Influence of Femtosecond Laser Modification on Biomechanical and Biofunctional Behavior of Porous Titanium Substrates
Abstract
:1. Introduction
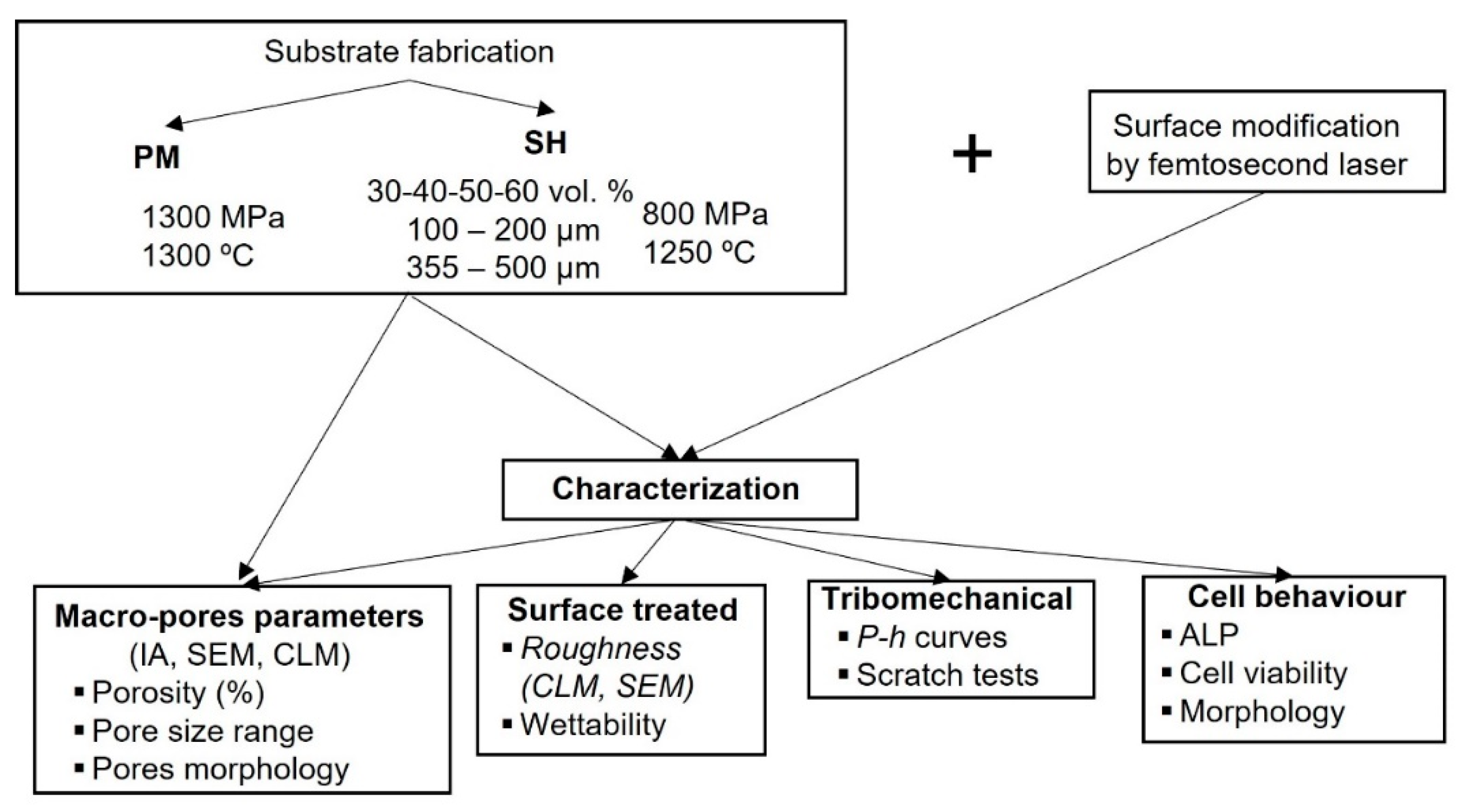
2. Materials and Methods
2.1. Substrates Preparation
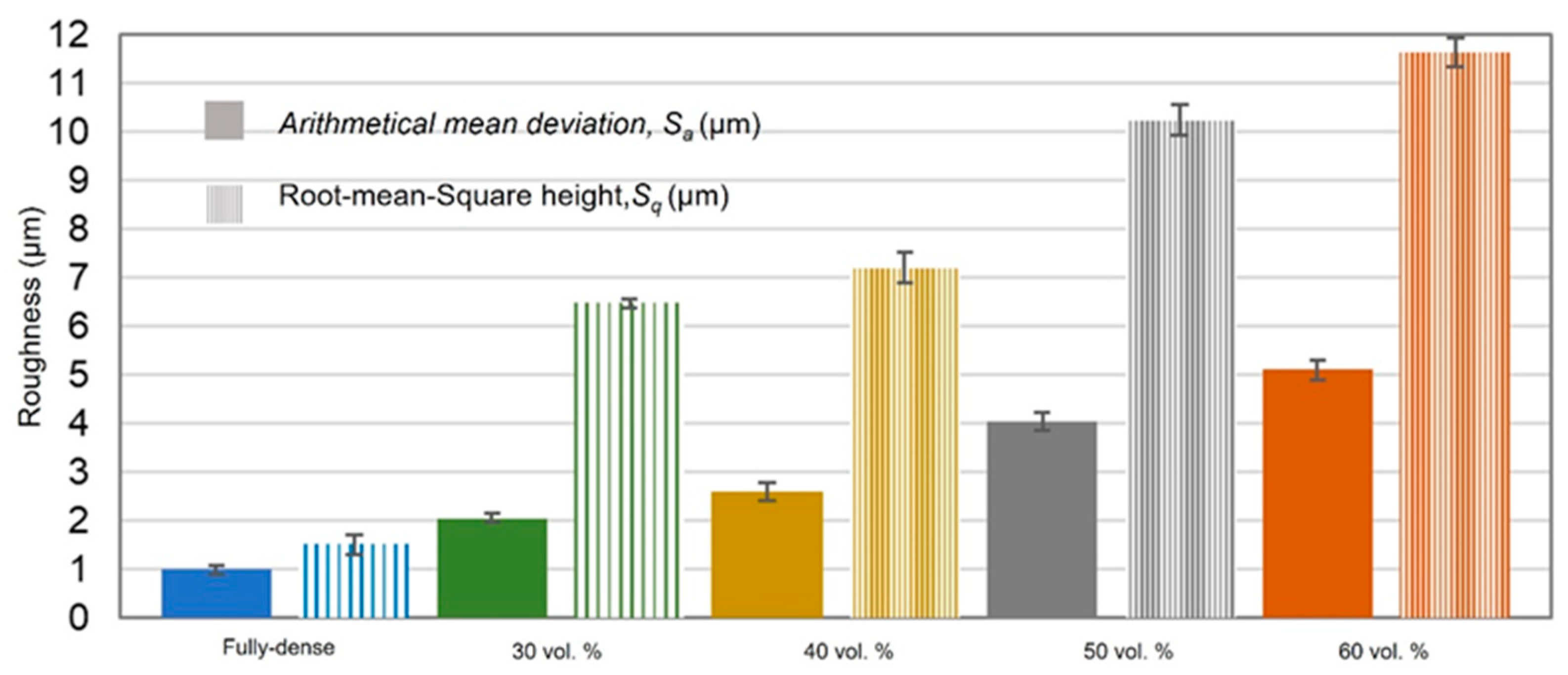
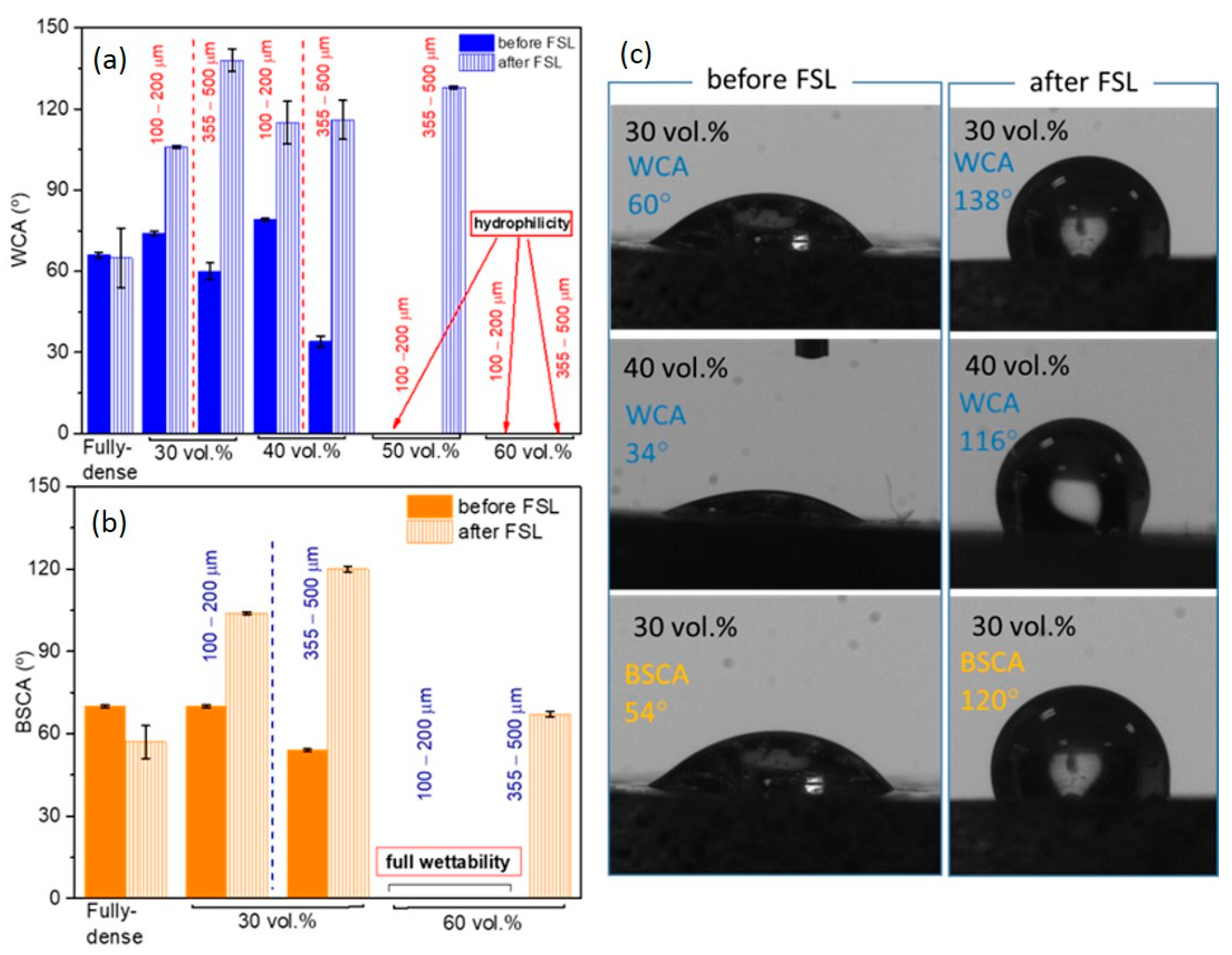
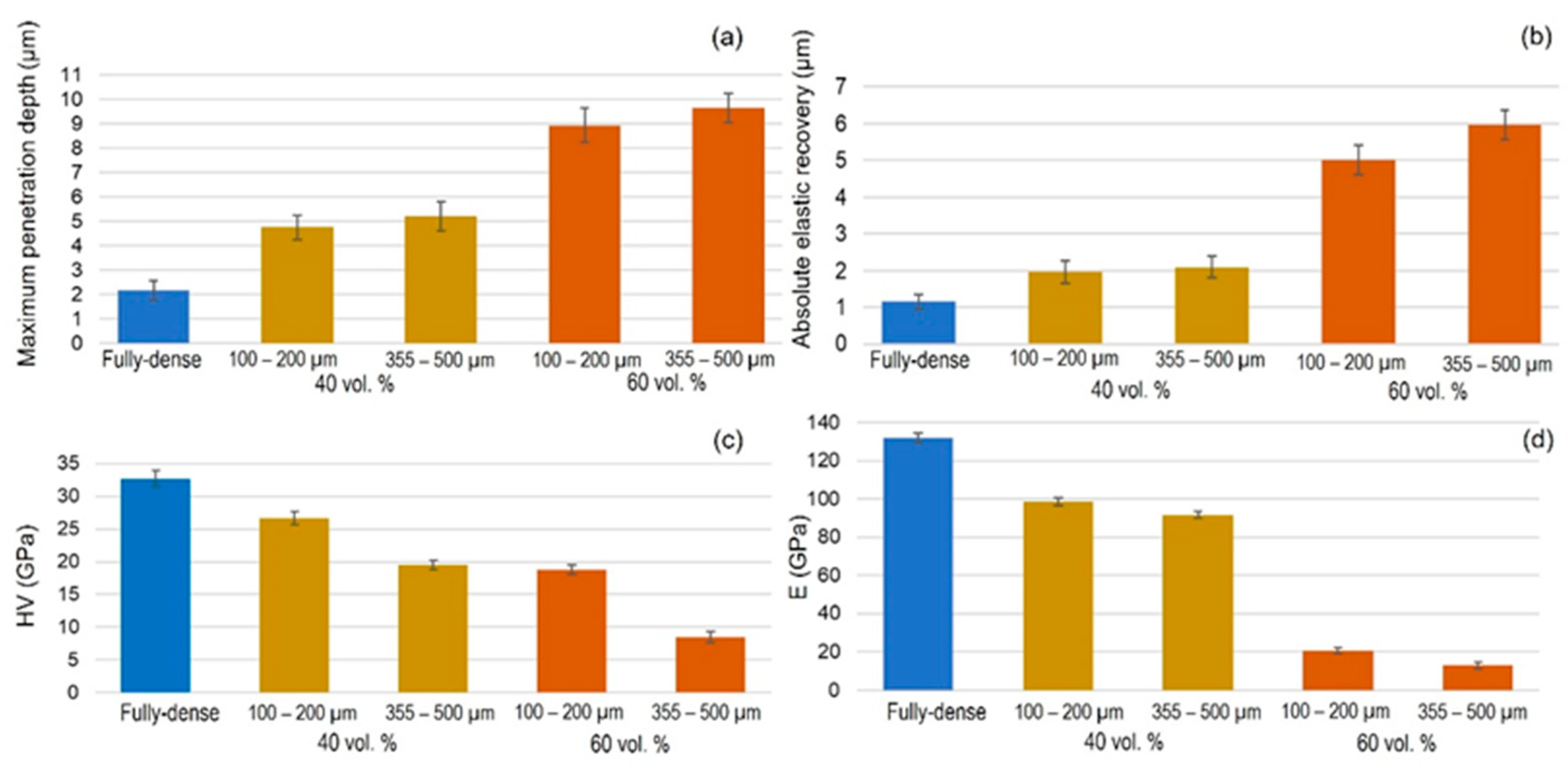
2.2. Tribo-Mechanical Characterization of Modified Substrates
2.3. Cellular Behavior of Modified Surfaces by Femtosecond Laser Texture
2.4. In Vitro Cell Culture Techniques
2.5. Cell Culture
2.6. Cell Viability and Proliferation Assay
2.7. Cell Differentiation by Alkaline Phosphatase (ALP) Evaluation
2.8. Cell Morphology
2.9. Statistical Analysis
3. Results and Discussion
3.1. Cell Viability and Proliferation Study
3.2. Cell Functional Activity
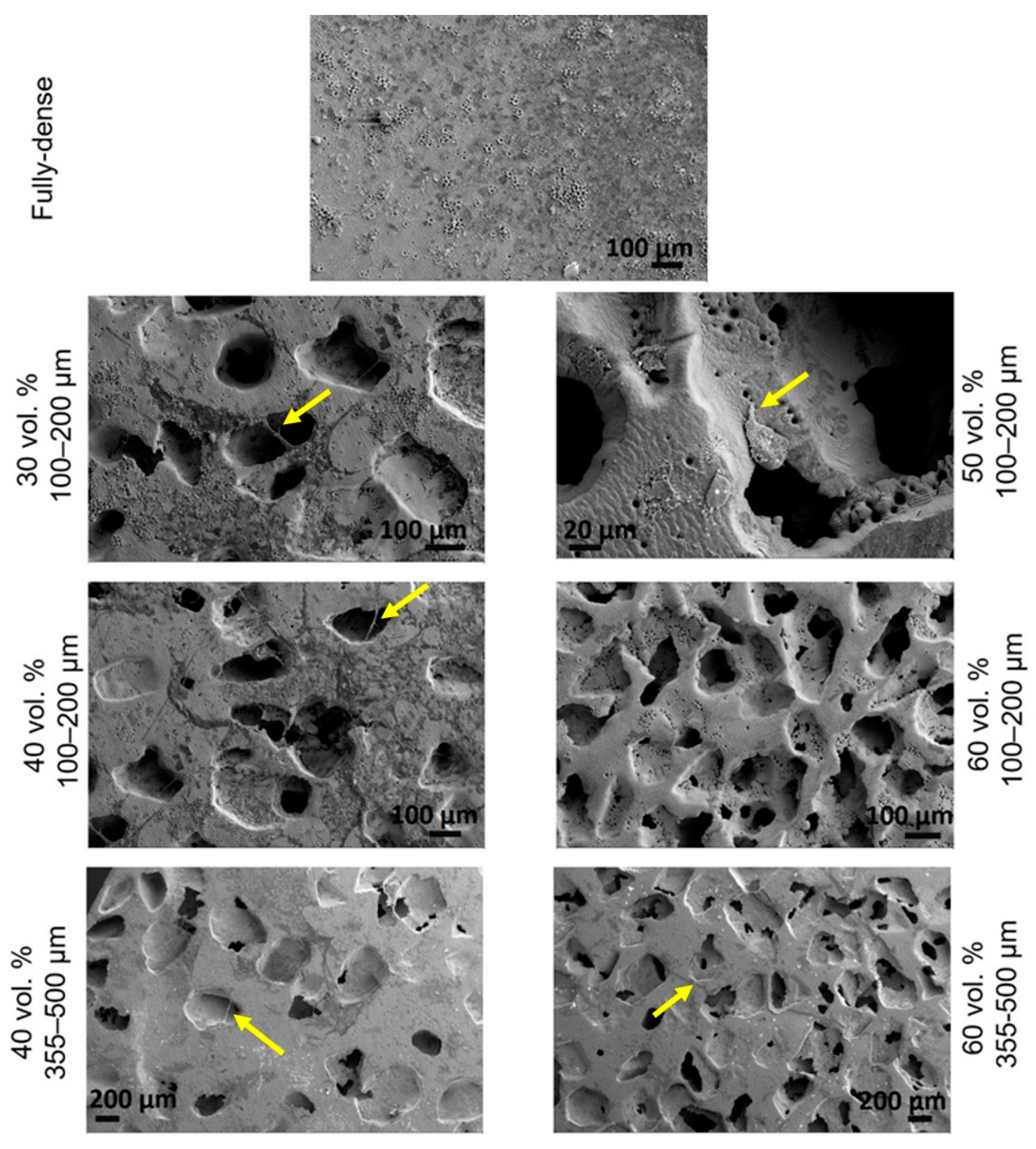
3.3. Morphological Study by SEM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in Biomedical Applications—Properties and Fabrication: A Review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [Green Version]
- Niinomi, M.; Liu, Y.; Nakai, M.; Liu, H.; Li, H. Biomedical titanium alloys with Young’s moduli close to that of cortical bone. Regen. Biomater. 2016, 3, 173–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.-Y.; Cui, Y.-W.; Zhang, L.-C. Recent Development in Beta Titanium Alloys for Biomedical Applications. Metals 2020, 10, 1139. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.S.; Singh, H.; Gepreel, M.A.-H. A review on alloy design, biological response, and strengthening of β-titanium alloys as biomaterials. Mater. Sci. Eng. C 2021, 121, 111661. [Google Scholar] [CrossRef]
- Zhao, B.; Gain, A.K.; Ding, W.; Zhang, L.; Li, X.; Fu, Y. A review on metallic porous materials: Pore formation, mechanical properties, and their applications. Int. J. Adv. Manuf. Technol. 2018, 95, 2641–2659. [Google Scholar] [CrossRef]
- Domínguez-Trujillo, C.; Peón, E.; Chicardi, E.; Pérez, H.; Rodríguez-Ortiz, J.A.; Pavón, J.; García-Couce, J.; Galvan, J.C.; García-Moreno, F.; Torres, Y. Sol-gel deposition of hydroxyapatite coatings on porous titanium for biomedical applications. Surf. Coat. Technol. 2018, 333, 158–162. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Chen, L.-Y. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef] [Green Version]
