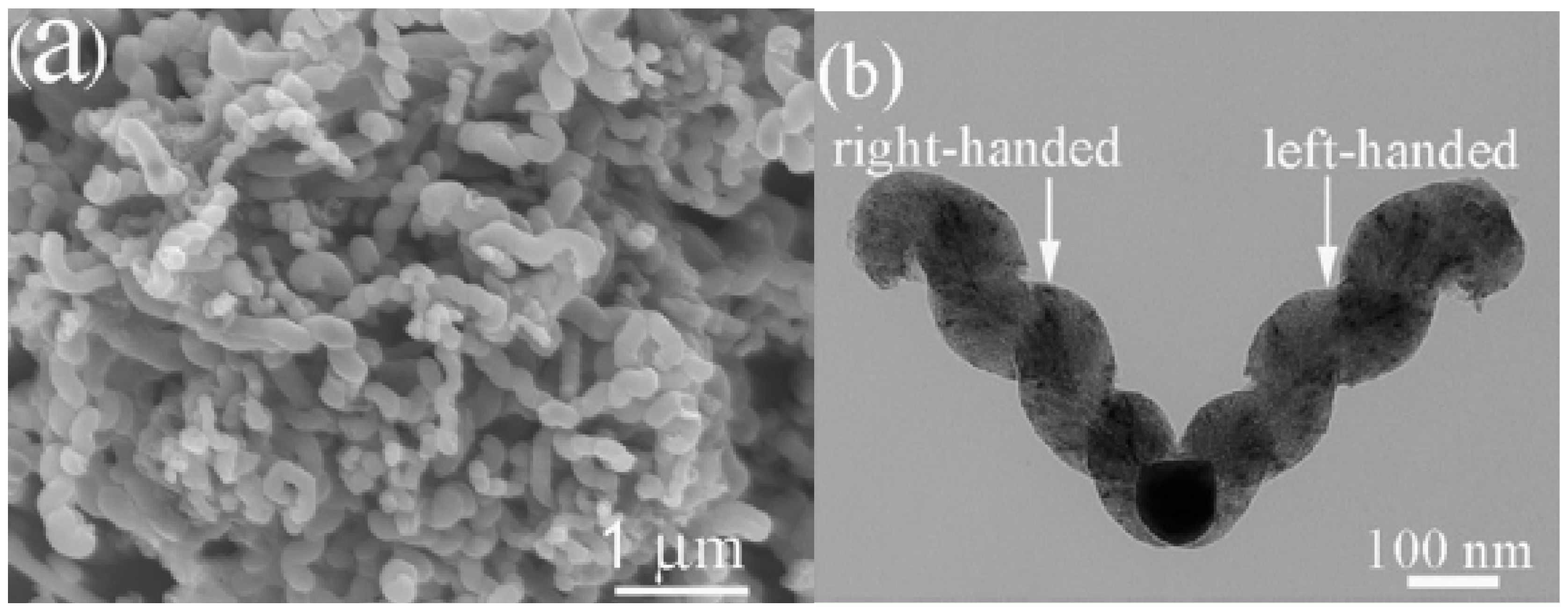
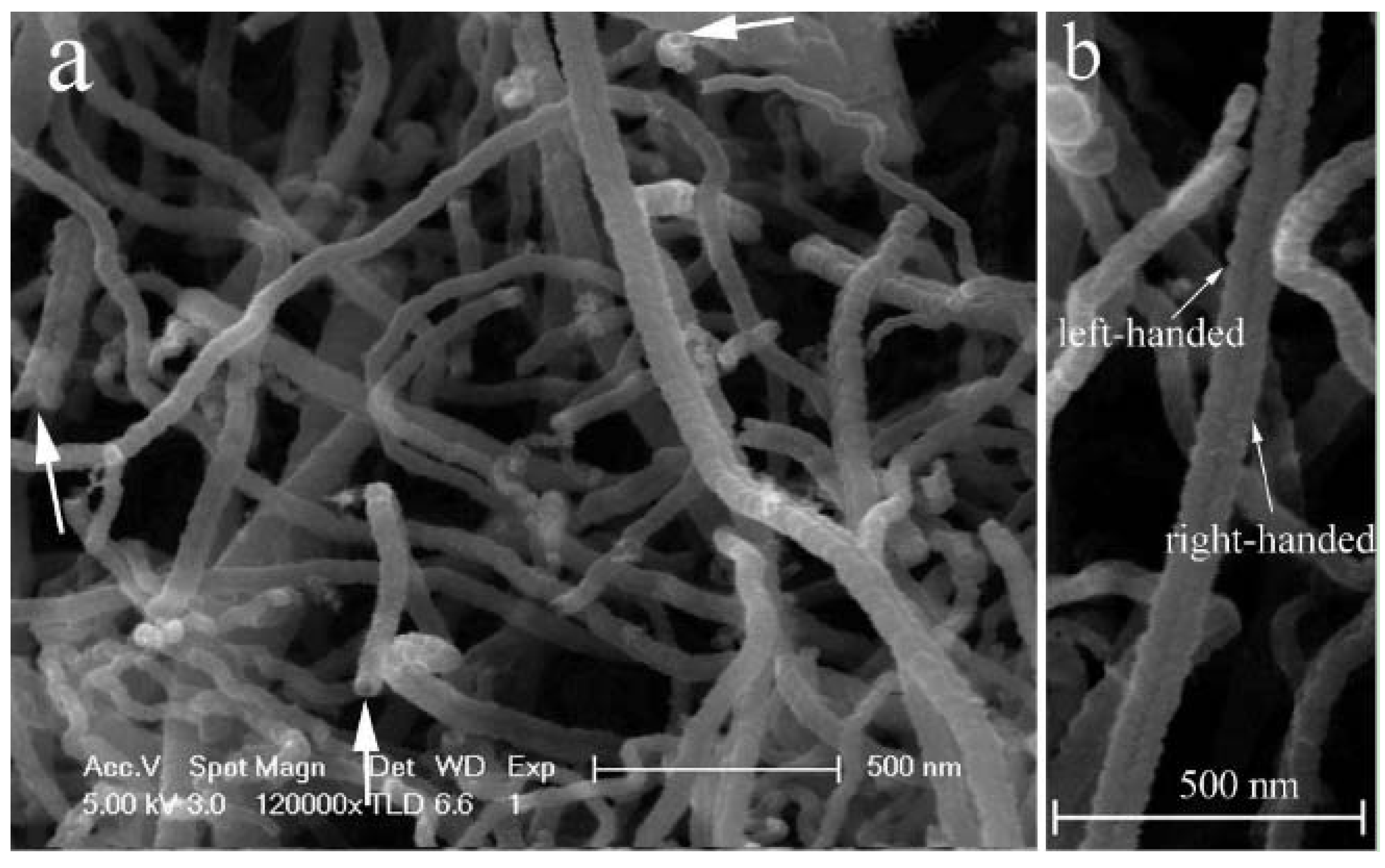
| Reactor setup | Sample | Optical RL value (dB) | (GHz) (optical RL) | Frequency range (RL<−10 and −20 dB) |
|---|---|---|---|---|
| quartz tube | plait-like CNCs | −20 | 15.96 | 9.01–18.0 GHz (−10 dB) |
| quartz tube placed inside a stainless steel tube | T-CNCs | −36.09 | 17.29 | 9.00–18.0 GHz (−10 dB) 9.00–17.2 GHz (−20 dB) |
2.2. Effect of Catalyst Category
| Catalyst | Product | Reference |
|---|---|---|
| Cu | aligned MWCNTs | [92] |
| Au | CNT junctions | [93] |
| Cu-Cr | CNTs | [94] |
| Pd-Cr-Pt | CNTs | [95] |
| Cs2CO3 | CNFs and CNTs | [96] |
| Li2CO3 | HCNFs | [97] |
| K2CO3 | carbon nanofoam | [97] |
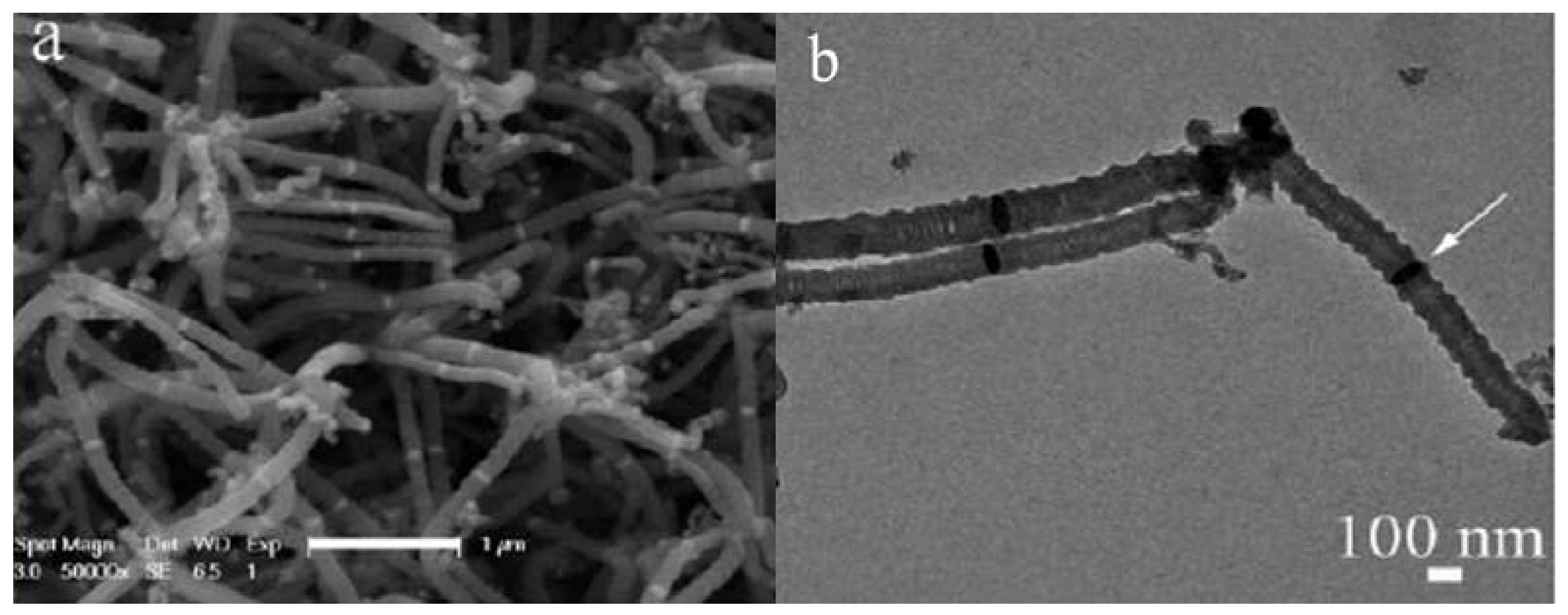
| Reactor | Product yield | Product | Saturation magnetization (MS) | Coercivity (Hc) |
|---|---|---|---|---|
| stainless steel tube | 4,420% | HCNFs | 4.99 emu/g | 91.61 Oe |
| quartz tube | 1,080% | HCNTs | 12.11 emu/g | 240.07 Oe |
2.3. Effect of Catalyst Preparation Procedure

2.4. Effect of Support Materials
2.5. Effect of Catalyst Promoters

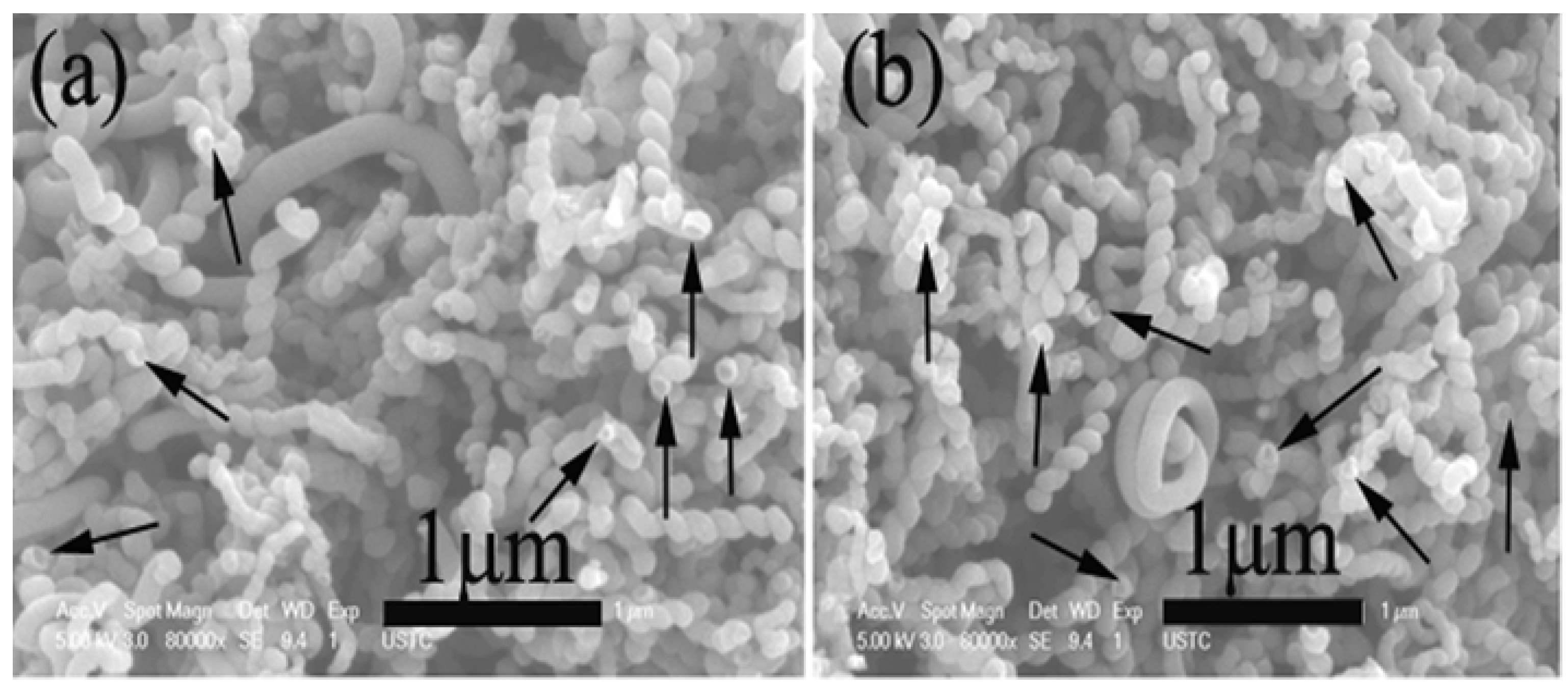
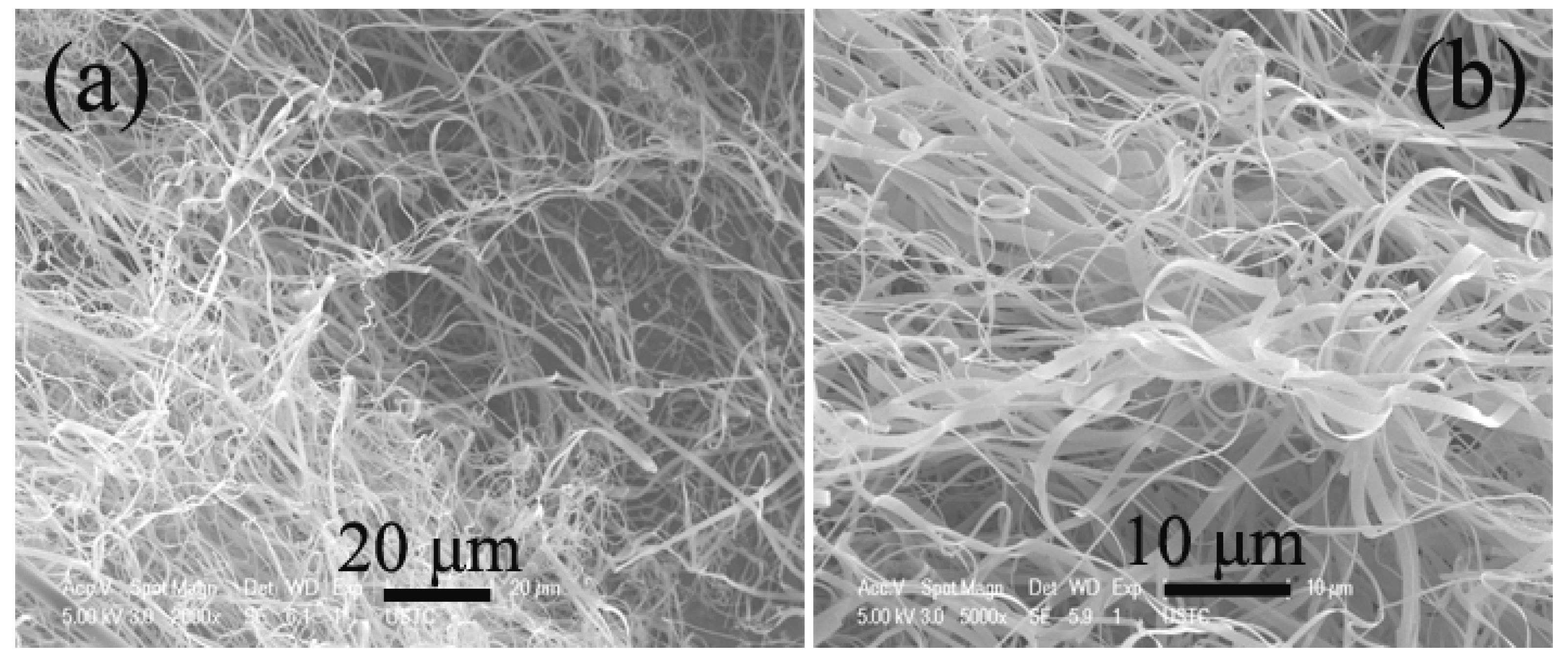
2.6. Effect of Reduction Temperature of Catalyst Precursors and Presence of Hydrogen in Carbon Source
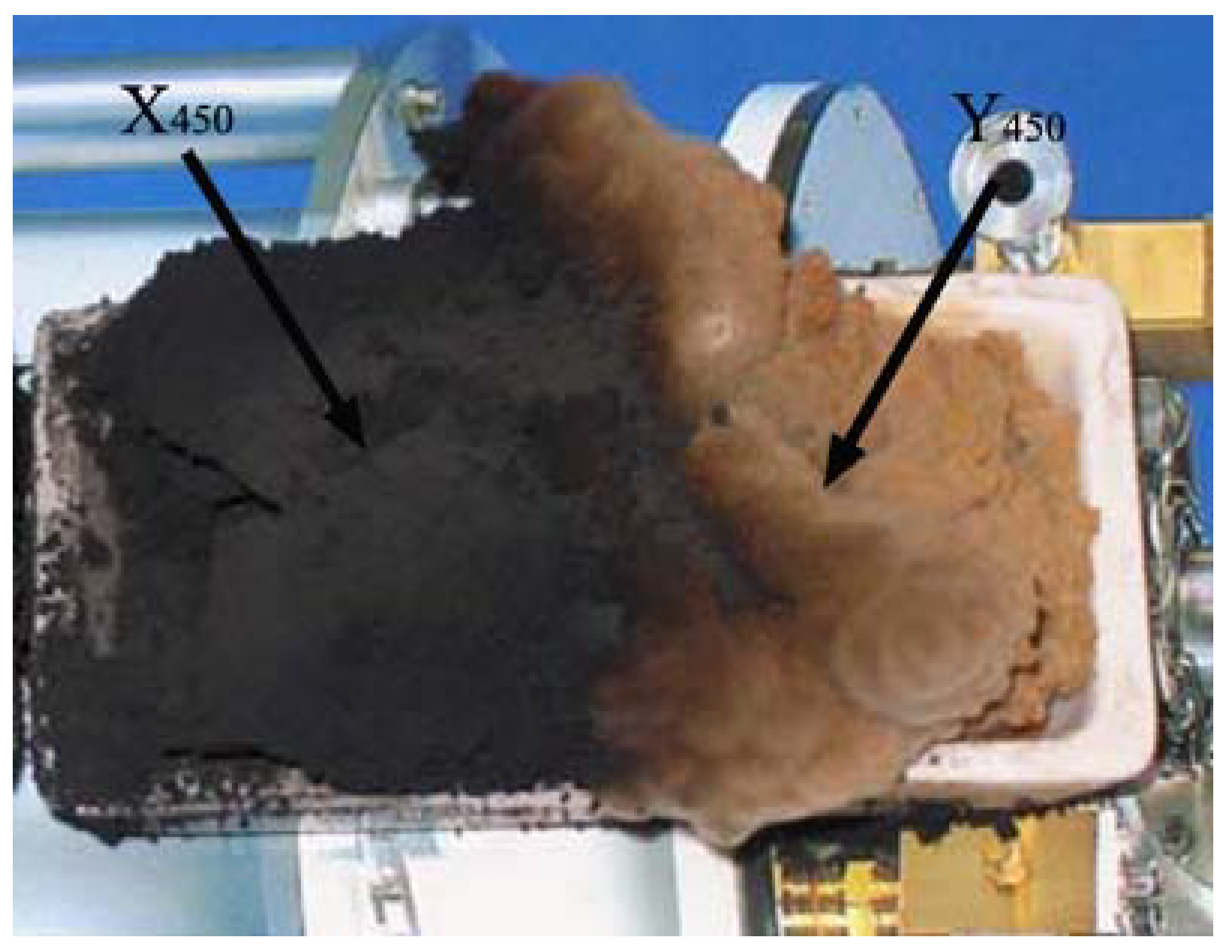

| Sample | Optical RL value (dB) | (mm) (RL<−20 dB) | (GHz) (optical RL) | Frequency range (GHz) (RL<−20 dB) |
|---|---|---|---|---|
| H-HCNTs | −8.25 | – | 11.8 | – |
| L-HCNTs | −25.78 | 2.0–3.0 | 7.18 | 7.18–10.68 |
| worm-like CNTs | −26.39 | 2.0–3.0 | 7.71 | 7.5–10.7 |
2.7. Effect of Reaction Temperature

2.8. Extra Effects
3. Conclusions
Acknowledgements
References and Notes
- Iijima, S. Helical microtubules of graphitic. Carbon 1991, 354, 56–58. [Google Scholar]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.M.; Ghaemi, H.F.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- De Heer, W.A.; Bacsa, W.S.; Chatelain, A.; Gergin, T.; Humphrey-Baker, R.; Forro, L.; Ugarte, D. Aligned carbon nanotube films-production and optical and electronic-properties. Science 1995, 268, 845–847. [Google Scholar]
- Delaney, P.; Choi, H.J.; Ihm, J.; Cohen, M.L. Broken symmetry and pseudogaps in ropes of carbon nanotubes. Nature 1998, 391, 466–468. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.S.; Chapline, M.G.; Franklin, N.R.; Tomber, T.W.; Cassell, A.M.; Dai, H.J. Self-oriented regular arrays of carbon nanotubes and their field emission properties. Science 1999, 283, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Kiang, C.H.; Choi, J.S.; Tran, T.T.; Bacher, A.D. Molecular nanowires of 1 nm diameter from capillary filling of single-walled carbon nanotubes. J. Phy. Chem. B 1999, 103, 7449–7451. [Google Scholar] [CrossRef]
- Tans, S.J.; Verschueren, A.R.M.; Dekker, C. Room-temperature transistor based on a single carbon nanotube. Nature 1998, 393, 49–52. [Google Scholar] [CrossRef]
- Yao, Z.; Postma, H.W.C.; Balents, L.; Dekker, C. Carbon nanotube intramolecular junctions. Nature 1999, 402, 273–276. [Google Scholar] [CrossRef]
- Rueckes, T.; Kim, K.; Joselevich, E.; Tseng, G.Y.; Cheung, C.L.; Lieber, C.M. Carbon nanotube-based nonvolatile random access memory for molecular computing. Science 2000, 289, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.; Joselevich, E.; Wooley, A.T.; Cheung, C.L.; Lieber, C.M. Covalently functionalized nanotubes as nanometre-sized probes in chemistry and biology. Nature 1998, 394, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Hafner, J.H.; Cheung, C.L.; Lieber, C.M. Direct growth of single-walled carbon nanotube scanning probe microscopy tips. J. Am. Chem. Soc. 1999, 121, 9750–9751. [Google Scholar] [CrossRef]
- Dai, H.J. Carbon nanotubes: opportunities and challenges. Surf. Sci. 2002, 500, 218–241. [Google Scholar] [CrossRef]
- Dillon, A.C.; Jones, K.M.; Bekkedahl, K.K.; Kiang, C.H.; Bethune, D.S.; Heben, M.J. Storage of hydrogen in single-walled carbon nanotubes. Nature 1997, 386, 377–379. [Google Scholar] [CrossRef]
- Vigolo, B.; Penicaud, A.; Coulon, C.; Sauder, C.; Pailler, R.; Journet, C.; Bernier, P.; Poulin, P. Macroscopic fibers and ribbons of oriented carbon nanotubes. Science 2000, 290, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Takagi, D.; Homma, Y.; Hibino, H.; Suzuki, S.; Kobayashi, Y. Single-walled carbon nanotube growth from highly activated metal nanoparticles. Nano Lett. 2006, 6, 2642–2645. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Bandaru, P.R. Enhanced optical absorption cross-section characteristics of multi-wall carbon nanotubes. Carbon 2009, 47, 2898–2903. [Google Scholar] [CrossRef]
- Cao, Q.; Rogers, J.A. Ultrathin films of single-walled carbon nanotubes for electronics and sensors: a review of fundamental and applied aspects. Adv. Mater. 2009, 21, 29–53. [Google Scholar] [CrossRef]
- Avouris, P. Molecular electronics with carbon nanotubes. Acc. Chem. Res. 2002, 35, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Huang, J.L.; Lieber, C.M. Fundamental electronic properties and applications of single-walled carbon nanotubes. Acc. Chem. Res. 2002, 35, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.N. Carbon nanotubes: properties and application. Mater. Sci. Eng. R 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Charlier, J.C.; Blase, X.; Roche, S. Electronic and transport of nanotubes. Rev. Mod. Phys. 2007, 79, 677–732. [Google Scholar] [CrossRef]
- Yamamoto, T.; Watanabe, K.; Hernandez, E.R. Mechanical properties, thermal stability and heat transport in carbon nanotubes. Carbon Nanotubes 2008, 111, 165–194. [Google Scholar]
- Tans, S.J.; Devoret, M.H.; Dai, H.J.; Thess, A.; Smalley, R.E.; Geerligs, L.J.; Dekker, C. Individual single-wall carbon nanotubes as quantum wires. Nature 1997, 386, 474–477. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kataura, H.; Shiraishi, M. Ambipolar single electron transistors using side-contacted single-walled carbon nanotubes. Chem. Phys. Lett. 2006, 417, 540–544. [Google Scholar] [CrossRef]
