Physical Analysis and Mathematical Modeling of the Hydrogen Storage Process in the MmNi4.2Mn0.8 Compound
Abstract
:1. Introduction
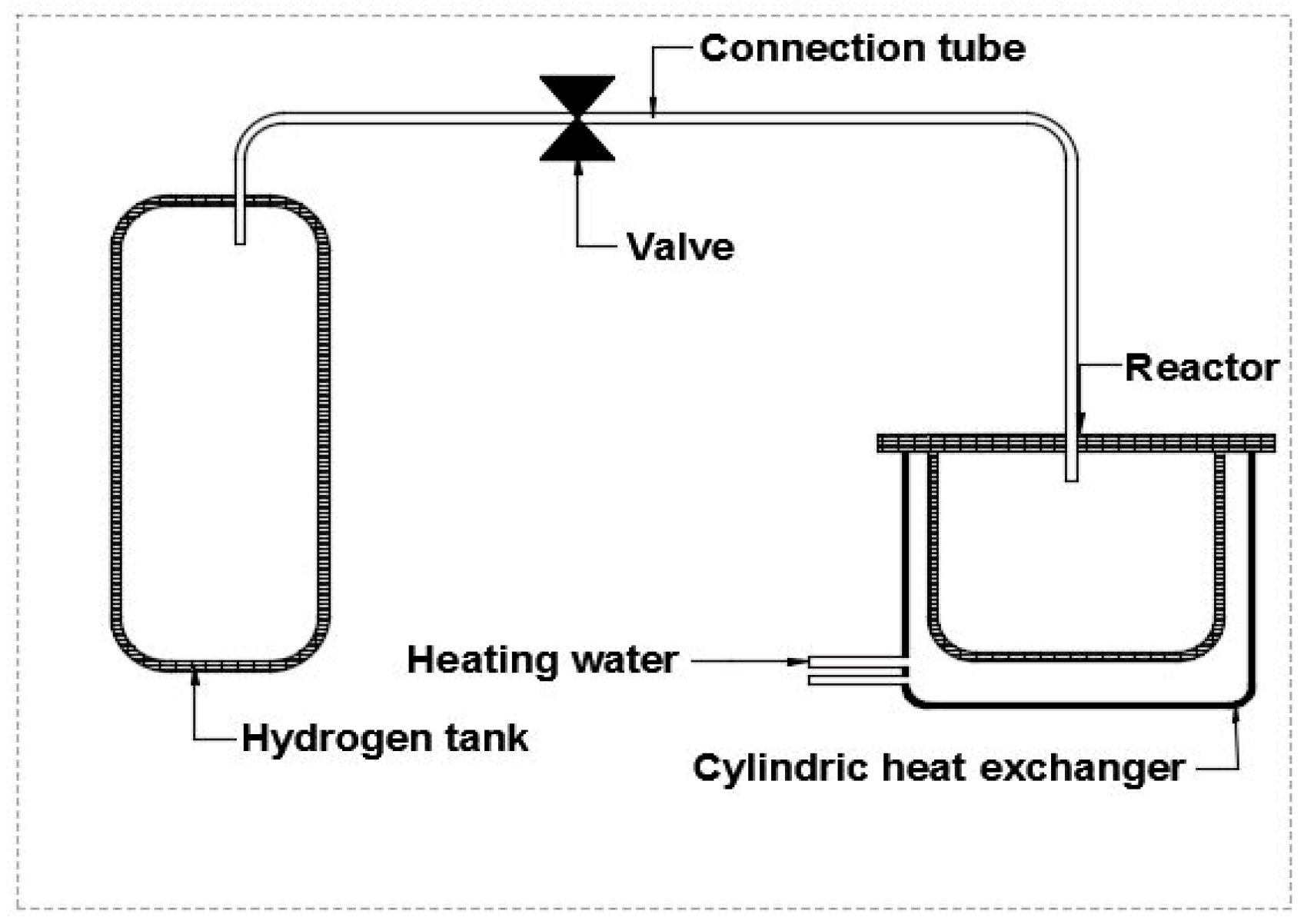
2. Equipment and Procedures
3. Experimental Isotherms
- * α phase: distinguished by a sharp rise in pressure against a slight change in H/M;
- * α + β phase: marked by a remarkable increase in H/M and a slight shift in pressure (equilibrium plateau);
- * β phase: characterized by a stabilization of H/M against a rise in pressure.
4. The Plot of Van’t Hoff
5. Modelling
5.1. Introduction of the Model
5.2. Adjustment of the Experimental P-C-T
5.3. Numerical Findings
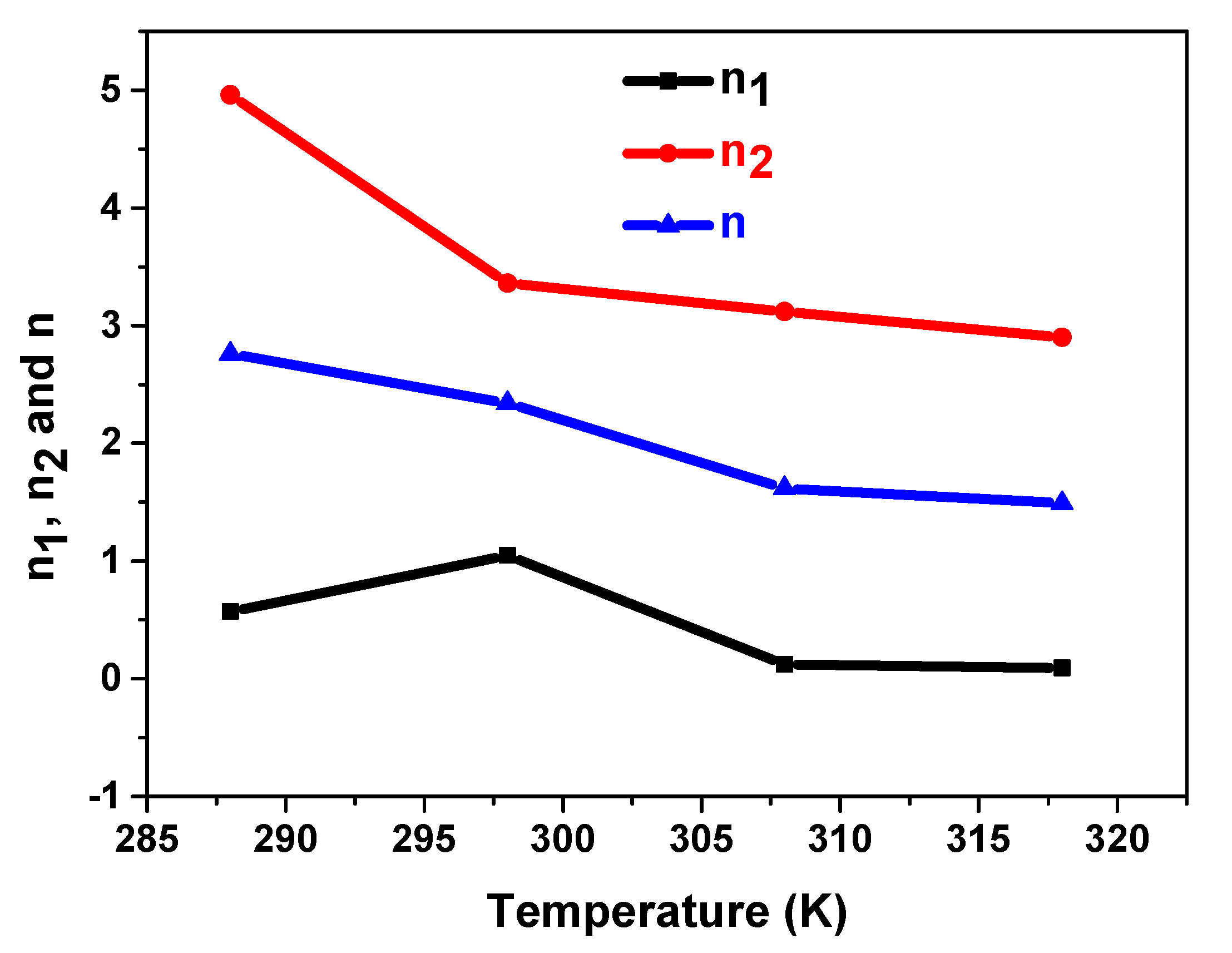
5.3.1. Temperature’s Effect on n1, n2, and n
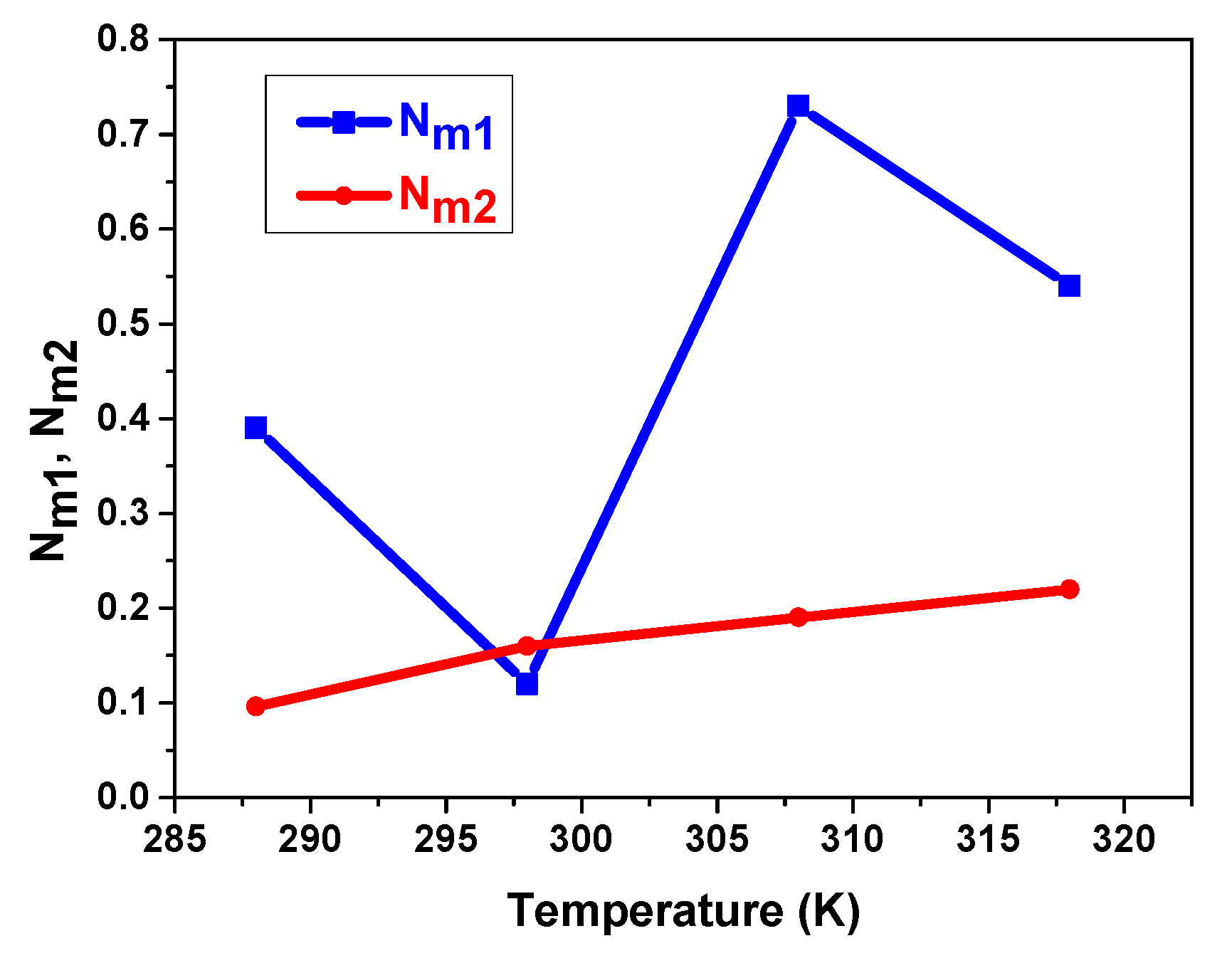
5.3.2. Temperature’s Influence on Interstitial Site Densities Nm1 and Nm2
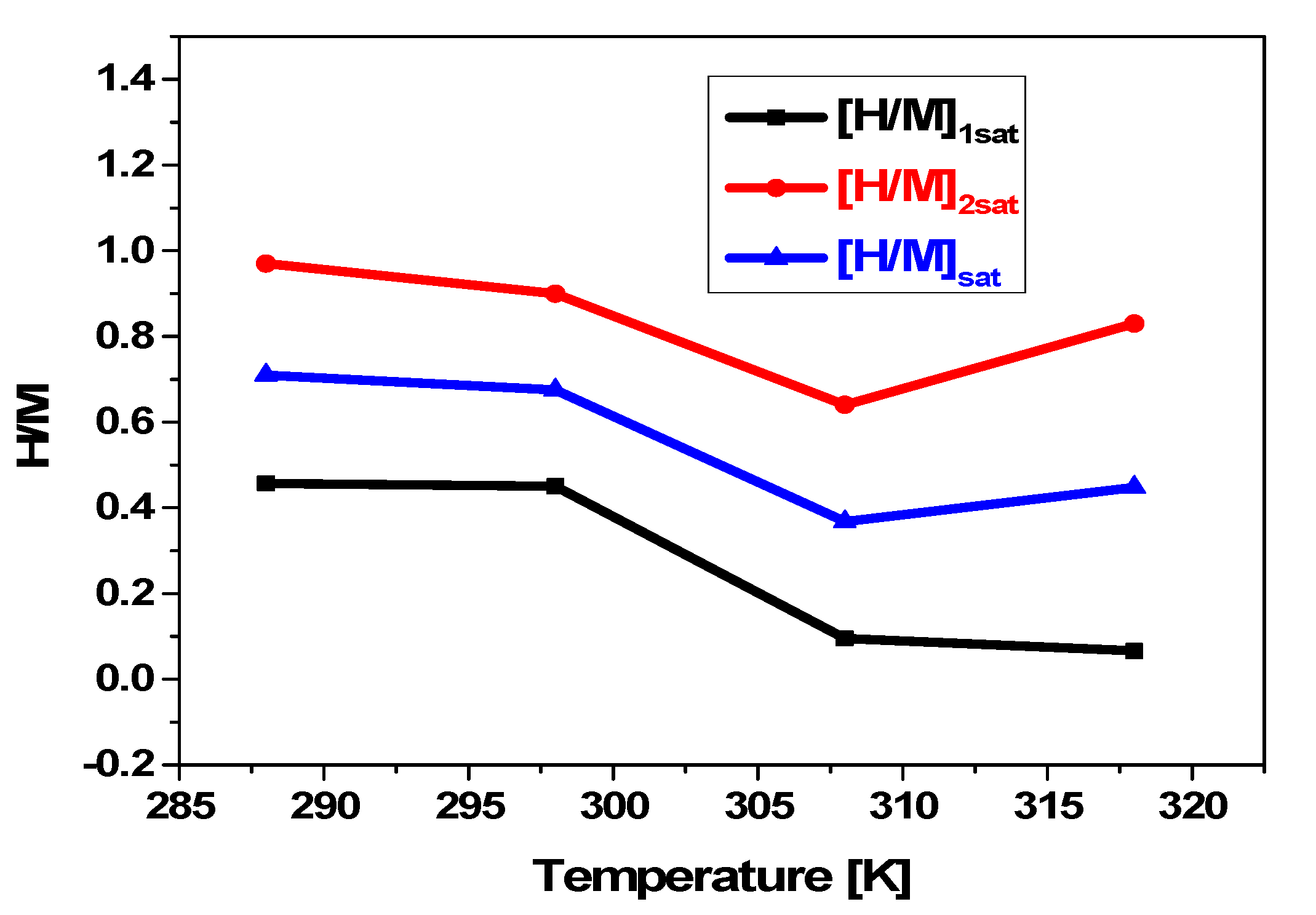
5.3.3. Temperature’s Influence on [H/M]1sat and [H/M]2sat
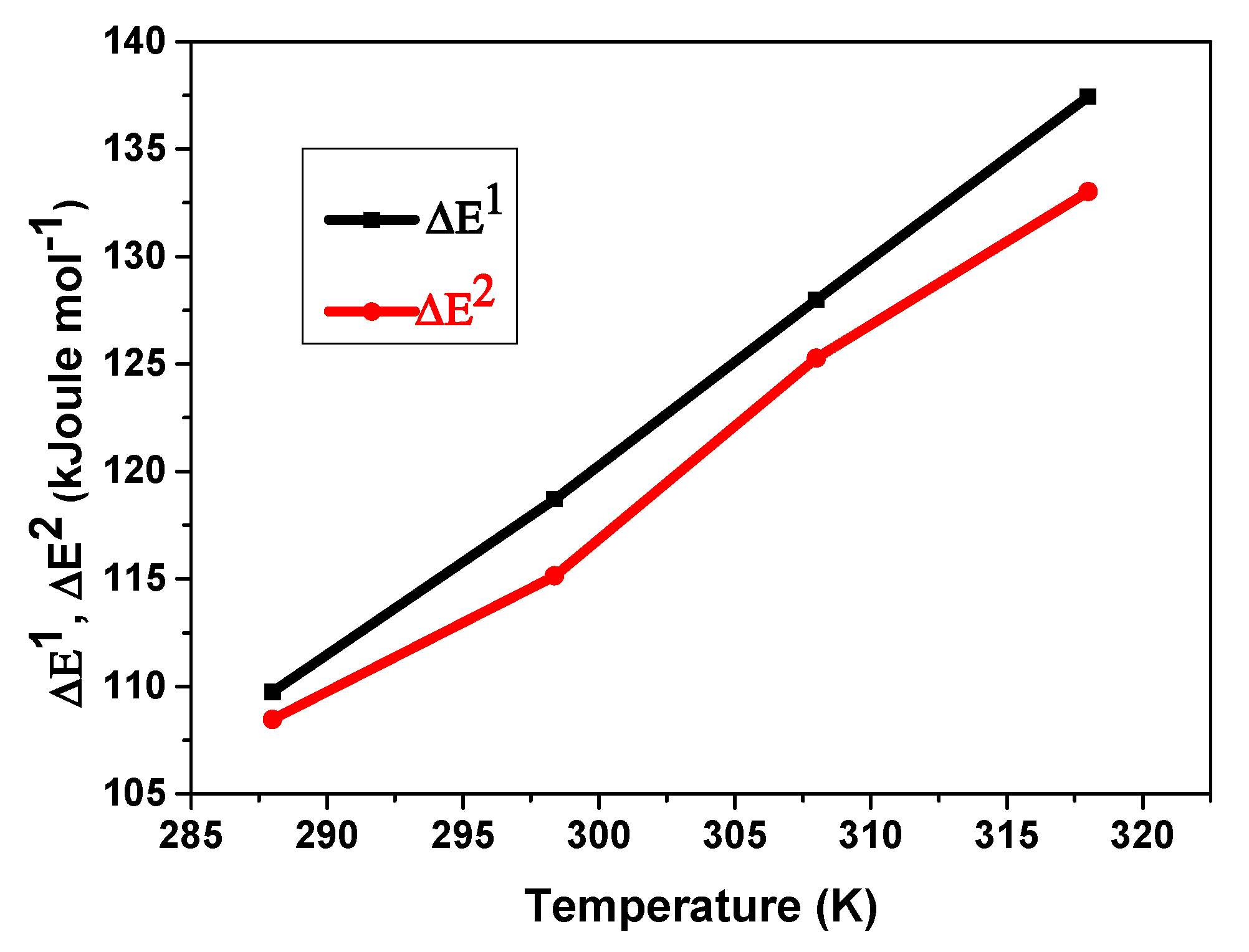
5.3.4. Temperature’s Influence on the ΔE1 and ΔE2
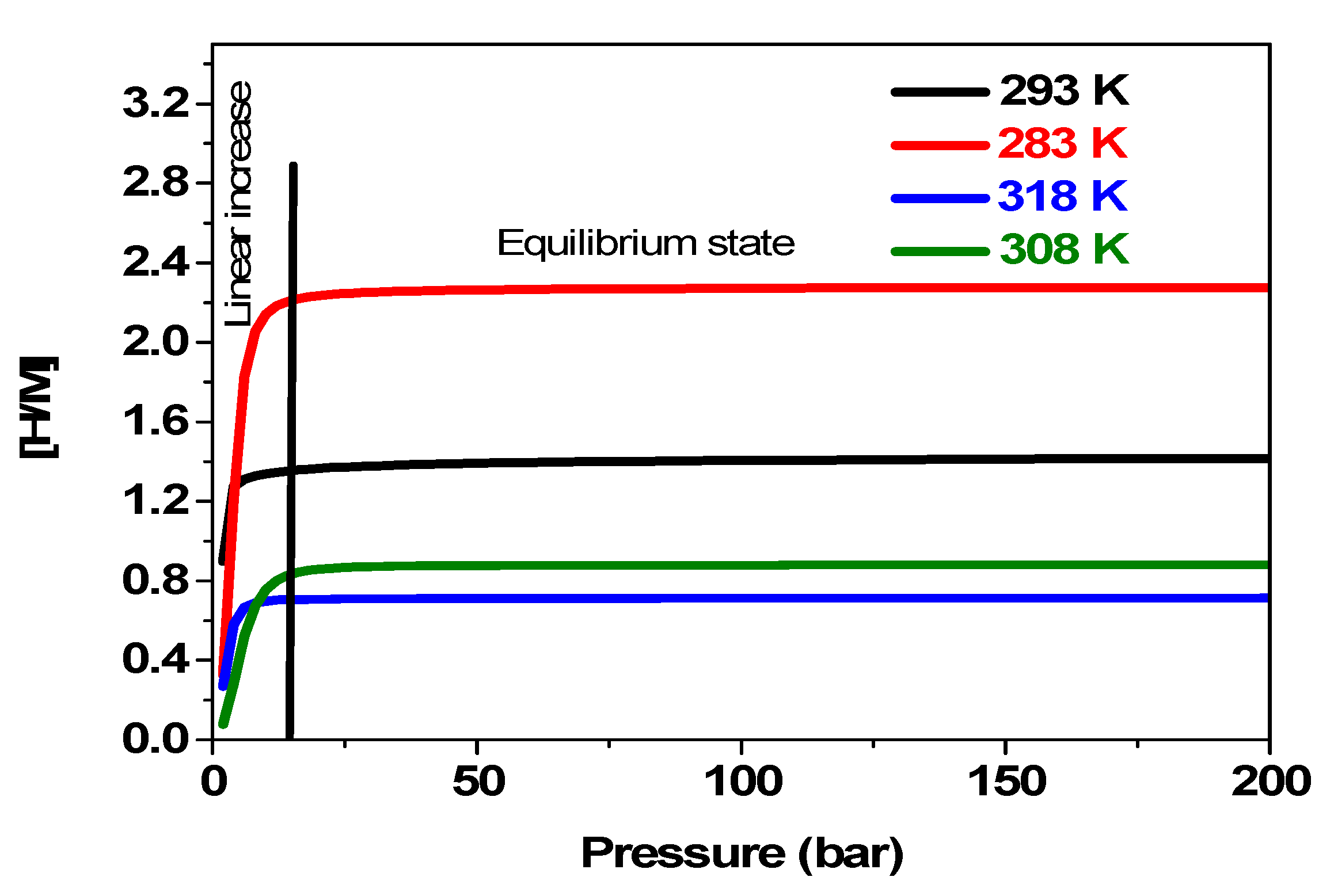
5.3.5. Variation in Hydrogen Concentration [H/M] with Pressure at Different Temperatures
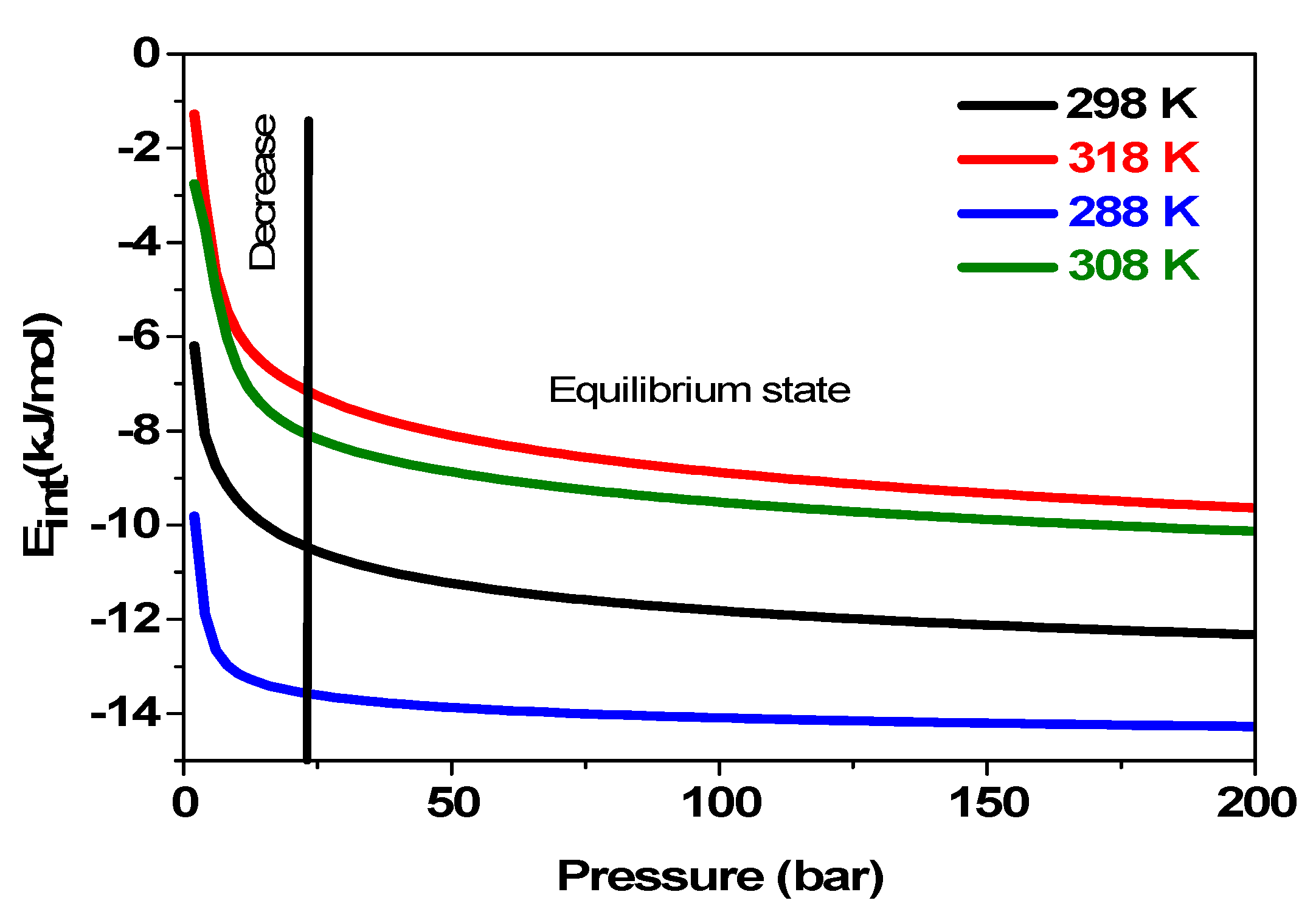
5.3.6. Effects of Pressure on the System’s Internal Energy Variation Uint versus Temperatures
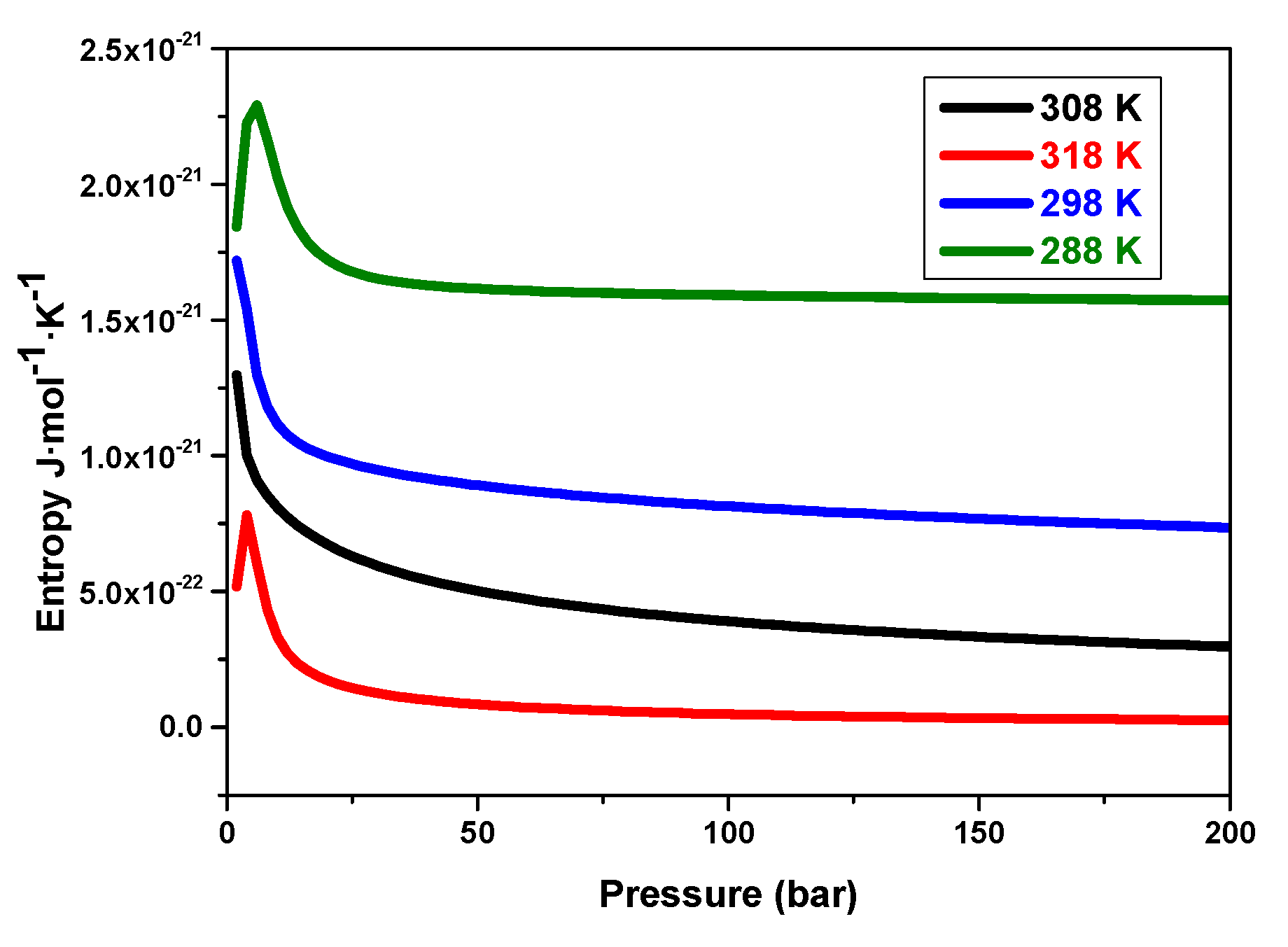
5.3.7. Effects of Pressure on the System’s Entropy Variation versus Temperatures
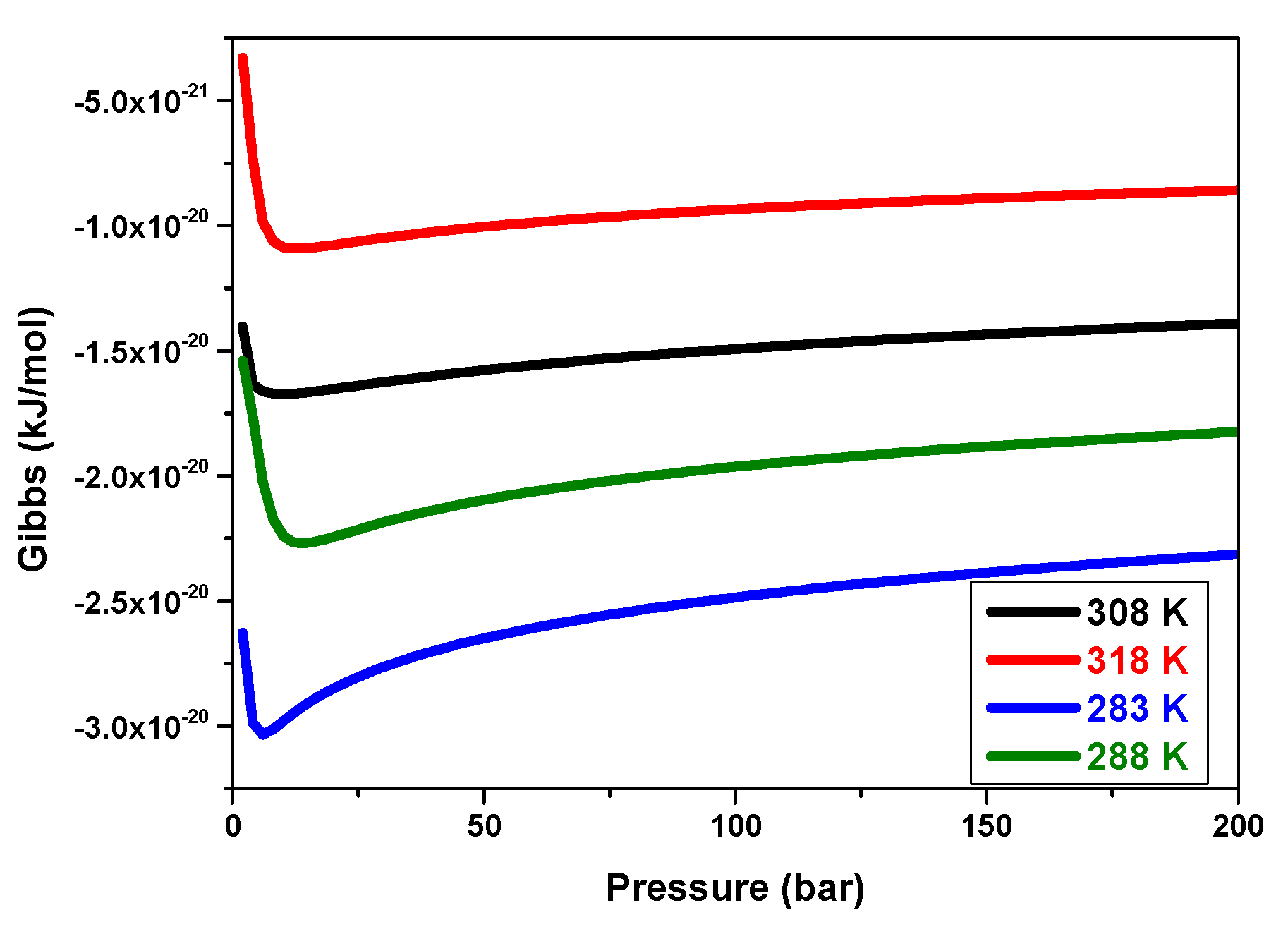
5.3.8. Effects of Pressure on the Gibbs Variation versus Temperatures
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yi, X.; Fang, Z. Impact of energy depletion, human development, and income distribution on natural resource sustainability. Resour. Policy 2023, 83, 103531. [Google Scholar]
- Iñaki, A.; Iñigo, C.P.; Rosa, L.; Gorka, B.; Roberto, B. The energy requirements of a developed world. Energy Sustain. Dev. 2016, 33, 1–13. [Google Scholar]
- Françoise, B.; Mara, M.; Alfredo, C.; Philipp, B.; Veneta, K. Sustainable energy transitions and social inequalities in energy access: A relational comparison of capabilities in three European countries. Glob. Transit. 2019, 1, 226–240. [Google Scholar]
- Oguz, O.Y. World energy outlook and state of renewable energy: 10-Year evaluation. Innov. Green Dev. 2023, 2, 100070. [Google Scholar]