- Murr, L.E. Strategies for creating living, additively manufactured, open-cellular metal and alloy implants by promoting osseointegration, osteoinduction and vascularization: An overview. J. Mater. Sci. Technol. 2018, 35, 231–241. [Google Scholar] [CrossRef]
- Mavrogenis, A.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet. Neuronal. Interact. 2009, 9, 61–71. [Google Scholar] [PubMed]
- de Vasconcellos, L.M.R.; Carvalho, Y.R.; do Prado, R.F.; de Vasconcellos, L.G.O.; de Alencastro Graça, M.L.; Cairo, C.A.A. Porous titanium by powder metallurgy for biomedical application: Characterization, cell citotoxity and in vivo tests of osseointegration. In Biomedical Engineering: Technical Applications in Medicine; Intech: London, UK, 2012; pp. 47–74. [Google Scholar]
- Devgan, S.; Sidhu, S.S. Evolution of surface modification trends in bone related biomaterials: A review. Mater. Chem. Phys. 2019, 233, 68–78. [Google Scholar] [CrossRef]
- Ibrahim, M.Z.; Sarhan, A.A.; Yusuf, F.; Hamdi, M. Biomedical materials and techniques to improve the tribological, mechanical and biomedical properties of orthopedic implants—A review article. J. Alloys Compd. 2017, 714, 636–667. [Google Scholar] [CrossRef]
- Accioni, F.; Vázquez, J.; Merinero, M.; Begines, B.; Alcudia, A. Latest Trends in Surface Modification for Dental Implantology: Innovative Developments and Analytical Applications. Pharmaceutics 2022, 14, 455. [Google Scholar] [CrossRef]
- Pavón, J.; Galvis, O.; Echeverría, F.; Castaño, J.; Echeverry, M.; Robledo, S.; Jiménez-Piqué, E.; Mestra, A.; Anglada, M. Anodic oxidation of titanium for implants and prosthesis: Processing, characterization and potential improvement of osteointegration. In Proceedings of the V Latin American Congress on Biomedical Engineering CLAIB 2011, Havana, Cuba, 16–21 May 2011; pp. 176–179. [Google Scholar]
- Saji, V.S. Superhydrophobic surfaces and coatings by electrochemical anodic oxidation and plasma electrolytic oxidation. Adv. Colloid Interface Sci. 2020, 283, 102245. [Google Scholar] [CrossRef] [PubMed]