- Babic, B.; Iqbal, M.; Schonenberger, C. Ambipolar field-effect transistor on as-grown single-wall carbon nanotubes. Nanotechnology 2003, 14, 327–331. [Google Scholar] [CrossRef]
- Li, C.Y.; Chou, T.W. Strain and pressure sensing using single-walled carbon nanotubes. Nanotechnology 2004, 15, 1493–1496. [Google Scholar] [CrossRef]
- Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.J.; Petit, P.; Robert., J.; Xu, C.H.; Lee, Y.H.; Kim, S.G.; Rinzler, A.G.; Colbert, D.T.; Scuseria, G.E; Tomanek, D.; Fischer, J.E.; Smalley, R.E. Crystalline ropes of metallic carbon nanotubes. Science 1996, 273, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Journet, C.; Maser, W.K.; Bernier, P.; Loiseau, A.; delaChapelle, M.L.; Lefrant, S.; Deniard, P.; Lee, P.; Fischer, J.E. Large-scale production of single-walled carbon nanotubes by the electric-arc technique. Nature 1997, 388, 756–758. [Google Scholar] [CrossRef]
- Grüneis, A.; Kramberger, C.; Grimm, D.; Gemming, T.; Rümmeli, M.H.; Barreiro, A.; Ayala, P.; Pichler, T.; Schaman, C.; Kuzmany, H.; Schumann, J.; Büchner, B. Eutectic limit for the growth of carbon nanotubes from a thin iron film by chemical vapor deposition of cyclohexane. Chem. Phys. Lett. 2006, 425, 301–305. [Google Scholar] [CrossRef]
- Meyyappan, M.; Delzeit, L.; Cassell, A.; Hash, D. Carbon nanotube growth by PECVD: a review. Plasma Sources Sci. Technol. 2003, 12, 205–216. [Google Scholar] [CrossRef]
- Setton, R.; Setton, N. Carbon nanotubes: III. Toroidal structures and limits of a model for the construction of helical and S-shaped nanotubes. Carbon 1997, 35, 497–505. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Du, J.H.; Cheng, H.M. Preparation of carbon microcoils by catalytic decomposition of acetylene using nickel foam as both catalyst and substrate. Carbon 2005, 43, 1874–1878. [Google Scholar] [CrossRef]
- Fonseca, A.; Hernadi, K.; Nagy, J.B.; Lambin, Ph.; Lucas, A.A. Model structure of perfectly graphitizable coiled nanotubes. Carbon 1995, 33, 1759–1775. [Google Scholar] [CrossRef]
- Qi, X.S.; Zhong, W.; Deng, Y.; Au, C.T.; Du, Y.W. Synthesis of helical carbon nanotubes, worm-like carbon nanotubes and nanocoils at 450 °C and their magnetic properties. Carbon 2010, 48, 365–376. [Google Scholar] [CrossRef]
- Chen, X.Q.; Zhang, S.L.; Dikin, D.A.; Ding, W.Q.; Ruoff, R.S.; Pan, L.J. Mechanics of a carbon nanocoil. Nano Lett. 2003, 3, 1299–304. [Google Scholar] [CrossRef]
- Chen, X.Q.; Motojima, S.; Iwanaga, H. Carbon coatings on carbon micro-coils by pyrolysis of methane and their properties. Carbon 1999, 37, 1825–1831. [Google Scholar] [CrossRef]
- Yan, S.M.; Chen, X.Q.; Motojima, S. Tactile sensing properties of protein-like single-helix carbon microcoils. Carbon 2006, 44, 3352–3355. [Google Scholar] [CrossRef]
- Akagi, K.; Tamura, R.; Tsukada, M.; Itoh, S.; Ihara, S. Electronic-structure of helically coiled cage of graphitic carbon. Phys. Rev. Lett. 1995, 74, 2307–2310. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.; Nagy, J.B.; Lambin, Ph.; Lucas, A.; Zhang, X.B.; Zhang, X.F.; Bernaerts, D.; Van Tendeloo, G.; Amelinckx, S.; Landuyt, J.V. The study of carbon nanotubules produced by catalytic method. Chem. Phys. Lett. 1994, 223, 329–335. [Google Scholar] [CrossRef]
- Hernadi, K.; Thien-Nga, L.; Forro, L. Growth and microstructure of catalytically produced coiled carbon nanotubes. J. Phys. Chem. B 2001, 105, 12464–12468. [Google Scholar] [CrossRef]
- Zhang, X.B.; Zhang, X.F.; Bernaerts, D.; Tendeloo, G.V.; Amelinckx, S.; Landuyt, J.V.; Ivanov, V.; Nagy, J.B.; Lambin, Ph.; Lucas, A. The texture of catalytically grown coil-shaped carbon nanotubules. Europhys. Lett. 1994, 27, 141–146. [Google Scholar] [CrossRef]
- Amelinckx, S.; Zhang, X.B.; Bernaerts, D.; Zhang, X.F.; Ivanov, V.; Nagy, J.B. A formation mechanism for catalytically grown helix-shaped graphite nanotubes. Science 1994, 265, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.K.; Shen, Z.M. Synthesis of regular coiled carbon nanotubes by Ni-catalyzed pyrolysis of acetylene and a growth mechanism analysis. Carbon 2001, 39, 2369–2374. [Google Scholar] [CrossRef]
- Bajpai, V.; Dai, L.M.; Ohashi, T. Large-scale synthesis of perpendicularly aligned helical carbon nanotubes. J. Am. Chem. Soc. 2004, 126, 5070–5071. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Liu, W.M.; Guo, X.Y.; Li, H.L. Coiled carbon nanotubes growth via reduced-pressure catalytic chemical vapor deposition. Carbon 2004, 42, 805–811. [Google Scholar] [CrossRef]
- Hou, H.Q.; Jun, Z.; Weller, F.; Greiner, A. Large-scale synthesis and characterization of helically coiled carbon nanotubes by use of Fe(CO)5 as floating catalyst precursor. Chem. Mater. 2003, 15, 3170–3175. [Google Scholar] [CrossRef]
- Bai, J.B. Growth of nanotubes/nanofibers coils by CVD on an alumina substrate. Mater. Lett. 2003, 57, 2629–2633. [Google Scholar] [CrossRef]
- Xie, J.N.; Mukhopadyay, K.; Yadev, J.; Varadan, V.K. Catalytic chemical vapor deposition synthesis and electron microscopy observation of coiled carbon nanotubes. Smart. Mater. Struct. 2003, 12, 744–748. [Google Scholar] [CrossRef]
- Zhong, D.Y.; Liu, S.; Wang, E.G. Patterned growth of coiled carbon nanotubes by a template-assisted technique. Appl. Phys. Lett. 2003, 83, 4423–4425. [Google Scholar] [CrossRef]
- Gao, R.P.; Wang, Z.L.; Fan, S.S. Kinetically controlled growth of helical and zigzag shapes of carbon nanotubes. J. Phys. Chem. B 2000, 104, 1227–1234. [Google Scholar] [CrossRef]
- Luo, T.; Liu, J.W.; Chen, L.Y.; Zeng, S.Y.; Qian, Y.T. Synthesis of helically coiled carbon nanotubes by reducing ethyl ether with metallic zinc. Carbon 2005, 43, 755–759. [Google Scholar] [CrossRef]