- Shiqi, Z.; Yupeng, W.; Xiaoqiang, G.; Zheng, L.; Xiaofei, S.; Frede, B. Overview of US patents for energy management of renewable energy systems with hydrogen. Int. J. Hydrogen Energy 2023, 48, 9574–9591. [Google Scholar]
- Joan, B.B.; Elisa, T.B. Impacts of intermittent renewable generation on electricity system costs. Energy Policy 2016, 94, 411–420. [Google Scholar]
- Chu, D.I.; Samuel, G.; Emmanuel, T.; Eric, E.D. Distributed Generation and Renewable Energy Integration into the Grid: Prerequisites, Push Factors, Practical Options, Issues and Merits. Energies 2021, 14, 5375. [Google Scholar]
- Antonio, S.; Qi, Z.; Mariano, M.; Pastora, V. Towards a new renewable power system using energy storage: An economic and social analysis. Energy Convers. Manag. 2022, 252, 115056. [Google Scholar]
- Fan, Z.; Pengcheng, Z.; Meng, N.; Jon, M. The survey of key technologies in hydrogen energy storage. Int. J. Hydrogen Energy 2016, 41, 14535–14552. [Google Scholar]
- Alka, P.; Rekha, D.; Jyoti, G.; Jyothi, C.; Vivek, A.; Pramod, H.B. Insights into renewable hydrogen energy: Recent advances and prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [Google Scholar]
- Meiling, Y.; Hugo, L.; Elodie, P.; Robin, R.; Samir, J.; Daniel, H. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar]
- Ujwal, S.M.; Nidhi, B.; Aditi, P.; Subramanya, K.N.; Lourdu, A.R. Challenges associated with hydrogen storage systems due to the hydrogen embrittlement of high strength steels. Int. J. Hydrogen Energy 2023, 47, 17894–17913. [Google Scholar]
- Ahmed, O.; Neha, N.; Ahmed, M.E.; Mahmoud, H.; Amer, A.H.; Ala’a, H.A.M.; David, W.R. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar]
- Nejc, K.; Ilena, G.; Franz, W.; Markus, S.; Alexander, T. A review on metal hydride materials for hydrogen storage. J. Energy Storage 2023, 72, 108456. [Google Scholar]
- Hoffman, K.C.; Reilly, J.J.; Salzano, F.J.; Waide, C.H.; Wiswall, R.H.; Winsche, W.E. Metal hydride storage for mobile and stationary applications. Int. J. Hydrogen Energy 1976, 1, 133–151. [Google Scholar] [CrossRef]
- Mykhaylo, V.L.; Ivan, T.; Moegamat, W.D.; Yevgeniy, V.K.; Adrian, P.; Dana, S.; Righardt, E.; Gerhard, L.; Vladimir, L. Metal hydride hydrogen storage and supply systems for electric forklift with low-temperature proton exchange membrane fuel cell power module. Int. J. Hydrogen Energy 2016, 41, 13831–13842. [Google Scholar]
