Microstructure of CEM II/B-S Pastes Modified with Set Accelerating Admixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Types of Cements
2.2. Accelerating Admixtures
- 20% sodium hydroxide (NaOH) solution. The solution was introduced to the batch water to obtain 5% NaOH according to cement’s mass.
- 20% calcium nitrate tetrahydrate (Ca(NO3)2·4H2O). The solution was added to reach 2% of calcium nitrate for the cement’s mass (symbol CN).
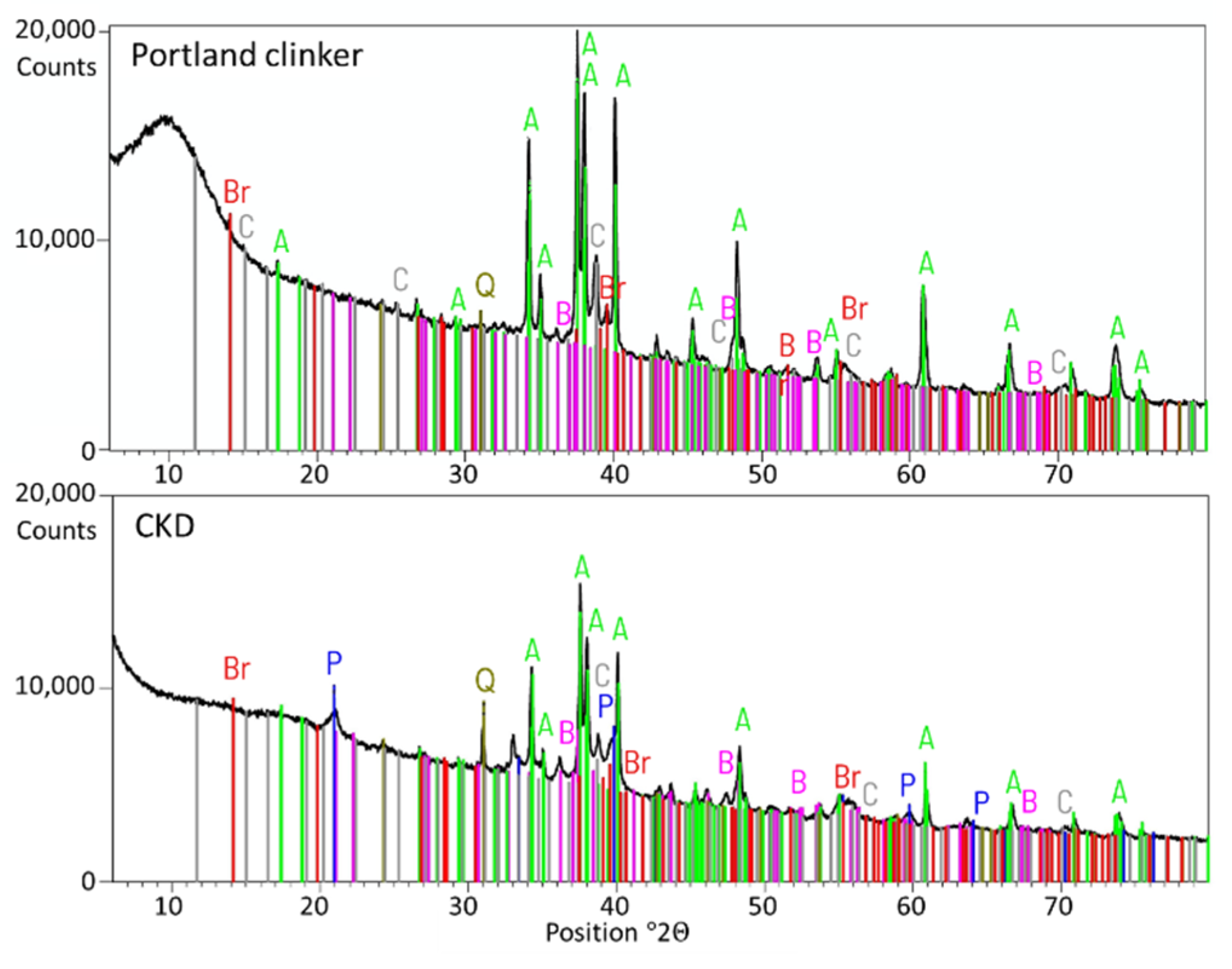
- Cement kiln dust (CKD)—obtained from one of the polish cement plants. CKD was added as an ingredient of the cement. The amount was established as 5% of total cement’s mass. The chemical composition of CKD is given in Table 2. The X-ray diffraction (XRD) analysis was conducted for CKD and Portland clinker from which manufacture the CKD was derived. The results are given in Figure 2. The similarity of constituents described in the Introduction section is visible.