- Motojima, S.; Kawaguchi, M.; Nozaki, K.; Iwanaga, H. Growth of regularly coiled carbon filaments by Ni catalyzed pyrolysis of acetylene, and their morphology and extension characteristic. Appl. Phys. Lett. 1990, 56, 321–323. [Google Scholar] [CrossRef]
- Yang, S.M.; Hasegawa, M.; Chen, X.Q.; Motojima, S. Synthesis and morphology of carbon microcoils produced using methane as a carbon source. Carbon 2007, 45, 1592–1595. [Google Scholar] [CrossRef]
- Pan, L.J.; Zhang, M.; Nakayama, Y. Growth mechanism of carbon nanocoils. J. Appl. Phys. 2002, 91, 10058–10061. [Google Scholar] [CrossRef]
- Duesberg, G.S.; Graham, A.P.; Kreupl, F.; Liebau, M.; Seidel, R.; Unger, E.; Hoenlein, W. Ways towards the scaleable integration of carbon nanotubes into silicon based technology. Diamond Relat. Mater. 2004, 13, 354–361. [Google Scholar] [CrossRef]
- Korneva, G.; Ye, H. H.; Gogotsi, Y.; Halverson, D.; Friedman, G.; Bradley, J. C.; Kornev, K. G. C. 2005, 5, 879.
- Tang, N.J.; Zhong, W.; Au, C.T.; Gedanken, A.; Yang, Y.; Du, Y.W. Large-scale synthesis, annealing, purification, and magnetic properties of crystalline helical carbon nanotubes with symmetrical structures. Adv. Funct. Mater. 2007, 17, 1542–1550. [Google Scholar] [CrossRef]
- Karwa, M.; Iqbal, Z.; Mitra, S. Scaled-up self-assembly of carbon nanotubes inside long stainless steel tubing. Carbon 2006, 44, 1235–1242. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, Y.; Yang, S.L.; Li, Y.S.; Hirose, A. Simultaneous growth of diamond thin films and carbon nanotubes at temperatures < = 550 °C. Carbon 2008, 46, 589–595. [Google Scholar] [CrossRef]
- Tang, N.J.; Zhong, W.; Gedanken, A.; Du, Y.W. High magnetization helical carbon nanofibers produced by nanoparticle catalysis. J. Phys. Chem. B 2006, 110, 11772–11774. [Google Scholar] [CrossRef]
- Tang, N.J.; Yang, Y.; Lin, K.J.; Zhong, W.; Au, C.T.; Du, Y.W. Synthesis of plait-like carbon nanocoils in ultrahigh yield, and their microwave absorption properties. J. Phys. Chem. C 2008, 112, 10061–10067. [Google Scholar] [CrossRef]
- Tang, N.J.; Zhong, W.; Au, C.T.; Yang, Y.; Han, M.G.; Lin, K.J.; Du, Y.W. Synthesis, microwave electromagnetic, and microwave absorption properties of twin carbon nanocoils. J. Phys. Chem. C 2008, 112, 19316–19323. [Google Scholar] [CrossRef]
- Arya, V.P.; Prasad, V.; Kumar, P.S.A. Magnetic properties of iron particles embedded in multiwall carbon nanotubes. J. Nanosci. Nanotechno. 2009, 9, 5406–5410. [Google Scholar] [CrossRef]
- Aguilo-Aguayo, N.; Castano-Bernal, J.L.; Garcia-Cespedes, J.; Bertran, E. Magnetic response of CVD and PECVD iron filled multi-walled carbon nanotubes. Diamond Relat. Mater. 2009, 18, 953–956. [Google Scholar] [CrossRef]
- Misra, A.; Daraio, C. Sharp carbon-nanotube tips and carbon-nanotube soldering irons. Adv. Mater. 2009, 21, 2305–2308. [Google Scholar] [CrossRef]
- Radhakrishnan, J.K.; Pandian, P.S.; Padaki, V.C.; Bhusan, H.; Rao, K.U.B.; Xie, J.; Abraham, J.K.; Varadan, V.K. Growth of multiwalled carbon nanotube arrays by chemical vapor deposition over iron catalyst and the effect of growth parameters. Appl. Surf. Sci. 2009, 255, 6325–6334. [Google Scholar] [CrossRef]
- Liu, Q.F.; Ren, W.C.; Chen, Z.G.; Liu, B.L.; Yu, B.; Li, F.; Cong, H.T.; Cheng, H.M. Direct synthesis of carbon nanotubes decorated with size-controllable Fe nanoparticles encapsulated by graphitic layers. Carbon 2008, 46, 1417–1423. [Google Scholar] [CrossRef]
- Yuan, D.N.; Ding, L.; Chu, H.B.; Feng, Y.Y.; McNicholas, T.P.; Liu, J. Horizontally aligned single-walled carbon nanotube on quartz from a large variety of metal catalysts. Nano Lett. 2008, 8, 2576–2579. [Google Scholar] [CrossRef] [PubMed]
- Kakehi, K.; Noda, S.; Maruyama, S.; Yamaguchi, Y. Individuals, grasses, and forests of single- and multi-walled carbon nanotubes grown by supported Co catalysts of different nominal thicknesses. Appl. Surf. Sci. 2008, 254, 6710–6714. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, B.; Huang, L.; He, L.; Ni, X.M. Novel composite of Co/carbon nanotubes: Synthesis, magnetism and microwave absorption properties. Solid. State. Sci. 2008, 3, 316–320. [Google Scholar] [CrossRef]
- Kakehi, K.; Noda, S.; Maruyama, S.; Yamaguchi, Y. Growth valley dividing single- and multi-walled carbon nanotubes: Combinatorial study of nominal thickness of Co catalyst. Jpn. J. Appl. Phys. 2008, 47, 1961–1965. [Google Scholar] [CrossRef]
- Khavrus, V.O.; Lemesh, N.V.; Gordijchuk, S.V.; Tripolsky, A.I.; Ivashchenko, T.S.; Biliy, M.M.; Strizhak, P.E. Chemical catalytic vapor deposition (CCVD) synthesis of carbon nanotubes by decomposition of ethylene on metal (Ni, Co, Fe) nanoparticles. React. Kinet. Catal. L. 2008, 93, 295–303. [Google Scholar] [CrossRef]
- Wei, S.; Kang, W.P.; Davidson, J.L.; Huang, J.H. Aligned carbon nanotubes fabricated by thermal CVD at atmospheric pressure using Co as catalyst with NH3 as reactive gas. Diamond Relat. Mater. 2006, 15, 1828–1833. [Google Scholar] [CrossRef]
- Kozhuharova, R.; Ritschel, M.; Elefant, D.; Graff, A.; Leonhardt, A.; Monch, I.; Muhl, T.; Groudeva-Zotova, S.; Schneider, C.M. Well-aligned Co-filled carbon nanotubes: preparation and magnetic properties. Appl. Surf. Sci. 2004, 238, 355–359. [Google Scholar] [CrossRef]
- Zaretskiy, S.N.; H6ong, Y.K.; Ha, D.H.; Yoon, J.H.; Cheon, J.; Koo, J.Y. Growth of carbon nanotubes from Co nanoparticles and C2H2 by thermal chemical vapor deposition. Chem. Phys. Lett. 2003, 372, 300–305. [Google Scholar] [CrossRef]
- Kar, K.K.; Rahaman, A.; Agnihotri, P.; Sathiyamoorthy, D. Synthesis of carbon nanotubes on the surface of carbon fiber/fabric by catalytic chemical vapor deposition and their characterization. Fuller Nanotub. Car. N. 2009, 17, 209–229. [Google Scholar] [CrossRef]
- Feng, H.; Ma, J.; Hu, Z. Six-membered-ring-based radical mechanism for catalytic growth of carbon nanotubes with benzene precursor. J. Phys. Chem. C 2009, 113, 6495–16502. [Google Scholar] [CrossRef]