- Inga, B.; Marc, L. Operation strategies for gas solid reactions in thermal energy storage systems. J. Energy Storage 2021, 40, 102767. [Google Scholar]
- Stefan, B.; Ratnesh, T.; Amir, S.; Michael, O. Heat/mass transfer enhancement of an exothermic absorption utilizing a spinning disk reactor. Int. J. Heat Mass Transf. 2019, 129, 326–341. [Google Scholar]
- Hassan, I.A.; Haitham, S.R.; Mohamed, A.S.; Daniel, H. Hydrogen storage technologies for stationary and mobile applications: Review, analysis and perspectives. Renew. Sustain. Energy Rev. 2021, 149, 111311. [Google Scholar] [CrossRef]
- Yuanlu, L.; Erika, T.; Fernando, Z.; Verónica, D. Design of a AB5-metal hydride cylindrical tank for hydrogen storage. Int. J. Hydrogen Energy 2021, 46, 33889–33898. [Google Scholar]
- Jean-Marc, J.; Valérie, P.-B.; Fermin, C.; Junxian, Z.; Michel, L. LaNi5 related AB5copounds: Structure, properties and applications. J. Alloys Compd. 2021, 862, 158163. [Google Scholar]
- Mahvash, A.; Rohit, M.; Pratibha, S. Heat transfer techniques in metal hydride hydrogen storage: A review. Int. J. Hydrogen Energy 2017, 42, 30661–30682. [Google Scholar]
- Joakim, A.; Stefan, G. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar]
- Liang, L.; Alexander, I.; Daniel, L.; Ashleigh, C.; Wendy, T.; Cherry, C.; Jon, Y.; Liezl, S. Metal Hydride Composite Structures for Improved Heat Transfer and Stability for Hydrogen Storage and Compression Applications. Inorganics 2023, 11, 181. [Google Scholar]
- Belkhiria, S.; Briki, C.; Dhaou, M.H.; Alresheedi, F.; Jemni, A. A study of the magnetic properties of LaNi5 and their effect on hydrogen desorption under the action of a magnetostatic field. Heliyon 2023, 9, 20311. [Google Scholar] [CrossRef] [PubMed]
- Briki, C.; Belkhiria, S.; Almoneef, M.; Mbarek, M.; Jemni, A. Experimental study of the microstructures and hydrogen storage properties of the LaNi4Mn0·5Co0.5 alloys. Heliyon 2023, 9, 17430. [Google Scholar] [CrossRef] [PubMed]
- Belkhiria, S.; Briki, C.; Dhaou, M.H.; Jemni, A. Experimental study of a metal–hydrogen reactor’s behavior under the action of an external magnetostatic field during absorption and desorption. Int. J. Hydrogen Energy 2020, 45, 4673–4684. [Google Scholar] [CrossRef]