- Available on market modern setting and hardening accelerating admixture containing crystal seeds in form of C-S-H nanoparticles (symbol CS).
2.3. Methods
3. Results and Discussion
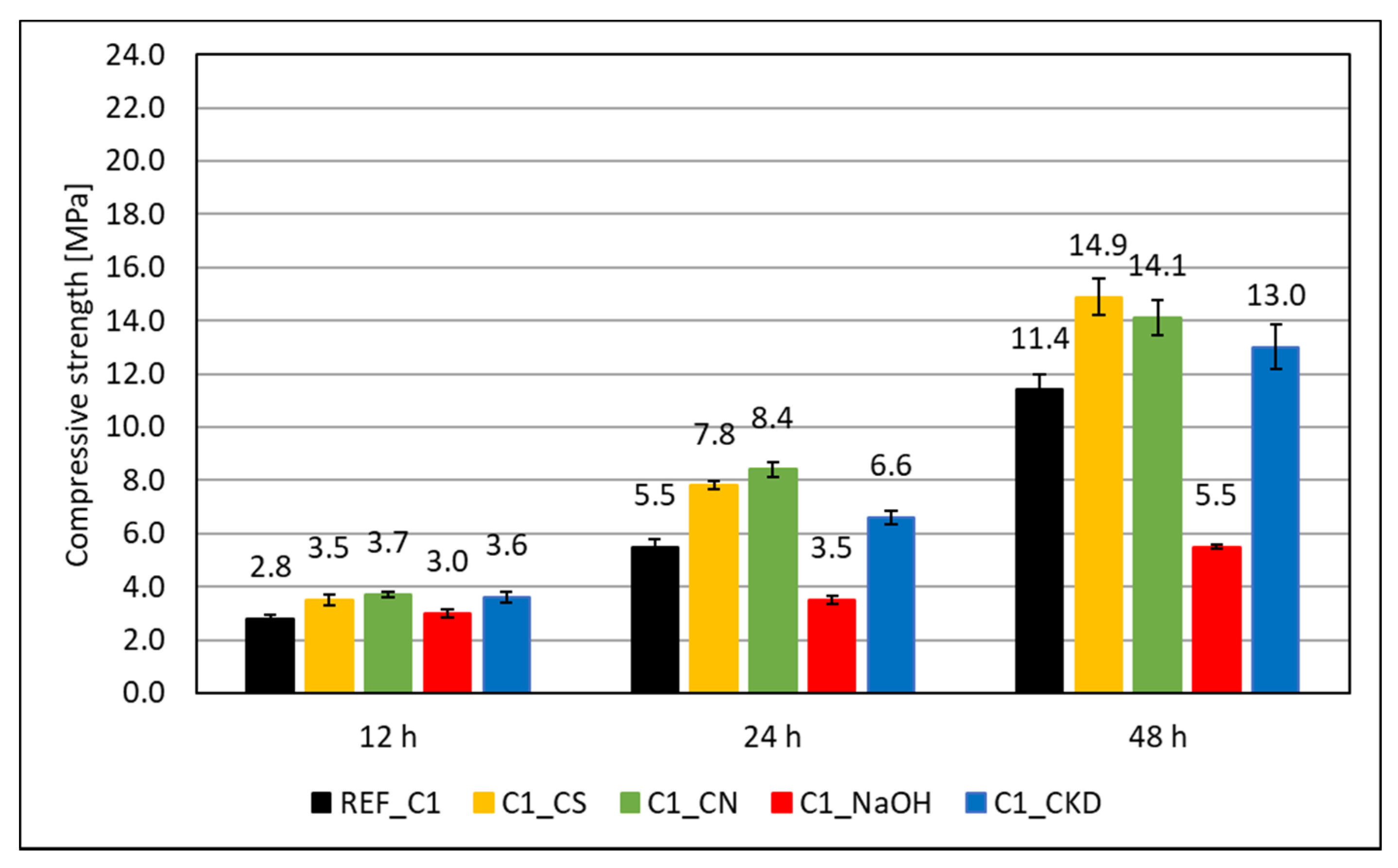