- Khavrus, V.O.; Lemesh, N.V.; Gordijchuk, S.V.; Tripolsky, A.I.; Ivashchenko, T.S.; Biliy, M.M.; Strizhak, P.E. Chemical catalytic vapor deposition (CCVD) synthesis of carbon nanotubes by decomposition of ethylene on metal (Ni, Co, Fe) nanoparticles. React. Kinet. Catal. L. 2008, 93, 295–303. [Google Scholar] [CrossRef]
- Guan, L.H.; Shi, Z.J.; Li, H.J.; You, L.P.; Gu, Z.N. Super-long continuous Ni nanowires encapsulated in carbon nanotubes. Chem. Commun. 2004, 17, 1988–1989. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, T.Y.; Choi, S.H.; Han, J.H.; Yoo, J.B.; Park, C.Y.; Jung, T.; Yu, S.G.; Yi, W.; Han, I.T.; Kim, J.M. Control of carbon nanotubes density through Ni nanoparticle formation using thermal and NH3 plasma treatment. Diamond Relat. Mater. 2003, 12, 794–798. [Google Scholar] [CrossRef]
- Gozzi, D.; Latini, A.; Capannelli, G.; Canepa, F.; Napoletano, M.; Cimberle, M.R.; Tropeano, M. Synthesis and magnetic characterization of Ni nanoparticles and Ni nanoparticles in multiwalled carbon nanotubes. J. Alloy. Comp. 2006, 419, 32–39. [Google Scholar] [CrossRef]
- Tsuchiya, N.; Ogino, T. Morphology of carbon nanostructures in alcohol chemical vapor deposition. Jpn. J. Appl. Phys. 2007, 46, 6091–6094. [Google Scholar] [CrossRef]
- Lv, R.T.; Kang, F.Y.; Wang, W.X.; Wei, J.Q.; Gu, J.L.; Wang, K.L.; Wu, D.H. Effect of using chlorine-containing precursors in the synthesis of FeNi-filled carbon nanotubes. Carbon 2007, 45, 1433–1438. [Google Scholar] [CrossRef]
- Jourdain, V.; Paillet, M.; Almairac, R.; Loiseau, A.; Bernier, P. Relevant synthesis parameters for the sequential catalytic growth of carbon nanotubes. J. Phys. Chem. B 2005, 109, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.T.; Kang, F.Y.; Gu, J.L.; Gui, X.C.; Wei, J.Q.; Wang, K.L.; Wu, D.H. Carbon nanotubes filled with ferromagnetic alloy nanowires: Lightweight and wide-band microwave absorber. Appl. Phys. Lett. 2008, 93, 223105:1–223105:3. [Google Scholar] [CrossRef]
- Buang, N.A.; Abd Majid, Z.; Sulaiman, Y.; Sanip, S.M.; Ismail, A.F. Effect of addition of Ni metal catalyst onto the Co and Fe supported catalysts for the formation of carbon nanotubes. J. Porous Mat. 2006, 13, 331–334. [Google Scholar] [CrossRef] [Green Version]
- Lv, R.T.; Kang, F.Y.; Cai, D.Y.; Wang, C.; Gu, J.L.; Wang, K.L.; Wu, D.H. Long continuous FeNi nanowires inside carbon nanotubes: Synthesis, property and application. J. Phys. Chem. Solids 2008, 69, 1213–1217. [Google Scholar] [CrossRef]
- Esconjauregui, S.; Whelan, C.M.; Maex, K. The reasons why metals catalyze the nucleation and growth of carbon nanotubes and other carbon nanomorphologies. Carbon 2009, 47, 659–669. [Google Scholar] [CrossRef]
- Seidel, R.; Duesberg, G.S.; Unger, E.; Graham, A.P.; Liebau, M.; Kreupl, F. Chemical vapor deposition growth of single-walled carbon nanotubes at 600 °C and a simple growth model. J. Phys. Chem. B 2004, 108, 1888–1893. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, J.; Pan, C.; Tuggle, D.W. Effects of catalysts on the internal structures of carbon nanotubes and corresponding electron field-emission properties. Appl. Phys. A 2004, 78, 9–14. [Google Scholar]
- Luo, G.H.; Li, Z.F.; Wei, F.; Xiang, L.; Deng, X.Y.; Jin, Y. Catalysts effect on morphology of carbon nanotubes prepared by catalytic chemical vapor deposition in a nano-agglomerate bed. Phys. B 2002, 323, 314–317. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Shakerzadeh, M.; Tay, B.K.; Li, X.C.; Tan, C.W.; Lin, L.F.; Guo, P.S.; Feng, T.; Sun, Z. Fabrication of aligned carbon nanotubes on Cu catalyst by dc plasma-enhanced catalytic decomposition. Appl. Surf. Sci. 2009, 255, 6404–6407. [Google Scholar] [CrossRef]
- Luo, C.X.; Liu, L.; Jiang, K.L.; Zhang, L.; Li, Q.Q.; Fan, S.S. Growth mechanism of Y-Junctions and related carbon nanotube junctions synthesized by Au-catalyzed chemical vapor deposition. Carbon 2008, 46, 440–444. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Chua, D.H.C.; Gao, Y.; Zhang, Y.P.; Tang, Z.; Tay, B.K.; Feng, T.; Sun, Z.; Chen, Y.W. Field-emission properties of carbon nanotubes grown using Cu-Cr catalysts. J. Vac. Sci. Technol. B 2009, 27, 41–46. [Google Scholar] [CrossRef]
- Lee, C.J.; Park, J.; Kim, J.M.; Huh, Y.; Lee, J.Y.; No, K.S. Low-temperature growth of carbon nanotubes by thermal chemical vapor deposition using Pd, Cr, and Pt as co-catalyst. Chem. Phys. Lett. 2000, 327, 277–283. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Qin, Y.H.; Sun, X.; Zhang, J.; Jing, C.B. Synthesis of carbon nanotube using cesium carbonate catalyst by chemical vapor deposition. Mater. Lett. 2008, 62, 3776–3778. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Sun, X. Synthesis of carbon nanofibers and foam by catalytic chemical vapor deposition using a water-soluble alkali salt catalyst. Adv. Mater. 2007, 19, 961–964. [Google Scholar] [CrossRef]
- Klinke, C.; Bonard, J.M.; Kern, K. Comparative study of the catalytic growth of patterned carbon nanotube films. Surf. Sci. 2001, 492, 195–201. [Google Scholar] [CrossRef]
- Lee, C.J.; Park, J.; Yu, J.A. Catalyst effect on carbon nanotubes synthesized by thermal chemical vapor deposition. Chem. Phys. Lett. 2002, 360, 250–255. [Google Scholar] [CrossRef]
- Chen, X.C.; Wang, H.; He, J.H. Synthesis of carbon nanotubes and nanospheres with controlled morphology using different catalyst precursors. Nanotechnology 2008, 19, 325607/1–6. [Google Scholar]
- Zou, G.F.; Lu, J.; Wang, D.B.; Xu, L.Q.; Qian, Y.T. High-yield carbon nanorods obtained by a catalytic copyrolysis process. Inorg. Chem. 2004, 43, 5432–5435. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Chen, X.Q.; Motojima, S.; Iwanaga, H. The phenomenon of changing coiling-chirality in carbon nanocoils obtained by catalytic pyrolysis of acetylene with various catalysts. J. Nanosci. Nanotechno. 2004, 4, 167–175. [Google Scholar] [CrossRef]