- Yehui, C.; Xiangguo, Z.; Junfeng, X.; Huaqin, K. The comprehensive review for development of heat exchanger configuration design in metal hydride bed. Int. J. Hydrogen Energy 2022, 47, 2461–2490. [Google Scholar]
- Saurabh, T.; Pratibha, S. Integration of metal hydride reactor with thermocline based heat storage system. J. Energy Storage 2023, 59, 106506. [Google Scholar]
- Puchanee, L.; Nick, S.B.; Zhen, L.; Robert, F.; Emilie, S.; Mohammad, S.I. A novel design for faster hydrogen storage: A combined semi-cylindrical and central return tube heat exchanger. J. Energy Storage 2023, 71, 108018. [Google Scholar]
- Shahin, S.; Mary, H.M.C. Different reactor and heat exchanger configurations for metal hydride hydrogen storage systems—A review. Int. J. Hydrogen Energy 2016, 41, 9462–9470. [Google Scholar]
- Busra, A.; Mustafa, I.; Selahattin, C. Experimental analysis of hydrogen storage performance of a LaNi5–H2 reactor with phase change materials. Int. J. Hydrogen Energy 2023, 48, 6010–6022. [Google Scholar]
- Yang, Y.; Jianfeng, L.; Jing, D.; Weilong, W.; Jinyue, Y. Numerical simulation on the storage performance of a phase change materials based metal hydride hydrogen storage tank. Appl. Energy 2020, 278, 115682. [Google Scholar]
- Nuaman, F.A.; Leqaa, A.M.; Abdulwahhab, H.M.; Shankar, S.; Bilal, J.M.A.; Mustafa, A.A. The effect of addition Ag and MnO2 nanoparticles in the hydrogen storage of ethyl 2-((5-methoxybenzo[d]thiazol-2-yl)thio) acetate (organic: Inorganic nanohybrids). J. Indian Chem. Soc. 2022, 99, 100734. [Google Scholar]
- Michael, U.N.; Sesha, S.S.; Ayala, R.P.; Ashok, K.; Yogi, G.; Elias, K.S. Nanomaterials for Hydrogen Storage Applications: A Review. J. Nanomater. 2008, 2008, 950967. [Google Scholar]
- Andrii, L.; Julien, O.F.; Mohammad, F.; Jin-Yoo, S.; Young, S.L.; Jae-Hyeok, S.; Jihye, P.; Young, W.C. Enhancing the Hydrogen Storage Properties of AxBy Intermetallic Compounds by Partial Substitution: A Short Review. Hydrogen 2020, 1, 38–63. [Google Scholar]
- Jain, R.K.; Ankur, J.; Shivani, A.; Lalla, N.P.; Ganesan, V.; Phase, D.M.; Jain, I.P. Characterization and hydrogenation of CeNi5−xCrx (x = 0, 1, 2) alloys. J. Alloys Compd. 2007, 430, 165–169. [Google Scholar] [CrossRef]