3.1. Compressive Strength
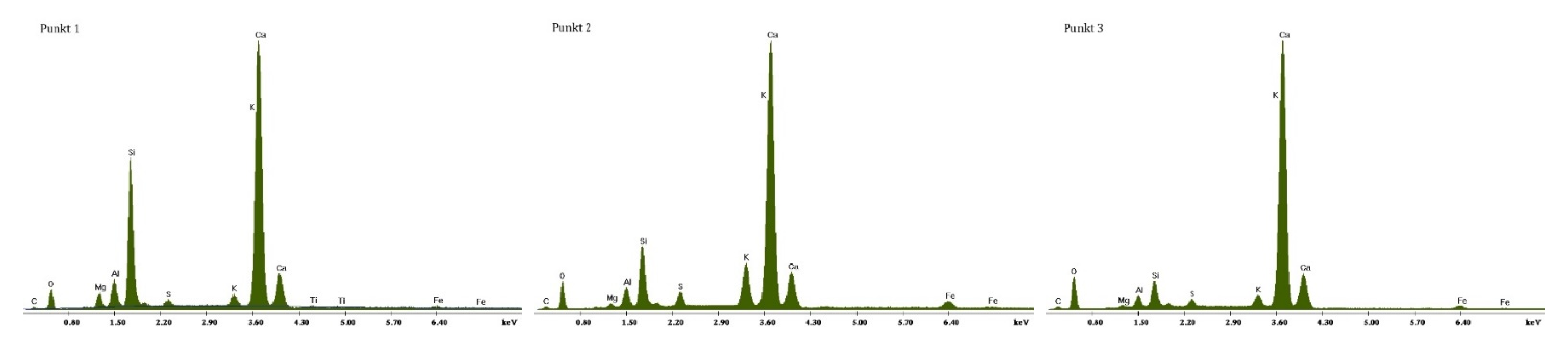
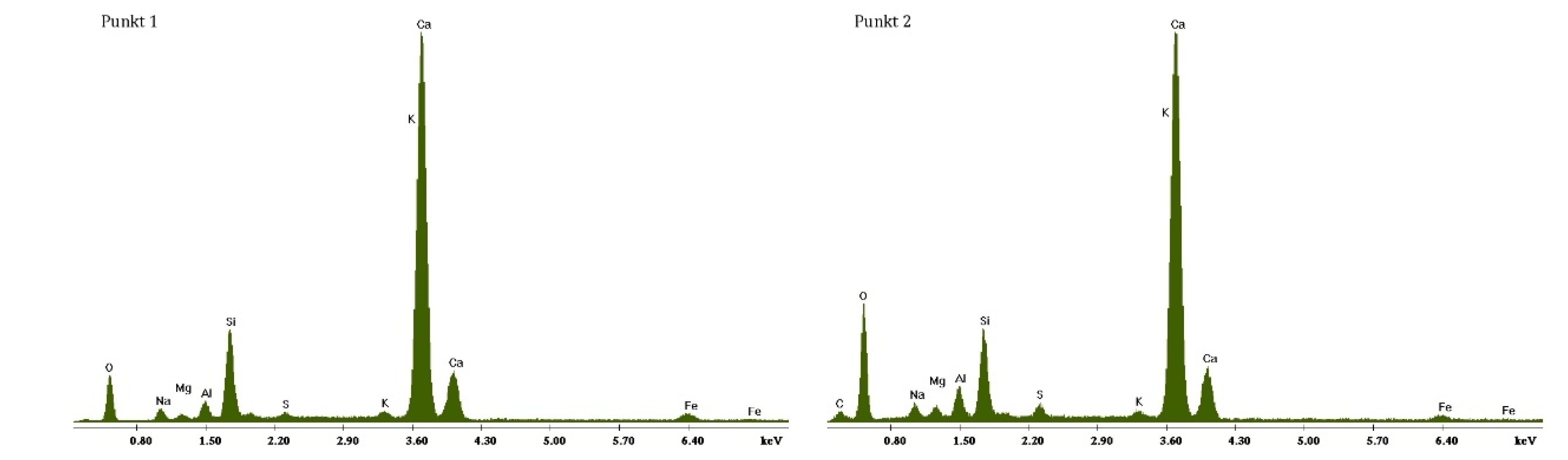