- Calderon-Moreno, J.M.; Labarta, A.; Batlle, X.; Pradell, T.; Crespo, D.; Binh, V.T. Magnetic properties of dense carbon nanospheres prepared by chemical vapor deposition. Chem. Phys. Lett. 2007, 447, 295–299. [Google Scholar] [CrossRef]
- Xi, G.C.; Zhang, M.; Ma, D.; Zhu, Y.C.; Zhang, H.B.; Qian, Y.T. Controlled synthesis of carbon nanocables and branched-nanobelts. Carbon 2006, 44, 734–741. [Google Scholar] [CrossRef]
- Motojima, S.; Chen, X.; Yang, S.; Hasegawa, M. Properties and potential applications of carbon microcoils/nanocoils. Diamond Relat. Mater. 2004, 13, 1989–1992. [Google Scholar] [CrossRef]
- Yang, S.; Chen, X.; Motojima, S. Morphology of the growth tip of carbon microcoils/nanocoils. Diamond Relat. Mater. 2004, 13, 2152–2155. [Google Scholar] [CrossRef]
- Zhao, D.L.; Shen, Z.M. Preparation and microwave absorption properties of carbon nanocoils. Mater. Lett. 2008, 62, 3704–3706. [Google Scholar] [CrossRef]
- Kuzuya, C.; In-Hwang, W.; Hirako, S.; Hishikawa, Y.; Motojima, S. Preparation, morphology, and growth. mechanism of carbon nanocoils. Chem. Vapor. Depos. 2002, 8, 57–62. [Google Scholar] [CrossRef]
- Chen, X.Q.; Hasegawa, M.; Yang, S.M.; Nitta, Y.; Katsuno, T.; Motojima, S. Preparation of carbon microcoils by catalytic methane hot-wire CVD process. Thin Solid Films 2008, 516, 714–717. [Google Scholar] [CrossRef]
- Su, M.; Zheng, B.; Liu, J. A scalable CVD method for the synthesis of single-walled carbon nanotubes with high catalyst producitivity. Chem. Phys. Lett. 2000, 322, 321–326. [Google Scholar] [CrossRef]
- Kong, J.; Cassell, A.; Dai, H. Chemical vapor deposition of methane for single-walled carbon nanotubes. Chem. Phys. Lett. 1998, 292, 567–574. [Google Scholar] [CrossRef]
- Planeix, J.M.; Coustel, N.; Coq, B.; Brotons, V.; Kumbhar, P.S.; Dutartre, R.; Geneste, P.; Bernier, P.; Ajayan, P.M. Application of carbon nanotubes as supports in heterogeneous catalysis. J. Am. Chem. Soc. 1994, 116, 7935–7936. [Google Scholar] [CrossRef]
- Gao, R.; Tan, C.D.; Baker, R.T.K. Ethylene hydroformylation on graphite nanofiber supported rhodium catalysts. Catal. Today 2001, 65, 19–29. [Google Scholar] [CrossRef]
- Vieira, R.; Pham-Huu, C.; Keller, N.; Ledoux, M.J. New carbon nanofiber/graphite felt composite for use as a catalyst support for hydrazine catalytic decomposition. Chem. Commun. 2002, 9, 954–955. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, H.B.; Lin, G.D.; Hong, Q.; Tsai, K.R. Growth of carbon nanotubes by catalytic decomposition of CH4 or CO on a Ni–MgO catalyst. Carbon 1997, 35, 1495–1501. [Google Scholar] [CrossRef]
- Bacsa, R.R.; Laurent, C.; Peigney, A.; Bacsa, W.S.; Vaugien, T.; Rousset, A. High specific surface area carbon nanotubes from catalytic chemical vapor deposition process. Chem. Phys. Lett. 2000, 323, 566–571. [Google Scholar] [CrossRef]
- Pinheiro, J.P.; Schouler, M.C.; Gadelle, P. Nanotubes and nanofilaments from carbon monoxide disproportionation over Co/MgO catalysts I. Growth versus catalyst state. Carbon 2003, 41, 2949–2959. [Google Scholar] [CrossRef]
- Ivanov, V.; Nagy, J.B.; Lambin, P.; Lucas, A.; Zhang, X.F.; Bernaerts, D.; Vantendelod, G.; Amelinckx, S.; Vanlanduyt, J. The study of carbon nanotubules produced by catalytic method. Chem. Phys. Lett. 1994, 223, 329–335. [Google Scholar] [CrossRef]
- Hernadi, K.; Fonseca, A.; Nagy, J.B.; Bernaerts, D.; Fudala, A.; Lucas, A.A. Catalytic synthesis of carbon nanotubes using zeolite support. Zeolites 1996, 17, 416–423. [Google Scholar] [CrossRef]
- Ros, T.G.; Keller, D.E.; van Dillen, A.J.; Geus, J.W.; Koningsberger, D.C. Preparation and activity of small rhodium metal particles on fishbone carbon nanofibers. J. Catal. 2002, 211, 85–102. [Google Scholar] [CrossRef]
- Häffner, M.; Haug, A.; Weitz, R.T.; Fleischer, M.; Burghard, M.; Peisert, H.; Chassé.; Kern, D.P. E-beam lithography of catalyst patterns for carbon nanotube growth on insulating substrates. Microelectron. Eng. 2008, 85, 768–773. [Google Scholar] [CrossRef]
- Fonseca, A.; Hernadi, K.; Nagy, J.B.; Bernaerts, D.; Lucas, A.A. Optimization of catalytic production and purification of buckytubes. J. Mol. Catal. A: Chem 1996, 107, 159–168. [Google Scholar] [CrossRef]
- Aexiadis, V.I.; Verykios, X.E. Influence of structural and preparation parameters of Fe2O3/Al2O3 catalysts on rate of production and quality of carbon nanotubes. Mater. Chem. Phys. 2009, 117, 528–535. [Google Scholar] [CrossRef]
- Avdeeva, L.B.; Kochubey, D.I.; Shaikhutdinov, S.K. Cobalt catalysts of methane decomposition: accumulation of the filamentous carbon. Appl. Catal. A: Gen 1999, 177, 43–51. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Avdeeva, L.B.; Ismagilov, Z.R.; Chuvilin, A.L.; Ushakov, V.A. Carbon capacious Ni-Cu-Al2O3 catalysts for high-temperature methane decomposition. Appl. Catal. A: Gen 2003, 247, 51–63. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Avdeeva, L.B.; Ismagilov, Z.R.; Chuvilin, A.L.; Fenelonov, V.B. Catalytic filamentous carbons-supported Ni for low-temperature methane decomposition. Catal. Today 2005, 102, 115–120. [Google Scholar] [CrossRef]
- Inoue, M.; Asai, K.; Nagayasu, Y.; Takane, K.; Iwamoto, S.; Yagasaki, E.; Ishii, K.I. Formation of multi-walled carbon nanotubes by Ni-catalyzed decomposition of methane at 600–750 °C. Diamond Relat. Mater. 2008, 17, 1471–1475. [Google Scholar] [CrossRef]
- Yu, G.J.; Gong, J.L.; Zhu, D.Z.; He, S.X.; Cao, J.Q.; Zhu, Z.Y. Efficient synthesis of carbon nanotubes over rare earth zeolites by thermal chemical vapor deposition at low temperature. Diamond Relat. Mater. 2006, 15, 1261–1265. [Google Scholar] [CrossRef]