- Ryo, Y.; Takayuki, K.; Satoshi, K.; Daisuke, O.; Taku, J.S.; Chikashi, N.; An-Pang, T. Creating the hydrogen absorption capability of CeNi5 through the addition of Al. Int. J. Hydrogen Energy 2017, 42, 21832–21840. [Google Scholar]
- Ryota, T.; Ryo, Y.; Satoshi, K.; Chikashi, N.; An-Pang, T. Ability of hydrogen storage CeNi5-xGaxand Mg2Ni alloys to hydrogen ateacetylene. Sci. Technol. Adv. Mater. 2019, 20, 774–785. [Google Scholar]
- Sumita, S.; Kuldeep, P. Investigations on Microstructures of Ball–milled MmNi5 Hydrogen Storage Alloy. Mater. Res. Bull. 2016, 73, 284–289. [Google Scholar]
- Masumeh, S.; Mohammad, J.; Seyed, M.A.; Seyed, M.G.M.S. Microstructure and thermodynamic assessment of MmNi5-xAlx hydrogen storage alloys. Sādhanā 2021, 46, 127. [Google Scholar]
- Billur, S.; Farida, L.-D.; Michael, H. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar]
- Todorova, S.; Rangelova, V.; Mihaylov, L.; Spassov, T. Effect of hydrogen induced decrepitation on the hydrogen sorption properties of MmNi5. Int. J. Electrochem. Sci. 2020, 15, 4900–4907. [Google Scholar] [CrossRef]
- Sumita, S.; Kuldeep, P. Effect of transition metals on ball-milled MmNi5 hydrogen storage alloy. Mater. Renew. Sustain. Energy 2015, 4, 19. [Google Scholar]
- Anil Kumar, E.; Prakash Maiya, M.; Srinivasa Murthy, S.; Viswanathan, B. Structural, hydrogen storage and thermodynamic properties of some mischmetal–nickel alloys with partial substitutions for nickel. J. Alloys Compd. 2009, 476, 92–97. [Google Scholar] [CrossRef]
- Toyoto, S.; Hiroyuki, S.; Reina, U.; Junya, I.; Yuki, N.; Kazuki, O.; Shigeyuki, T.; Shin-ichi, O. Hydrogen Absorption Reactions of Hydrogen Storage Alloy LaNi5 under High Pressure. Molecules 2023, 28, 1256. [Google Scholar]
- Mingyi, C.; Dongxu, O.; Jingwen, W.; Jiahao, L.; Jian, W. Environmental pressure effects on thermal runaway and fire behaviors of lithium-ion battery with different cathodes and state of charge. Process Saf. Environ. Prot. 2019, 130, 250–256. [Google Scholar]
- Rupali, N.; Sumita, S.; Sterlin, L.H.; Sandra, L.A.; Ashish, T.; Meenu, S.; Ramesh, A.; Sanjiv, S.; Varsha, K.; Sesha, S.S. Recent developments in state-of-the-art hydrogen energy technologies—Review of hydrogen storage materials. Sol. Compass 2023, 5, 100033. [Google Scholar]