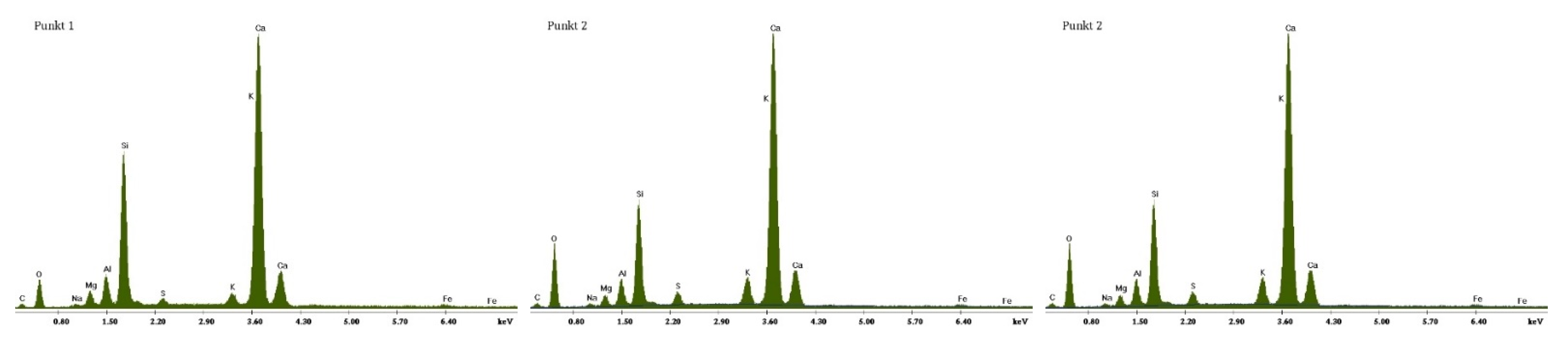
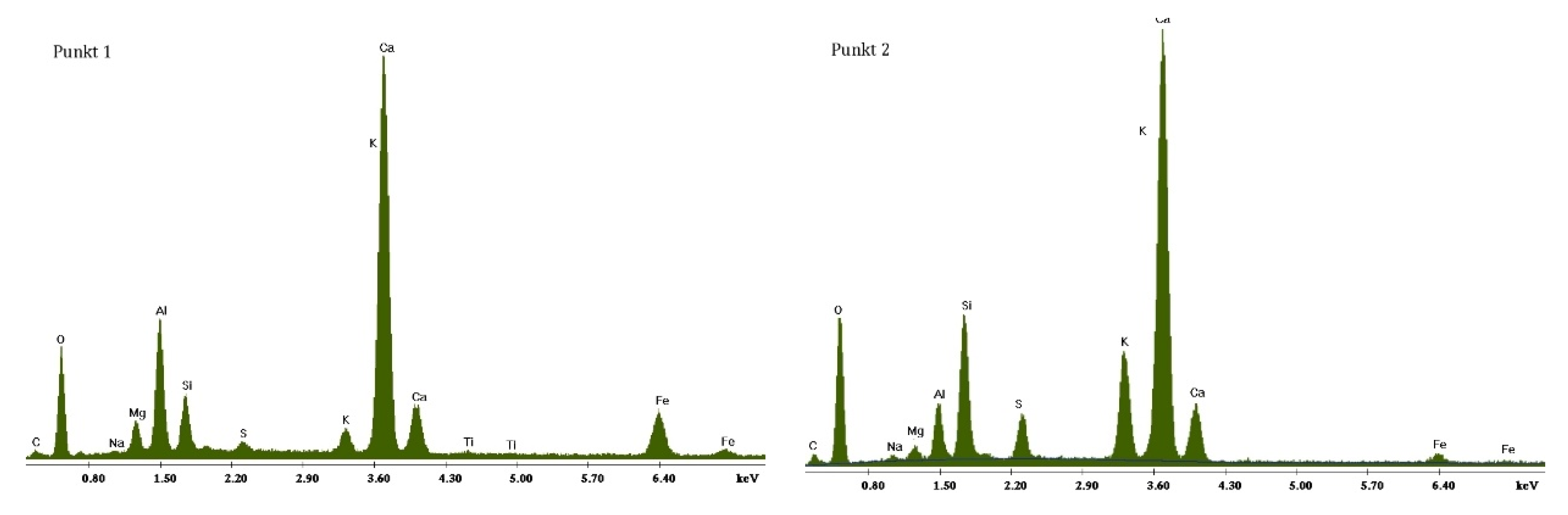