- Tang, T.; Chen, X.C.; Meng, X.Y.; Chen, H.; Ding, Y.P. Synthesis of multiwalled carbon nanotubes by catalytic combustion of polypropylene. Angew Chem. Int. Edit. 2005, 44, 1517–1520. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, B.; Lee, B.; Chung, H.; Lee, C.J.; Yoon, H. G.; Kim, W. Synthesis of aligned few-walled carbon nanotubes on conductive substrates. J. Phys. Chem. C 2009, 113, 17983–17988. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhu, H.W.; Suenaga, K.; Wei, J.Q.; Wang, K.L.; Wu, D.H. Diameter dependent growth mode of carbon nanotubes on nanoporous SiO2 substrates. Mater. Lett. 2009, 63, 1366–1369. [Google Scholar] [CrossRef]
- Lee, W.Y.; Lin, H.; Gu, L.; Leou, K.C.; Tsai, C.H. CVD catalytic growth of single-walled carbon nanotubes with a selective diameter distribution. Diamond Relat. Mater. 2008, 17, 66–71. [Google Scholar] [CrossRef]
- Landois, P.; Peigney, A.; Laurent, C.; Frin, L.; Datas, L.; Flahaut, E. CCVD synthesis of carbon nanotubes with W/Co-MgO catalysts. Carbon 2009, 47, 789–794. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Li, Z.R.; Dervishi, E.; Saini, V.; Cui, J.B.; Biris, A.R.; Lupu, D.; Biris, A.S. Surface area and thermal stability effect of the MgO supported catalysts for the synthesis of carbon nanotubes. J. Mater. Chem 2008, 18, 5738–5745. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Tang, Z.X.; Li, W.L.; Bai, J.B. Simultaneous production of hydrogen and multi-walled carbon nanotubes by ethanol decomposition over Ni/Al2O3 catalysts. Appl. Catal. B-Environ 2009, 88, 142–151. [Google Scholar] [CrossRef]
- Wang, X.Q.; Li, L.; Chu, N.J.; Liu, Y.P.; In, H.X.; Ge, H.L. Lamellar Fe/Al2O3 catalyst for high-yield production of multi-walled carbon nanotubes bundles. Mater. Res. Bull. 2009, 44, 422–425. [Google Scholar] [CrossRef]
- Li, C.H.; Zhao, Y.; Yao, K.F.; Liang, J. Application of carbon nanotube supported nickel/aluminum mixed oxide in the synthesis of carbon nanotubes. Carbon 2003, 41, 2443–2446. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Z. Preparation of carbon microcoils and nanocoils using activated carbon nanotubes as catalyst support. Carbon 2005, 43, 1574–1577. [Google Scholar] [CrossRef]
- Changa, N.K.; Changa, S.H. High-yield synthesis of carbon nanocoils on stainless steel. Carbon 2008, 46, 1091–1109. [Google Scholar] [CrossRef]
- Masarapu, C.; Wei, B.Q. Direct growth of aligned multiwalled carbon nanotubes on treated stainless steel substrates. Langmuir 2007, 23, 9046–9049. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.J.; Gong, J.L.; Zhu, D.Z.; He, S.X.; Zhu, Z.Y. Synthesis of carbon nanotubes over rare earth zeolites at low temperature. Carbon 2005, 43, 3015–3017. [Google Scholar] [CrossRef]
- Hiraoka, T.; Kawakubo, T.; Kimura, J.; Taniguchi, R.; Okamoto, A.; Okazaki, T.; Sugai, T.; Ozeki, Y.; Yoshikawa, M.; Shinohara, H. Selective synthesis of double-wall carbon nanotubes by CCVD of acetylene using zeolite supports. Chem. Phys. Lett. 2003, 382, 679–685. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Lin, Y.T.; Lin, J.Y.; Wei, J.L. Synthesis of carbon nanotubes over Ni- and Co-supported CaCO3 catalysts using catalytic chemical vapor deposition. Mater. Chem. Phys. 2009, 114, 702–708. [Google Scholar] [CrossRef]
- Zhang, M.; Nakayama, Y.; Pan, L.J. Synthesis of carbon tubule nanocoils in high yield using iron-coated indium tin oxide as catalyst. Jpn. J. Appl. Phys. 2000, 39, L1242–L1244. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Ticich, T.M.; Curtis, V.E. Substrate–support interactions in metal-catalyzed carbon nanofiber growth. Carbon 2001, 39, 2277–2289. [Google Scholar] [CrossRef]
- Ortega-Cervantez, G.; Rueda-Morales, G.; Ortiz-Lopez, J. Catalytic CVD production of carbon nanotubes using ethanol. Microelectron. J. 2005, 36, 495–498. [Google Scholar] [CrossRef]
- Zhu, J.; Yudasaka, M.; Iijima, S. A catalytic chemical vapor deposition synthesis of double-walled carbon nanotubes over metal catalysts supported on a mesoporous material. Chem. Phys. Lett. 2003, 380, 496–502. [Google Scholar] [CrossRef]
- Qi, X.S.; Deng, Y.; Zhong, W.; Yang, Y.; Qin, C.; Au, C.T.; Du, Y.W. Controllable and large-scale synthesis of carbon nanofibers, bamboo–like nanotubes and chains of nanospheres over Fe/SnO2, and their microwave absorption properties. J. Phys. Chem. C 2010, 114, 808–814. [Google Scholar] [CrossRef]
- Colomer, J.F.; Piedigrosso, P.; Willems, I.; Journet, C.; Bernier, P.; Van Tendeloo, G.; Fonseca, A.; Nagy, J.B. Purification of catalytically produced multi-wall nanotubes. J. Chem. Soc. Faraday Trans. 1998, 94, 3753–3758. [Google Scholar] [CrossRef]
- Bian, J.; Xiao, M.; Wang, S.J.; Lu, Y.X.; Meng, Y.Z. Carbon nanotubes supported Cu-Ni bimetallic catalysts and their properties for the direct synthesis of dimethyl carbonate from methanol and carbon dioxide. Appl. Surf. Sci. 2009, 255, 7188–7196. [Google Scholar] [CrossRef]
- Chen, B.; Wu, P. Aligned carbon nanotubes by catalytic decomposition of C2H2 over Ni-Cr alloy. Carbon 2005, 43, 3172–3177. [Google Scholar] [CrossRef]
- Hu, J.L.; Yang, C.C.; Huang, J.H. Vertically-aligned carbon nanotubes prepared by water-assisted chemical vapor deposition. Diamond Relat. Mater. 2008, 17, 2084–2088. [Google Scholar] [CrossRef]
- Wang, W.; Yang, K.Q.; Gaillard, J.; Bandaru, P.R.; Rao, A.M. Rational synthesis of helically coiled carbon nanowires and nanotubes through the use of tin and indium catalysts. Adv. Mater. 2008, 20, 179–182. [Google Scholar] [CrossRef]
- Rodriguez, N.M. A review of catalytically growth carbon nanofibers. J. Mater. Res. 1993, 8, 3233–3250. [Google Scholar] [CrossRef]
- Li, Y.D.; Chen, J.L.; Ma, Y.M.; Zhao, J.B.; Qin, Y.N. Chang, L. Formation of bamboo-like nanocarbon and evidence for the quasi-liquid state of nanosized metal particles at moderate temperatures. Chem. Commun. 1999, 12, 1141–1142. [Google Scholar] [CrossRef]
- Chambers, A.; Rodriguez, N.M.; Baker, R.T.K. Influence of copper on the structural characteristics of carbon nanofibers produced from the cobalt-catalyzed decomposition of ethylene. J. Mater. Res. 1996, 11, 430–438. [Google Scholar] [CrossRef]