- Evangelos, I.G.; Chongming, W.; Simon, S.; Oliver, C. Metal-Hydride-Based Hydrogen Storage as Potential Heat Source for the Cold Start of PEM FC in Hydrogen-Powered Coaches: A Comparative Study of Various Materials and Thermal Management Techniques. Hydrogen 2022, 3, 418–432. [Google Scholar]
- Anil Kumar, E.; Prakash Maiya, M.; Srinivasa Murthy, S. Influence of aluminium content on the dynamic characterstics of mischmetal based hydrogen storage alloys. J. Alloys Compd. 2009, 470, 157–162. [Google Scholar] [CrossRef]
- Molinas, B.; Pontarollo, A.; Scapin, M.; Peretti, H.; Melnichuk, M.; Corso, H.; Aurora, A.; Mirabile, D.G.; Montone, A. The optimization of MmNi5−xAlx hydrogen storage alloy for sea or lagoon navigation and transportation. Int. J. Hydrogen Energy 2016, 24, 14484–14490. [Google Scholar] [CrossRef]
- Apostolov, A.; Stanev, N.; Tcholakov, P. Hydrogen desorption charactersistics of MmNi5-xFex compounds. J. Less Common Metals 1985, 110, 127–129. [Google Scholar] [CrossRef]
- Alok, K.; Nithin, N.R.; Muthukumar, P. Parametric studies on MmNi4.7Fe0.3 based reactor with embedded cooling tubes for hydrogen storage and cooling application. J. Energy Storage 2021, 35, 102317. [Google Scholar]
- Miao, M.L.; Chang, C.W.; Chun, C.Y. Development of high-performance hydrogen storage alloys for applications in nickel-metal hydride batteries at ultra-low temperature. J. Power Sources 2021, 491, 229585. [Google Scholar]
- Vinod, K.; Pukazhselvan, D.; Singh, S.K. Hydrogen Storage Characteristics of MmNi5−xMx (M = Cu, Mn and Al). Adv. Sci. Lett. 2012, 18, 164–169. [Google Scholar]
- Dhanesh, C.; Wen-Ming, C.; Anjali, T. Metal Hydrides for NiMH Battery Applications. Solution thermodynamics of hydrogen in the mischmetal-Niδ system with aluminium, manganese and tin substituations. J. Alloys Compd. 1992, 185, 259–271. [Google Scholar]
- Xiaobo, M.; Xuedong, W.; Hui, D.; Yongning, L. The relationship between discharge capacity of LaNi5 type hydrogen storage alloys and formation enthalpy. J. Alloys Compd. 2010, 490, 548–551. [Google Scholar]
- Kwo-hsiung, Y.; Jean, N. The Current Status of Hydrogen Storage Alloy Development for Electrochemical Applications. Materials 2013, 6, 4574–4608. [Google Scholar]
- Leah, M.; James, J.H.; Michel, L.T.; Peter, G.; Jan, P.E.; Juergen, E.; Nikolas, K.; David, M.A. A manganese hydride molecular sieve for practical hydrogen storage under ambient conditions. Energy Environ. Sci. 2019, 12, 1580–1591. [Google Scholar]