3.2. Scanning Electron Microscopy and Energy Dispersive Spectroscopy
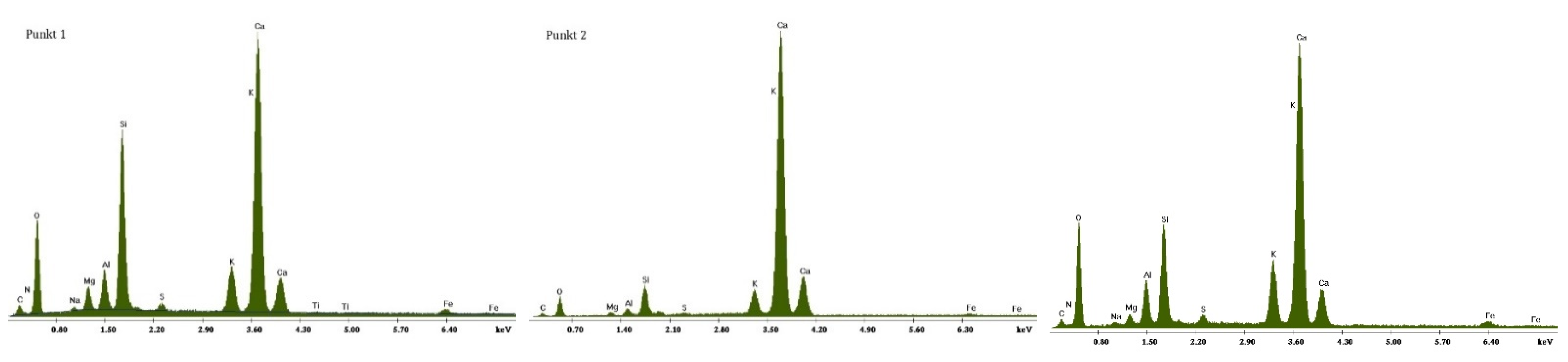