- Chen, B.; Wu, P. Aligned carbon nanotubes by catalytic decomposition of C2H2 over Ni–Cr alloy. Carbon 2005, 43, 3172–3177. [Google Scholar] [CrossRef]
- Topsoe, H.; Clausen, B.S.; Candia, R.; Wivel, C.; Morup, S. Insitu Mossbauer emission-spectroscopy studies of unsupported and supported sulfided Co-Mo hydrodesulfurization catalysts – evidence for and nature of a Co-Mo-S phase. J. Catal. 1981, 68, 433–452. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kubota, T. A model catalyst approach to the effects of the support on Co-Mo hydrodesulfurization catalysts. Catal. Today 2003, 86, 31–43. [Google Scholar] [CrossRef]
- Kitiyanan, B.; Alvarez, W.E.; Harwell, J.H.; Resasco, D.E. Controlled production of single-wall carbon nanotubes by catalytic decomposition of CO on bimetallic Co-Mo catalysts. Chem. Phys. Lett. 2000, 317, 497–503. [Google Scholar] [CrossRef]
- Ago, H.; Ohshima, S.; Uchida, K.; Yumura, M. Gas-phase synthesis of single-wall carbon nanotubes from colloidal solution of metal nanoparticles. J. Phys. Chem. B 2001, 105, 10453–10456. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Bae, J.C.; Baik, H.K.; Cho, S.J.; Lee, S.; Song, K.M.; Myung, N.S. Nucleation and growth control of carbon nanotubes in CVD process. Phys. B 2002, 323, 318–320. [Google Scholar] [CrossRef]
- Alvarez, W.E.; Pompeo, F.; Herrera, J.E.; Balzano, L.; Resasco, D.E. Characterization of single-walled carbon nanotubes (SWNTs) produced by CO disproportionation on Co-Mo catalysts. Chem. Mater. 2002, 14, 1853–1858. [Google Scholar] [CrossRef]
- Maruyama, S.; Kojima, R.; Miyauchi, Y.; Chiashi, S.; Kohno, M. Low-temperature synthesis of high-purity single-walled carbon nanotubes from alcohol. Chem. Phys. Lett. 2002, 360, 229–234. [Google Scholar] [CrossRef]
- Murakami, Y.; Miyauchi, Y.; Chiashi, S.; Maruyama, S. Direct synthesis of high-quality single-walled carbon nanotubes on silicon and quartz substrates. Chem. Phys. Lett. 2003, 377, 49–54. [Google Scholar] [CrossRef]
- Murakami, Y.; Chiashi, S.; Miyauchi, Y.; Hu, M.; Ogura, M.; Okubo, T.; Maruyama, S. Growth of vertically aligned single-walled carbon nanotube films on quartz substrates and their optical anisotropy. Chem. Phys. Lett. 2004, 385, 298–303. [Google Scholar] [CrossRef]
- Murakami, Y.; Chiashi, S.; Miyauchi, Y.; Maruyama, S. Direct synthesis of single-walled carbon nanotubes on silicon and quartz-based systems. Jpn. J. Appl. Phys. 2004, 43, 1221–1126. [Google Scholar] [CrossRef]
- Herrera, J.E.; Balzano, L.; Borgna, A.; Alvarez, W.E.; Resasco, D.E. Relationship between the structure/composition of Co-Mo catalysts and their ability to produce single-walled carbon nanotubes by CO disproportionation. J. Catal. 2001, 204, 129–145. [Google Scholar] [CrossRef]
- Alvarez, W.E.; Kitiyanan, B.; Borgna, A.; Resasco, D.E. Synergism of Co and Mo in the catalytic production of single-wall carbon nanotubes by decomposition of CO. Carbon 2001, 39, 547–548. [Google Scholar] [CrossRef]
- Resasco, D.E.; Alvarez, W.E.; Pompeo, F.; Balzano, L.; Herrera, J.E.; Kitiyanan, B.; Borgna, A. A scalable process for production of single-walled carbon nanotubes (SWNTs) by catalytic disproportionation of CO on a solid catalyst. J. Nanopart. Res. 2002, 4, 131–136. [Google Scholar] [CrossRef]
- Herrera, J.E.; Resasco, D.E. Loss of single-walled carbon nanotubes selectivity by disruption of the Co-Mo interaction in the catalyst. J. Catal. 2004, 221, 354–364. [Google Scholar] [CrossRef]
- Kitiyanan., B.; Alvarez, W.E.; Harwell, J.H.; Resasco, D.E. Controlled production of single-wall carbon nanotubes by catalytic decomposition of CO on bimetallic Co–Mo catalysts. Chem. Phys. Lett. 2000, 317, 497–503. [Google Scholar] [CrossRef]
- Okazaki, N.; Hosokawa, S.; Goto, T.; Nakayama, Y. Synthesis of carbon tubule nanocoils using Fe-In-Sn-O fine particles as catalysts. J. Phys. Chem. B 2005, 109, 17366–17371. [Google Scholar] [CrossRef]
- Pan, L.J.; Hayashida, T.; Harada, A.; Nakayama, Y. Effects of iron and indium tin oxide on the growth of carbon tubule nanocoils. Phys. B 2002, 323, 350–351. [Google Scholar] [CrossRef]
- Qi, X.S.; Zhong, W.; Deng, Y.; Au, C.T.; Du, Y.W. Characterization and magnetic properties of helical carbon nanotubes and carbon nanobelts synthesized in acetylene decomposition over Fe-Cu nanoparticles at 450 °C. J. Phys. Chem. C 2009, 113, 15934–15940. [Google Scholar] [CrossRef]
- Donato, M.G.; Galvagno, S.; Lanza, M.; Messina, G.; Milone, C.; Piperopoulos, E.; Pistone, A.; Santangelo, S. Influence of carbon source and Fe-catalyst support on the growth of multi-walled carbon nanotubes. J. Nanosci. Nanotechno. 2009, 9, 3815–3823. [Google Scholar] [CrossRef]
- Nourbakhsh, A.; Ganjipour, B.; Zahedifar, M.; Arzi, E. Morphology optimization of CCVD-synthesized multiwall carbon nanotubes, using statistical design of experiments. Nanotechnology 2007, 18, 115715. [Google Scholar] [CrossRef]
- Signore, M.A.; Rizzo, A.; Rossi, R.; Piscopiello, E.; Di Luccio, T.; Capodieci, L.; Dikonimos, T.; Giorgi, R. Role of iron catalyst particles density in the growth of forest-like carbon nanotubes. Diamond Relat. Mater. 2008, 17, 1936–1942. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Hall, L.J.; Berger, G.M. Optimization of flame synthesis for carbon nanotubes using supported catalyst. J. Phys. Chem. B 2002, 106, 13122–13132. [Google Scholar] [CrossRef]
- Piao, L.Y.; Li, Y.D.; Chen, J.L.; Chang, L.; Lin, J.Y.S. Methane decomposition to carbon nanotubes and hydrogen on an alumina supported nickel aerogel catalyst. Catal. Today 2002, 74, 145–155. [Google Scholar] [CrossRef]
- Chai, S.P.; Zein, S.H.S.; Mohamed, A.R. The effect of reduction temperature on Co-Mo/Al2O3 catalysts for carbon nanotubes formation. Appl. Catal. A-Gen 2007, 326, 173–179. [Google Scholar] [CrossRef]
- Chen, X.Q.; Motojima, S. The growth patterns and morphologies of carbon micro-coils produced by chemical vapor deposition. Carbon 1999, 37, 1817–1823. [Google Scholar] [CrossRef]