- Echeverry-Rendón, M.; Galvis, O.; Giraldo, D.A.Q.; Pavón-Palacio, J.-J.; López-Lacomba, J.L.; Jimenez-Pique, E.; Anglada, M.; Robledo, S.M.; Castaño, J.G.; Echeverria, F. Osseointegration improvement by plasma electrolytic oxidation of modified titanium alloys surfaces. J. Mater. Sci. Mater. Electron. 2015, 26, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Tsai, D.-S.; Chou, C.-C. Review of the Soft Sparking Issues in Plasma Electrolytic Oxidation. Metals 2018, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Aditya, T.; Allain, J.P.; Jaramillo, C.; Restrepo, A.M. Surface Modification of Bacterial Cellulose for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 610. [Google Scholar] [CrossRef]
- Echeverry-Rendon, M.; Allain, J.P.; Robledo, S.M.; Echeverria, F.; Harmsen, M.C. Coatings for biodegradable magnesium-based supports for therapy of vascular disease: A general view. Mater. Sci. Eng. C 2019, 102, 150–163. [Google Scholar] [CrossRef]
- Mahajan, A.; Sidhu, S.S. Surface modification of metallic biomaterials for enhanced functionality: A review. Mater. Technol. 2018, 33, 93–105. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Wang, L. Surface Modification of Biomedical Titanium Alloy: Micromorphology, Microstructure Evolution and Biomedical Applications. Coatings 2019, 9, 249. [Google Scholar] [CrossRef] [Green Version]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Burgui, S.; Langheinrich, D.; Gil, C.; Solano, C.; Toledo-Arana, A.; Helbig, R.; Lasagni, A.; Lasa, I. Evaluation of surface microtopography engineered by direct laser interference for bacterial anti-biofouling. Macromol. Biosci. 2015, 15, 1060–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera-Costa, D.; Bruque, J.M.; González-Martín, M.L.; Gómez-García, A.C.; Vadillo-Rodríguez, V. Studying the Influence of Surface Topography on Bacterial Adhesion using Spatially Organized Microtopographic Surface Patterns. Langmuir 2014, 30, 4633–4641. [Google Scholar] [CrossRef]
- Li, X.; Chen, T. Enhancement and suppression effects of a nanopatterned surface on bacterial adhesion. Phys. Rev. E 2016, 93, 052419. [Google Scholar] [CrossRef]
- Cunha, A.; Serro, A.P.; Oliveira, V.; Almeida, A.; Vilar, R.; Durrieu, M.-C. Wetting behaviour of femtosecond laser textured Ti–6Al–4V surfaces. Appl. Surf. Sci. 2013, 265, 688–696. [Google Scholar] [CrossRef] [Green Version]
- Vorobyev, A.Y.; Guo, C. Femtosecond laser structuring of titanium implants. Appl. Surf. Sci. 2007, 253, 7272–7280. [Google Scholar] [CrossRef]