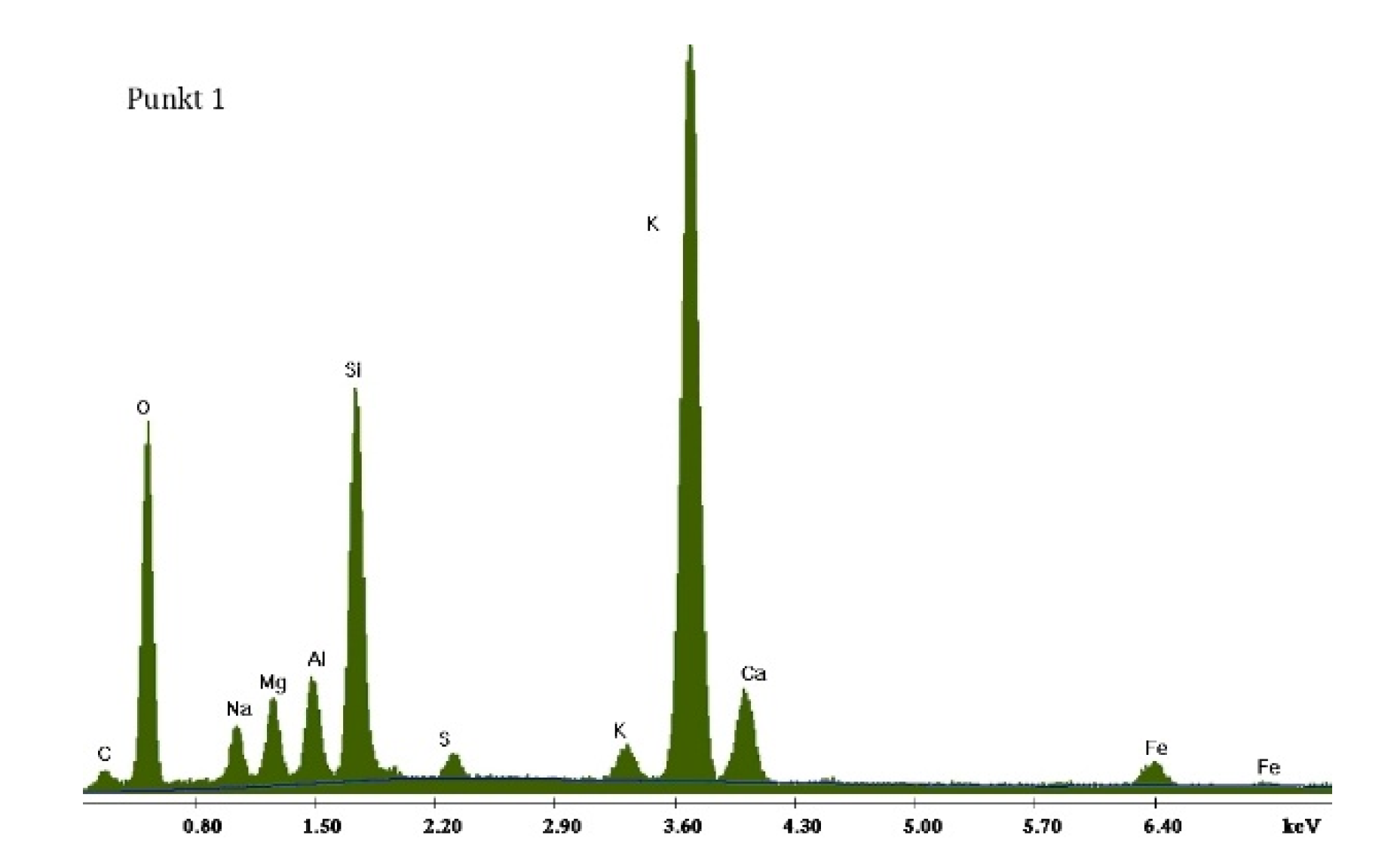
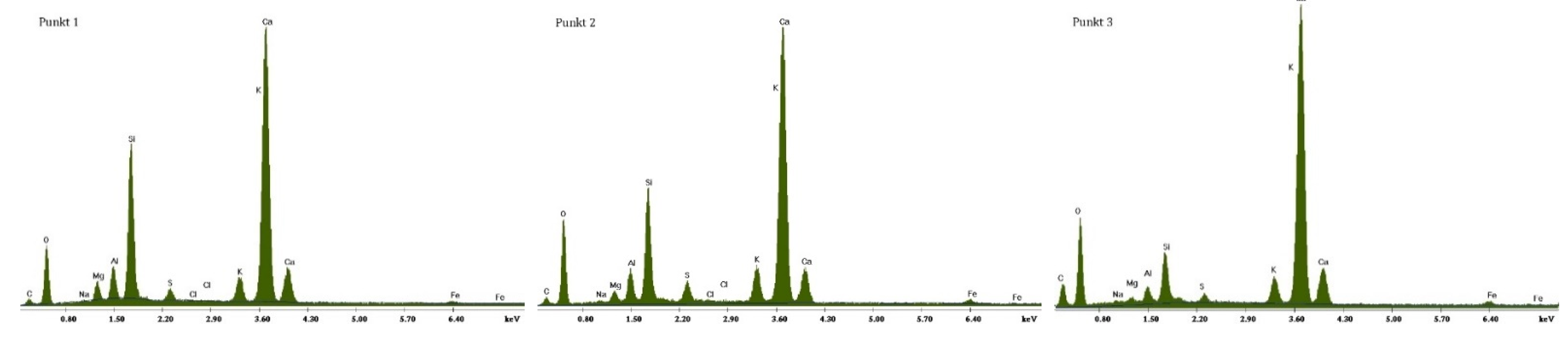