- Chen, X.Q.; Yang, S. M.; Kato, Y.; Motojima, S. Influence of CVD conditions on the growth of carbon microcoils with circular cross-sections. Mater. Lett. 2007, 61, 2900–2903. [Google Scholar] [CrossRef]
- Qi, X.S.; Yang, Y.; Zhong, W.; Deng, Y.; Au, C.T.; Du., Y.W. Large-scale synthesis, characterization and microwave absorption properties of carbon nanotubes of different helicities. J Solid State Chem 2009, 182, 2691–2697. [Google Scholar] [CrossRef]
- Messina, G.; Santangelo, S.; Donato, M.G.; Lanza, M.; Milone, C.; Pistone, A.; Galvagno, S. Multi-walled carbon nanotubes production by ethane decomposition over silica-supported iron-catalysts. Phys. Status. Solidi. A 2008, 205, 2422–2427. [Google Scholar] [CrossRef]
- Liu, Y.F.; Shen, Z.M. Preparation of carbon microcoils and nanocoils using activated carbon nanotubes as catalyst support. Carbon 2005, 43, 1557–158. [Google Scholar] [CrossRef]
- Xu, G.C.; Chen, B.B.; Shiki, H.; Katsumata, T.; Takikawa, H.; Sakakibara, T.; Itoh, S.; Ina, T. Parametric study on growth of carbon nanocoil by catalytic chemical vapor deposition. Jpn. J. Appl. Phys. 2005, 44, 1569–1576. [Google Scholar] [CrossRef]
- Xi, G.C.; Zhang, M.; Ma, D.K.; Zhu, Y.C.; Zhang, H.B.; Qian, Y.T. Controlled synthesis of carbon nanocables and branched-nanobelts. Carbon 2006, 44, 734–741. [Google Scholar] [CrossRef]
- Borowiak-Palen, E.; Rümmeli, M.H. Activated Cu catalysts for alcohol CVD synthesized non-magnetic bamboo-like carbon nanotubes and branched bamboo-like carbon nanotubes. Superlattice Microst 2009, 46, 374–378. [Google Scholar] [CrossRef]
- Li, H.P.; Shi, C.S.; Du, X.W.; He, C.N.; Li, J.J.; Zhao, N.Q. The influences of synthesis temperature and Ni catalyst on the growth of carbon nanotubes by chemical vapor deposition. Mater. Lett. 2008, 62, 1472–1475. [Google Scholar] [CrossRef]
- Singh, C.; Shaffer, M.S.; Windle, A.H. Production of controlled architectures of aligned carbon nanotubes by an injection chemical vapor deposition method. Carbon 2003, 41, 359–368. [Google Scholar] [CrossRef]
- Qi, X.S.; Xu, M.H.; Zhong, W.; Ye, X.J.; Deng, Y.; Au, C.T.; Jin, C.Q.; Du, Y.W. Magnetic properties and large-scale synthesis of novel carbon nanocomposites via benzene decomposition over Ni nanoparticles. J. Phys. Chem. C 2009, 113, 2267–2272. [Google Scholar] [CrossRef]
- Jin, Y.Z.; Gao, C.; Hsu, W.K.; Zhu, Y.Q.; Huczko, A.; Bystrzejewski, M.; Roe, M.; Lee, C.Y.; Acquah, S.; Kroto, H.; Walton, D.R.M. Large-scale synthesis and characterization of carbon spheres prepared by direct pyrolysis of hydrocarbons. Carbon 2005, 43, 1944–1953. [Google Scholar] [CrossRef]
- Yamada, M.; Kawana, M.; Miyake, M. Synthesis and diameter control of multi-walled carbon nanotubes over gold nanoparticle catalysts. Appl. Catal. A-Gen 2006, 302, 201–207. [Google Scholar] [CrossRef]
- Yang, S.M.; Ozeki, I.; Chen, X.Q.; Katsuno, T.; Motojima, S. Preparation of single-helix carbon microcoils by catalytic CVD process. Thin Solid Films 2008, 516, 718–721. [Google Scholar] [CrossRef]
- Chen, X.Q.; Hasegawa, M.; Yang, S.M.; Nitta, Y.; Katsuno, T.; Motojima, S. Preparation of carbon microcoils by catalytic methane hot-wire CVD process. Thin Solid Films 2008, 516, 714–717. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Du, J.H.; Cheng, H.M. Preparation of carbon microcoils by catalytic decomposition of acetylene using nickel foam as both catalyst and substrate. Carbon 2005, 43, 1874–1878. [Google Scholar]
- Li, Q.; Yan, H.; Zhang, J.; Liu, Z. Effect of hydrocarbons precursors on the formation of carbon nanotubes in chemical vapor deposition. Carbon 2004, 42, 829–835. [Google Scholar] [CrossRef]
- Lv, R.; Kang, F.Y.; Wang, W.X.; Wei, J.Q.; Gu, J.L.; Wang, K.L.; Wu, D.H. Effect of using chlorine-containing precursors in the synthesis of FeNi-filled carbon nanotubes. Carbon 2007, 45, 1433–1438. [Google Scholar] [CrossRef]
- Motojima, S.; Hasegawa, I.; Kaziya, S.; Momiyama, M.; Kawaguch, M.; Iwanaga, H. Preparation of coiled carbon fibers by pyrolysis of acetylene using a Ni catalyst and sulfur or phosphrous compounds impurity. Appl. Phys. Lett. 1993, 62, 2322–2323. [Google Scholar] [CrossRef]
- Iwanaga, H.; Kawaguchi, M.; Motojima, S. Growth mechanisms and properties of coiled whiskers of silicon nitride and carbon. Jpn. J. Appl. Phys. 1993, 32, 105–115. [Google Scholar] [CrossRef]
- Zhao, D.L.; Shen, Z.M. Preparation and microwave absorption properties of carbon nanocoils. Mater. Lett. 2008, 62, 3704–3706. [Google Scholar] [CrossRef]
- Chen, X.; Yang, S.; Takeuchi, K.; Hashishin, T.; Iwanaga, H.; Motojima, S. Conformation and growth mechanism of the carbon nanocoils with twisting form in comparison with that of carbon microcoils. Diamond Relat. Mater. 2003, 12, 1836–1840. [Google Scholar] [CrossRef]
- Motojima, S.; Chen, X.; Yang, S.; Hasegawa, M. Properties and potential applications of carbon microcoils/nanocoils. Diamond Relat. Mater. 2004, 13, 1989–1992. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qi, X.; Qin, C.; Zhong, W.; Au, C.; Ye, X.; Du, Y. Large-Scale Synthesis of Carbon Nanomaterials by Catalytic Chemical Vapor Deposition: A Review of the Effects of Synthesis Parameters and Magnetic Properties. Materials 2010, 3, 4142-4174. https://doi.org/10.3390/ma3084142
Qi X, Qin C, Zhong W, Au C, Ye X, Du Y. Large-Scale Synthesis of Carbon Nanomaterials by Catalytic Chemical Vapor Deposition: A Review of the Effects of Synthesis Parameters and Magnetic Properties. Materials. 2010; 3(8):4142-4174. https://doi.org/10.3390/ma3084142
Chicago/Turabian StyleQi, Xiaosi, Chuan Qin, Wei Zhong, Chaktong Au, Xiaojuan Ye, and Youwei Du. 2010. "Large-Scale Synthesis of Carbon Nanomaterials by Catalytic Chemical Vapor Deposition: A Review of the Effects of Synthesis Parameters and Magnetic Properties" Materials 3, no. 8: 4142-4174. https://doi.org/10.3390/ma3084142