- Oliveira, V.; Ausset, S.; Vilar, R. Surface micro/nanostructuring of titanium under stationary and non-stationary femtosecond laser irradiation. Appl. Surf. Sci. 2009, 255, 7556–7560. [Google Scholar] [CrossRef]
- Bonse, J.; Höhm, S.; Koter, R.; Hartelt, M.; Spaltmann, D.; Pentzien, S.; Rosenfeld, A.; Krüger, J. Tribological performance of sub-100-nm femtosecond laser-induced periodic surface structures on titanium. Appl. Surf. Sci. 2016, 374, 190–196. [Google Scholar] [CrossRef]
- Shinonaga, T.; Tsukamoto, M.; Kawa, T.; Chen, P.; Nagai, A.; Hanawa, T. Formation of periodic nanostructures using a femtosecond laser to control cell spreading on titanium. Appl. Phys. A 2015, 119, 493–496. [Google Scholar] [CrossRef]
- Schnell, G.; Duenow, U.; Seitz, H. Effect of Laser Pulse Overlap and Scanning Line Overlap on Femtosecond Laser-Structured Ti6Al4V Surfaces. Materials 2020, 13, 969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menci, G.; Demir, A.G.; Waugh, D.; Lawrence, J.; Previtali, B. Laser surface texturing of β-Ti alloy for orthopaedics: Effect of different wavelengths and pulse durations. Appl. Surf. Sci. 2019, 489, 175–186. [Google Scholar] [CrossRef]
- Schweitzer, L.; Cunha, A.; Pereira, T.; Mika, K.; Rego, A.M.B.D.; Ferraria, A.M.; Kieburg, H.; Geissler, S.; Uhlmann, E.; Schoon, J. Preclinical In Vitro Assessment of Submicron-Scale Laser Surface Texturing on Ti6Al4V. Materials 2020, 13, 5342. [Google Scholar] [CrossRef]
- Klos, A.; Sedao, X.; Itina, T.E.; Helfenstein-Didier, C.; Donnet, C.; Peyroche, S.; Vico, L.; Guignandon, A.; Dumas, V. Ultrafast Laser Processing of Nanostructured Patterns for the Control of Cell Adhesion and Migration on Titanium Alloy. Nanomaterials 2020, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Calderon, M.; Martín-Palma, R.J.; Rodríguez, A.; Gómez-Aranzadi, M.; García-Ruiz, J.P.; Olaizola, S.M.; Manso-Silván, M. Biomimetic hierarchical micro/nano texturing of TiAlV alloys by femtosecond laser processing for the control of cell adhesion and migration. Phys. Rev. Mater. 2020, 4, 056008. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.; Yang, L.; Shi, Z.; Yang, P.; Cheng, G. In Vitro Bioactivity and Biocompatibility of Bio-Inspired Ti-6Al-4V Alloy Surfaces Modified by Combined Laser Micro/Nano Structuring. Molecules 2020, 25, 1494. [Google Scholar] [CrossRef] [Green Version]