- Snigdha, S.; Amrish, K.P.; Tripathi, M.M. Storage technologies for electric vehicles. J. Traffic Transp. Eng. (Engl. Ed.) 2020, 7, 340–361. [Google Scholar]
- Shammya, A.; Sumon, R.; Kairat, K.; Asset, K.; Marzhan, M.K.; Kenzhebatyr, Z.B.; Abul, K.A. Emerging and Recycling of Li-Ion Batteries to Aid in Energy Storage, A Review. Recycling 2023, 8, 48. [Google Scholar]
- Poojan, M.; Kondo-Francois, A.-Z. Titanium-iron-manganese (TiFe0.85Mn0.15) alloy for hydrogen storage: Reactivation upon oxidation. Int. J. Hydrogen Energy 2019, 44, 16757–16764. [Google Scholar]
- Satya, P.P.; Amritendu, R.; Soobhankar, P. Role of Mn-substitution towards the enhanced hydrogen storage performance in FeTi. Int. J. Hydrogen Energy 2022, 47, 9357–9371. [Google Scholar]
- Peng, L.; Changlin, Z.; Dongfang, H.; Xingsheng, Z.; Zhichen, L.; Dejuan, H. Effect of introducing manganese as additive on microstructure, hydrogen storage properties and rate limiting step of Ti–Cr alloy. Int. J. Hydrogen Energy 2022, 47, 459–469. [Google Scholar]
- Ben Lamine, A.; Bouazra, Y. Application of statistical thermodynamics to the olfaction mechanism. Chem. Senses 1997, 22, 67–75. [Google Scholar] [CrossRef]
- Sghaier, W.; Ben Torkia, Y.; Bouzid, M.; Ben Lamine, A. Thermodynamic analysis of cooling cycles based on statistical physics modeling of ethanol adsorption isothermsAnalysethermodynamique des cycles de refroidissementbasée sur la modélisation physique statistique des isothermes à adsorption d’éthanol. Int. J. Refrig. 2022, 141, 119–131. [Google Scholar] [CrossRef]
- Belkhiria, S.; Briki, C.; Almoneef, M.; Dhaou, M.H.; Mbarek, M.; Jemni, A. Experimental and numerical study of hydrogen absorption in the MmNi5−xMx compound. Int. J. Hydrogen Energy 2023, 51, 29–40. [Google Scholar] [CrossRef]
- Cousins, A.; Zohra, F.T.; Gray, E.M.; Webb, C.J.; Kochanek, M.; Edwards, S.; Schoeman, L. Alloy selection for dual stage metal-hydride hydrogen compressor: Using a thermodynamic model to identify metal-hydride pairs. Int. J. Hydrogen Energy 2023, 48, 28453–28459. [Google Scholar] [CrossRef]
- Magda, P.; Julita, D.-W.; Tomasz, P.; Marek, P. The Influence of Cerium on the Hydrogen Storage Properties of La1-xCexNi5 Alloys. Energies 2020, 13, 1437. [Google Scholar]