- Dumas, V.; Guignandon, A.; Vico, L.; Mauclair, C.; Zapata, X.; Linossier, M.T.; Bouleftour, W.; Granier, J.; Peyroche, S.; Dumas, J.-C.; et al. Femtosecond laser nano/micro patterning of titanium influences mesenchymal stem cell adhesion and commitment. Biomed. Mater. 2015, 10, 055002. [Google Scholar] [CrossRef]
- Liang, C.; Wang, H.; Yang, J.; Cai, Y.; Hu, X.; Yang, Y.; Li, B.; Li, H.; Li, H.; Li, C.; et al. Femtosecond Laser-Induced Micropattern and Ca/P Deposition on Ti Implant Surface and Its Acceleration on Early Osseointegration. ACS Appl. Mater. Interfaces 2013, 5, 8179–8186. [Google Scholar] [CrossRef]
- Wang, C.; Hu, H.; Li, Z.; Shen, Y.; Xu, Y.; Zhang, G.; Zeng, X.; Deng, J.; Zhao, S.; Ren, T.; et al. Enhanced Osseointegration of Titanium Alloy Implants with Laser Microgrooved Surfaces and Graphene Oxide Coating. ACS Appl. Mater. Interfaces 2019, 11, 39470–39483. [Google Scholar] [CrossRef]
- Webb, H.; Crawford, R.; Ivanova, E.P. Wettability of natural superhydrophobic surfaces. Adv. Colloid Interface Sci. 2014, 210, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Raimbault, O.; Benayoun, S.; Anselme, K.; Mauclair, C.; Bourgade, T.; Kietzig, A.-M.; Girard-Lauriault, P.-L.; Valette, S.; Donnet, C. The effects of femtosecond laser-textured Ti-6Al-4V on wettability and cell response. Mater. Sci. Eng. C 2016, 69, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rui, Z.; Cheng, W.; Song, L.; Xu, Y.; Li, R.; Zhang, X. Characterization and evaluation of a femtosecond laser-induced osseointegration and an anti-inflammatory structure generated on a titanium alloy. Regen. Biomater. 2021, 8, rbab006. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.; Elie, A.-M.; Plawinski, L.; Serro, A.; Rego, A.M.B.D.; Almeida, A.; Urdaci, M.C.; Durrieu, M.-C.; Vilar, R. Femtosecond laser surface texturing of titanium as a method to reduce the adhesion of Staphylococcus aureus and biofilm formation. Appl. Surf. Sci. 2016, 360, 485–493. [Google Scholar] [CrossRef]
- Rodríguez, Á.; Trueba, P.; Amado, J.M.; Tobar, M.J.; Giner, M.; Amigó, V.; Torres, Y. Surface Modification of Porous Titanium Discs Using Femtosecond Laser Structuring. Metals 2020, 10, 748. [Google Scholar] [CrossRef]
- ASTM F67-00, Standard Specification for Unalloyed Titanium for Surgical Implant Applications (UNS R50250, UNS R50400, UNS R50550, UNS R50700); ASTM: West Conshohocken, PA, USA, 2002.
- Torres, Y.; Trueba, P.; Pavón-Palacio, J.-J.; Montealegre-Melendez, I.; Rodríguez-Ortiz, J.A. Designing, processing and characterisation of titanium cylinders with graded porosity: An alternative to stress-shielding solutions. Mater. Des. 2014, 63, 316–324. [Google Scholar] [CrossRef]
- Lascano, S.; Arévalo, C.; Montealegre-Melendez, I.; Muñoz, S.; Rodriguez-Ortiz, J.A.; Trueba, P.; Torres, Y. Porous Titanium for Biomedical Applications: Evaluation of the Conventional Powder Metallurgy Frontier and Space-Holder Technique. Appl. Sci. 2019, 9, 982. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Brinson, L. Finite element modeling of porous titanium. Int. J. Solids Struct. 2007, 44, 320–335. [Google Scholar] [CrossRef] [Green Version]
- Imwinkelried, T. Mechanical properties of open-pore titanium foam. J. Biomed. Mater. Res. Part A 2007, 81, 964–970. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Hasan, J.; Crawford, R.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gavilan, A.; de Castro, J.V.; Arana, A.; Merino, S.; Retolaza, A.; Alves, S.A.; Francone, A.; Kehagias, N.; Sotomayor-Torres, C.M.; Cocina, D.; et al. Antibacterial activity testing methods for hydrophobic patterned surfaces. Sci. Rep. 2021, 11, 6675. [Google Scholar] [CrossRef] [PubMed]
- Neoh, K.G.; Hu, X.; Zheng, D.; Kang, E.-T. Balancing osteoblast functions and bacterial adhesion on functionalized titanium surfaces. Biomaterials 2012, 33, 2813–2822. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Wang, H.; Wang, W.; Tong, L.; Pan, H.; Ruan, C.; Ma, Q.; Liu, M.; Yang, H.; Zhang, L.; et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials 2014, 35, 4255–4265. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Qian, S.; Guo, G.; Wang, Q.; Tang, J.; Shen, H.; Liu, X.; Zhang, X.; Chu, P. Antibacterial Surface Design of Titanium-Based Biomaterials for Enhanced Bacteria-Killing and Cell-Assisting Functions Against Periprosthetic Joint Infection. ACS Appl. Mater. Interfaces 2016, 8, 11162–11178. [Google Scholar] [CrossRef]
- Chen, P.; Miyake, M.; Tsukamoto, M.; Tsutsumi, Y.; Hanawa, T. Response of preosteoblasts to titanium with periodic micro/nanometer scale grooves produced by femtosecond laser irradiation. J. Biomed. Mater. Res. Part A 2017, 105, 3456–3464. [Google Scholar] [CrossRef]