- Alok, K.; Muthukumar, P.; Pratibha, S.; Anil Kumar, E. Absorption based solid state hydrogen storage system: A review. Sustain. Energy Technol. Assess. 2022, 5, 102204. [Google Scholar]
- Bouaziz, N.; Briki, C.; Dhahri, E.; Jemni, A.; Ben Lamine, A. Experimental and theoretical investigation of absorption and desorption of hydrogen in the LaNi4Co0.5Mn0.5 alloy. Chem. Eng. Sci. 2022, 251, 117453. [Google Scholar] [CrossRef]
- Bouaziz, N.; Ben Manaa, M.; Ben Lamine, A. Physicochemical and thermodynamic investigation of hydrogen absorption and desorption in LaNi3.8Al1.0Mn0.2 using the statistical physics modeling. Results Phys. 2018, 9, 1323–1334. [Google Scholar] [CrossRef]
- Bajahzar, A.; Bouzid, M.; Briki, C.; Nasri, F.; Belmabrouk, H.; Jemni, A. Experimental and numerical study of the isotherms and determination of physicochemical parameters of the hydrogen absorption/desorption process by the metal hydrides. Int. J. Hydrogen Energy 2020, 45, 15281–15293. [Google Scholar] [CrossRef]



| ΔH0 (kJ mol−1) | ΔS0 (J mol−1 K−1) |
|---|---|
| −12.4 | −81.18 |
| Absorption Process | |||||||
|---|---|---|---|---|---|---|---|
| Temperature (K) | n1 | n2 | n | Nm1 | Nm2 | P1 | P2 |
| 288 | 0.57 | 4.96 | 2.76 | 0.39 | 0.096 | 1.05 | 1.8 |
| 298 | 1.05 | 3.63 | 2.34 | 0.12 | 0.16 | 2.47 | 3.99 |
| 308 | 0.12 | 3.12 | 1.62 | 0.73 | 0.19 | 0.04 | 2.55 |
| 318 | 0.093 | 2.9 | 1.49 | 0.54 | 0.22 | 1.01 | 5.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belkhiria, S.; Alsawi, A.; Briki, C.; Altarifi, S.M.; Dhaou, M.H.; Jemni, A. Physical Analysis and Mathematical Modeling of the Hydrogen Storage Process in the MmNi4.2Mn0.8 Compound. Materials 2024, 17, 2237. https://doi.org/10.3390/ma17102237
Belkhiria S, Alsawi A, Briki C, Altarifi SM, Dhaou MH, Jemni A. Physical Analysis and Mathematical Modeling of the Hydrogen Storage Process in the MmNi4.2Mn0.8 Compound. Materials. 2024; 17(10):2237. https://doi.org/10.3390/ma17102237
Chicago/Turabian StyleBelkhiria, Sihem, Abdulrahman Alsawi, Chaker Briki, Saleh M. Altarifi, Mohamed Houcine Dhaou, and Abdelmajid Jemni. 2024. "Physical Analysis and Mathematical Modeling of the Hydrogen Storage Process in the MmNi4.2Mn0.8 Compound" Materials 17, no. 10: 2237. https://doi.org/10.3390/ma17102237