- Civantos, A.; Giner, M.; Trueba, P.; Lascano, S.; Montoya-García, M.-J.; Arévalo, C.; Vázquez, M.; Allain, J.P.; Torres, Y. In Vitro Bone Cell Behavior on Porous Titanium Samples: Influence of Porosity by Loose Sintering and Space Holder Techniques. Metals 2020, 10, 696. [Google Scholar] [CrossRef]
- Wo, J.; Huang, S.-S.; Wu, D.-Y.; Zhu, J.; Li, Z.-Z.; Yuan, F. The integration of pore size and porosity distribution on Ti-6A1-4V scaffolds by 3D printing in the modulation of osteo-differentation. J. Appl. Biomater. Funct. Mater. 2020, 18. [Google Scholar] [CrossRef]
- Van den Beucken, J.J.J.P.; Walboomers, X.F.; Boerman, O.C.; Vos, M.R.J.; Sommerdijk, N.A.J.M.; Hayakawa, T.; Fukushima, T.; Okahata, Y.; Nolte, R.J.M.; Jansen, J.A. Functionalization of multilayered DNA-coatings with bone morphogenetic protein. J. Control Release 2006, 113, 63–72. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, X.; Zhang, Y.; Yuan, F. Osteoblast differentiation of bone marrow stromal cells by femtosecond laser bone ablation. Biomed. Opt. Express 2020, 11, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-J.; Kim, S.-N.; Cho, S.-A. Comparison of alkaline phosphatase activity of MC3T3-E1 cells cultured on different Ti surfaces: Modified sandblasted with large grit and acid-etched (MSLA), laser-treated, and laser and acid-treated Ti surfaces. J. Adv. Prosthodont. 2016, 8, 235–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, A.; Thibeaux, R.; Toptan, F.; Pinto, A.; Ponthiaux, P.; David, B. Influence of macroporosity on NIH/3T3 adhesion, proliferation, and osteogenic differentiation of MC3T3-E1 over bio-functionalized highly porous titanium implant material. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 73–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]



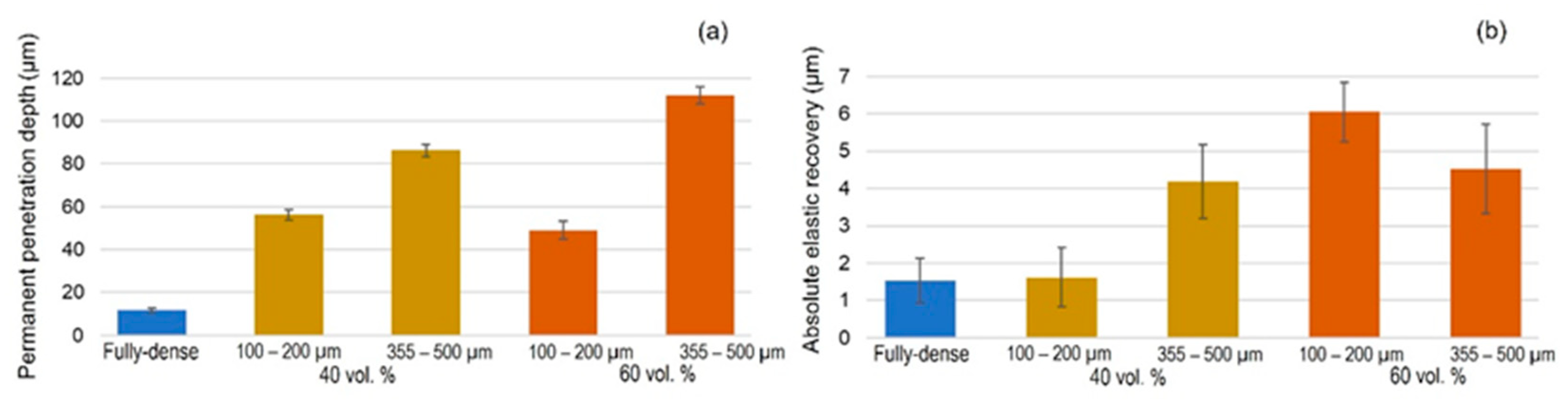
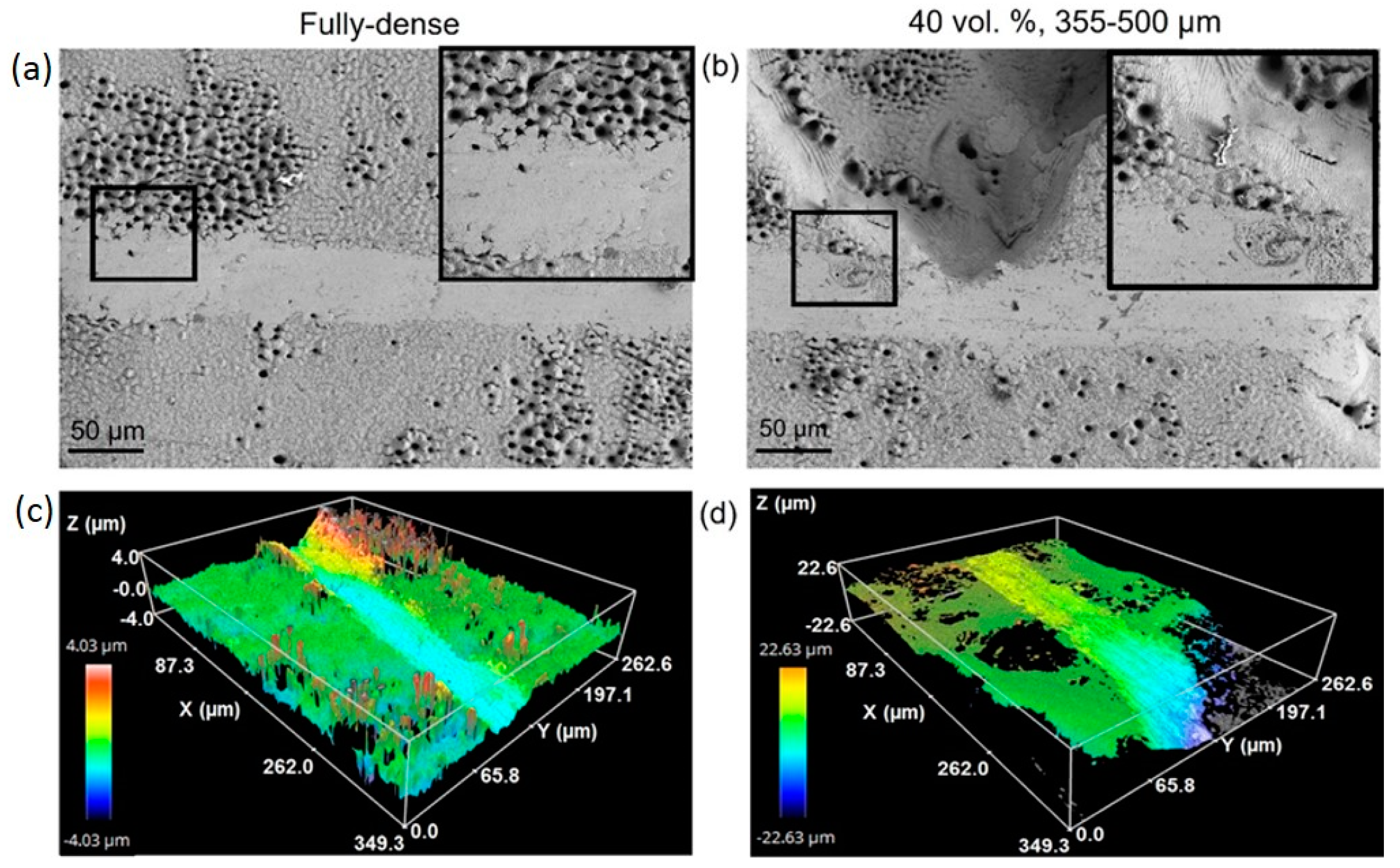
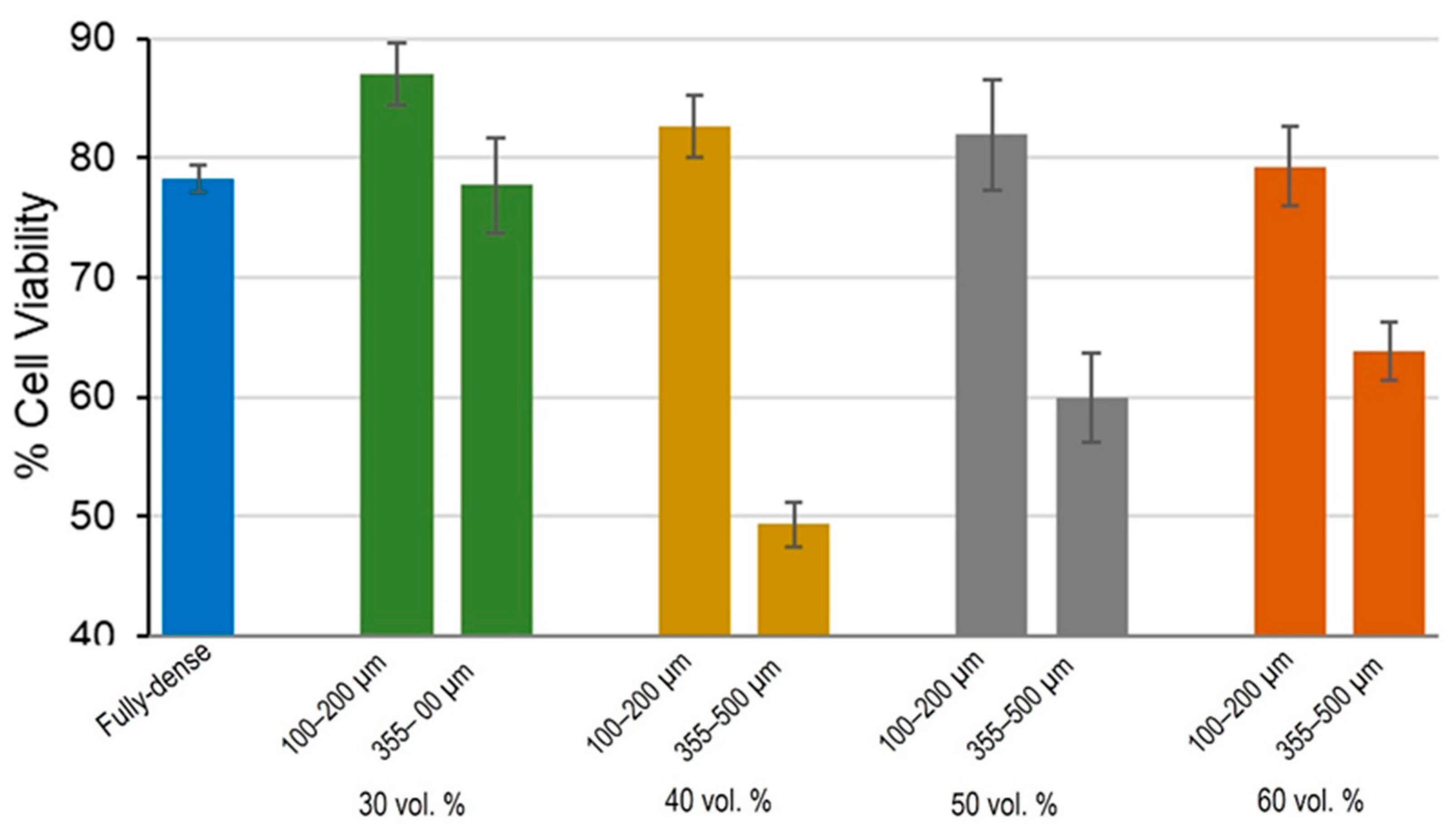
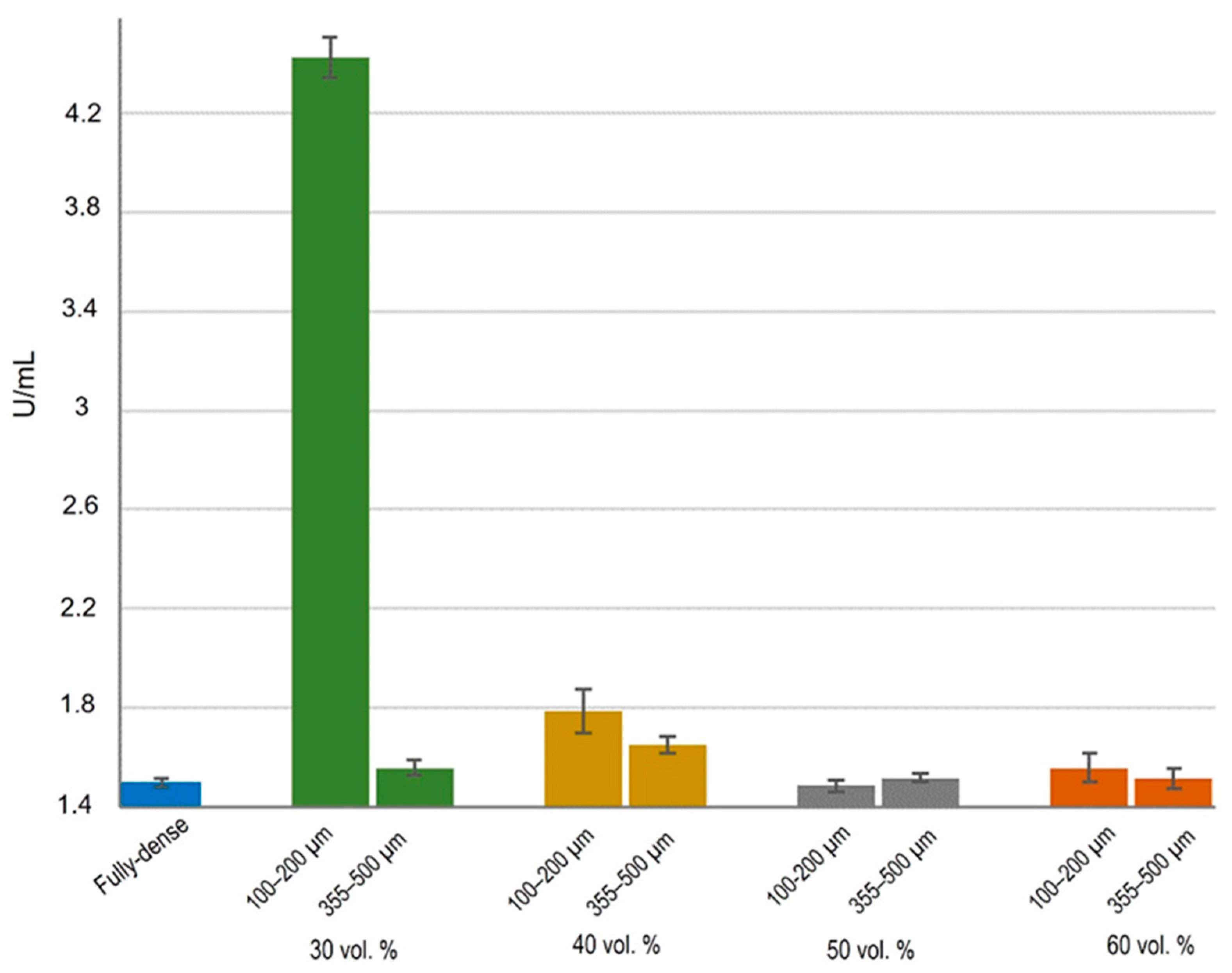
| Discs Tested | Before FSL | After FSL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PT (%) | Deq (μm) | Ed (GPa) | σy (MPa) * | PT (%) | Deq (μm) | Ed (GPa) ** | ||||
| Conventional PM | 1.6 ± 0.3 | 3.9 ± 0.2 | 103.2 ± 1.3 | 739 ± 6 | 25.6 ± 0.9 | 6 ± 1.2 | 62.6 ± 1.2 | |||
| Space-holder technique | 30 vol.% | 100–200 μm | 30.2 ± 0.2 | 192 ± 117 | 56.8 ± 0.7 | 354 ± 26 | PF *** | 9.8 ± 0.7 | 10 ± 3 | 50.2 ± 1.5 |
| PSH | 27.2 ± 0.9 | 111 ± 2 | ||||||||
| 355–500 μm | 30.1 ± 0.1 | 335 ± 40 | 57.0 ± 1.4 | 341 ± 37 | PF *** | 9.4 ± 0.8 | 11 ± 3 | 44.6 ± 1.1 | ||
| PSH | 32.2 ± 0.9 | 250 ± 5 | ||||||||
| 40 vol.% | 100–200 μm | 40.2 ± 1.1 | 226 ± 178 | 45.9 ± 1.2 | 234 ± 28 | PF *** | 6.7 ± 1.1 | 11 ± 3 | 35.7 ± 1.0 | |
| PSH | 45.4 ± 0.6 | 138 ± 3 | ||||||||
| 355–500 μm | 40.8 ± 1.3 | 359 ± 123 | 45.3 ± 1.1 | 206 ± 26 | PF *** | 6.4 ± 0.9 | 12 ± 3 | 39.7 ± 1.2 | ||
| PSH | 40.3 ± 1.2 | 322 ± 4 | ||||||||
| 50 vol.% | 100–200 μm | 52.3 ± 1.2 | 164 ± 28 | 35.4 ± 1.9 | 95 ± 30 | PF *** | 5.8 ± 1.5 | 12 ± 4 | 30.0 ± 1.0 | |
| PSH | 56.3 ± 1.1 | 197 ± 3 | ||||||||
| 355–500 μm | 50.1 ± 1.0 | 365 ± 34 | 37.1 ± 1.6 | 118 ± 22 | PF *** | 3.8 ± 1.3 | 11 ± 4 | 35.4 ± 1.4 | ||
| PSH | 46.5 ± 1.4 | 340 ± 5 | ||||||||
| 60 vol.% | 100–200 μm | 56.4 ± 0.5 | 189 ± 105 | 32.3 ± 1.5 | 91 ± 27 | PF *** | 2.7 ± 1.0 | 9 ± 2 | 26.5 ± 1.4 | |
| PSH | 63.6 ± 0.9 | 205 ± 4 | ||||||||
| 355–500 μm | 57.8 ± 0.6 | 395 ± 131 | 31.3 ± 1.6 | 84 ± 31 | PF *** | 2.5 ± 1.3 | 8 ± 2 | 25.4 ± 1.6 | ||
| PSH | 64.5 ± 1.5 | 325 ± 2 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán, A.M.; Giner, M.; Rodríguez, Á.; Trueba, P.; Rodríguez-Albelo, L.M.; Vázquez-Gámez, M.A.; Godinho, V.; Alcudia, A.; Amado, J.M.; López-Santos, C.; et al. Influence of Femtosecond Laser Modification on Biomechanical and Biofunctional Behavior of Porous Titanium Substrates. Materials 2022, 15, 2969. https://doi.org/10.3390/ma15092969
Beltrán AM, Giner M, Rodríguez Á, Trueba P, Rodríguez-Albelo LM, Vázquez-Gámez MA, Godinho V, Alcudia A, Amado JM, López-Santos C, et al. Influence of Femtosecond Laser Modification on Biomechanical and Biofunctional Behavior of Porous Titanium Substrates. Materials. 2022; 15(9):2969. https://doi.org/10.3390/ma15092969
Chicago/Turabian StyleBeltrán, Ana M., Mercè Giner, Ángel Rodríguez, Paloma Trueba, Luisa M. Rodríguez-Albelo, Maria Angeles Vázquez-Gámez, Vanda Godinho, Ana Alcudia, José M. Amado, Carmen López-Santos, and et al. 2022. "Influence of Femtosecond Laser Modification on Biomechanical and Biofunctional Behavior of Porous Titanium Substrates" Materials 15, no. 9: 2969. https://doi.org/10.3390/ma15092969