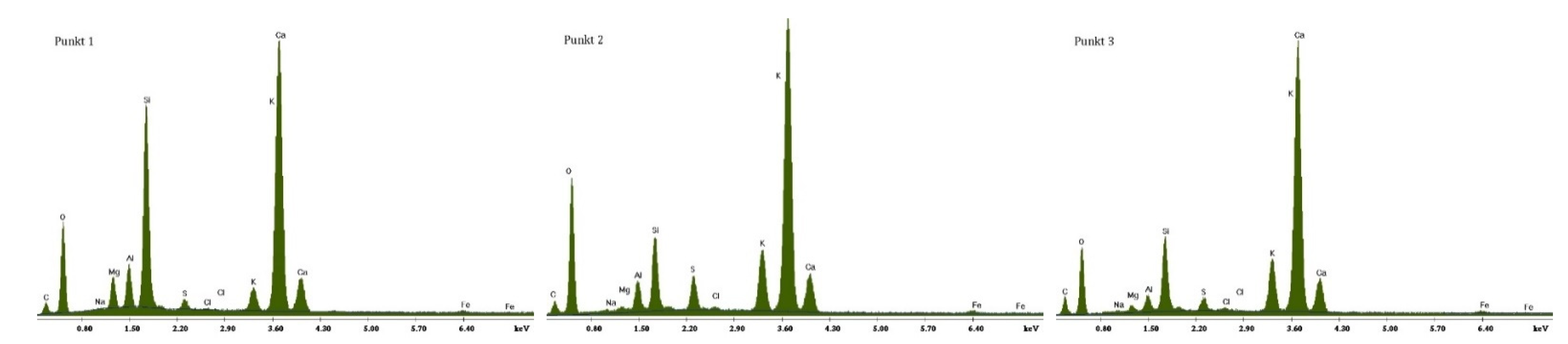
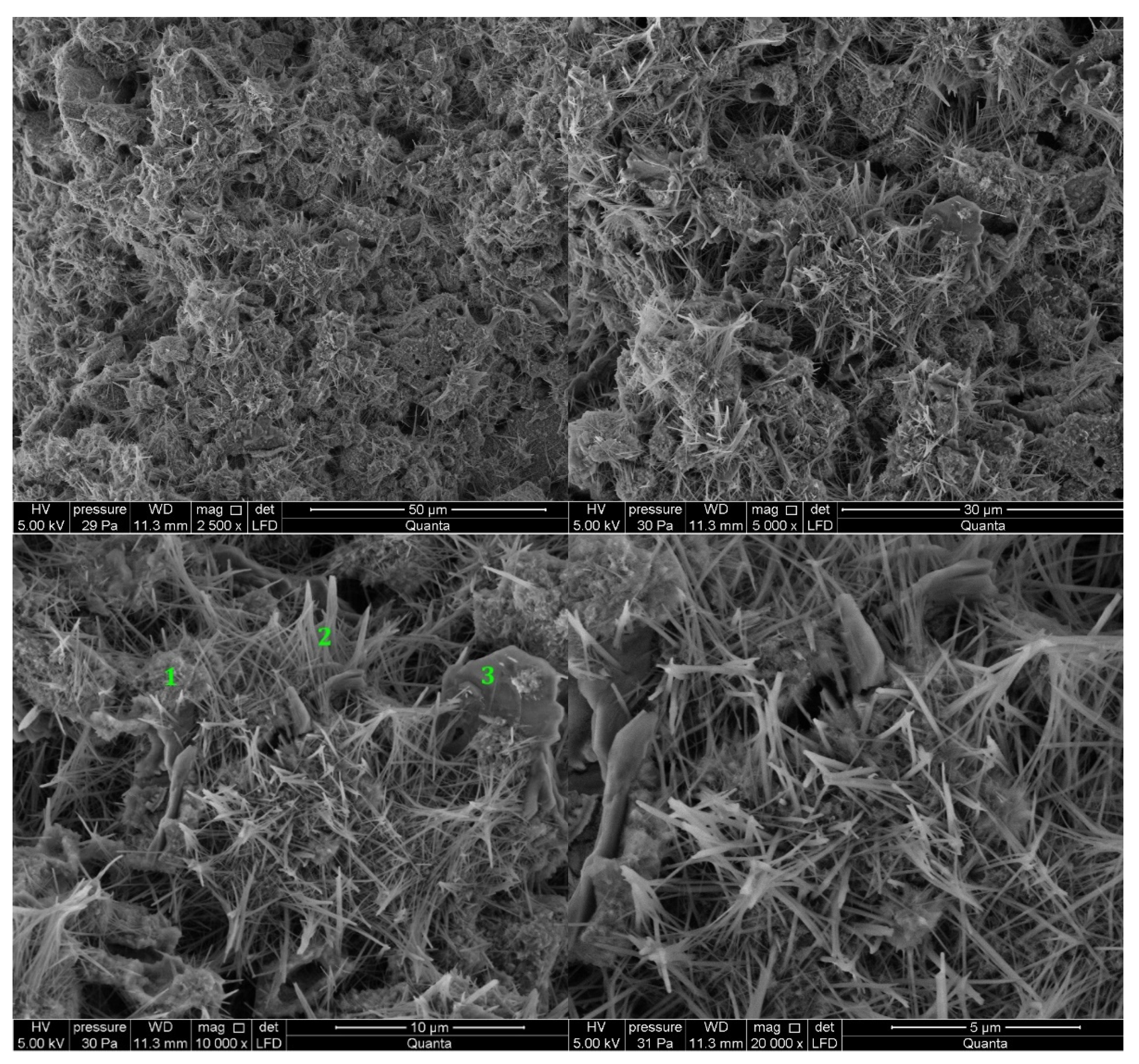
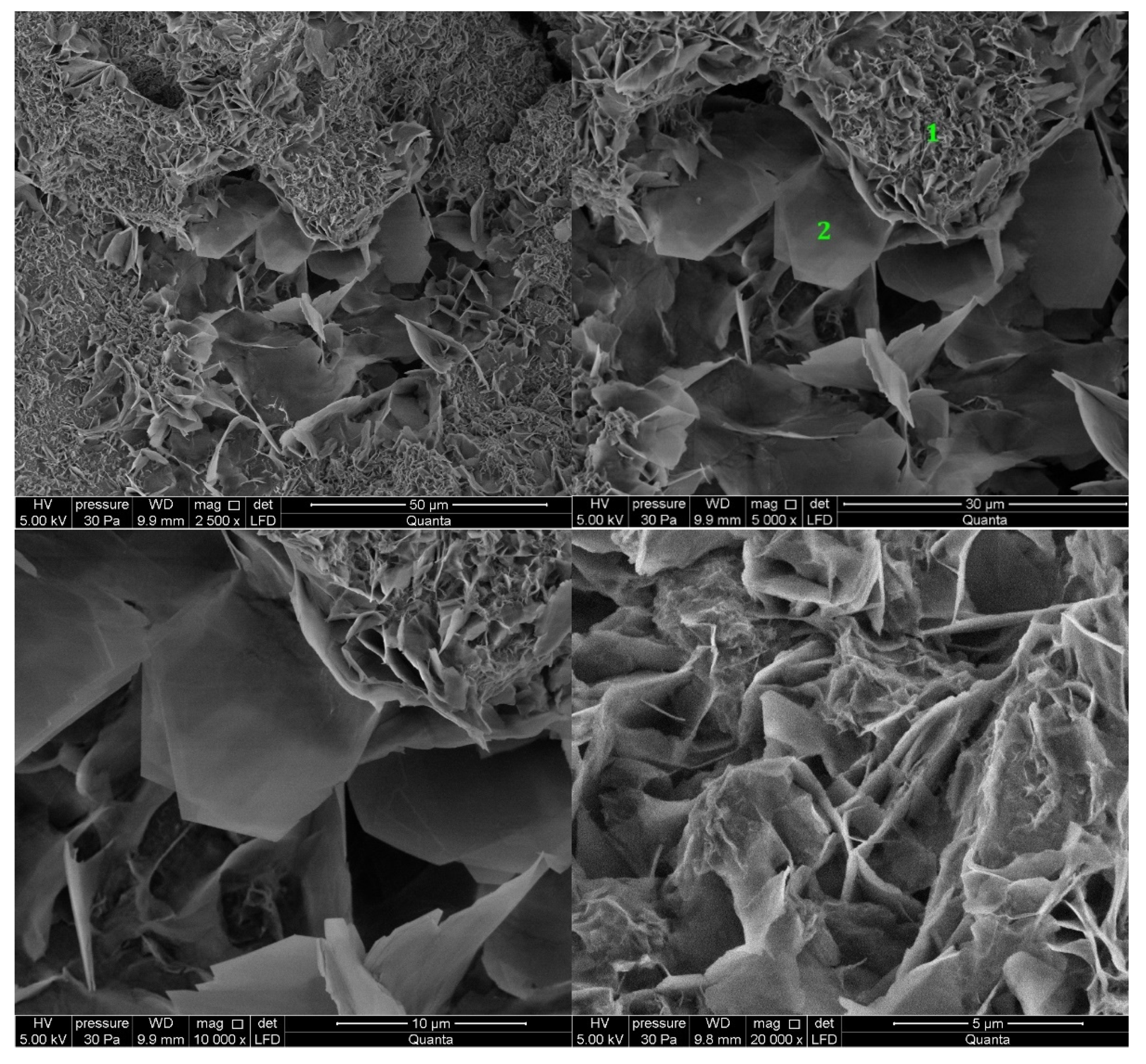
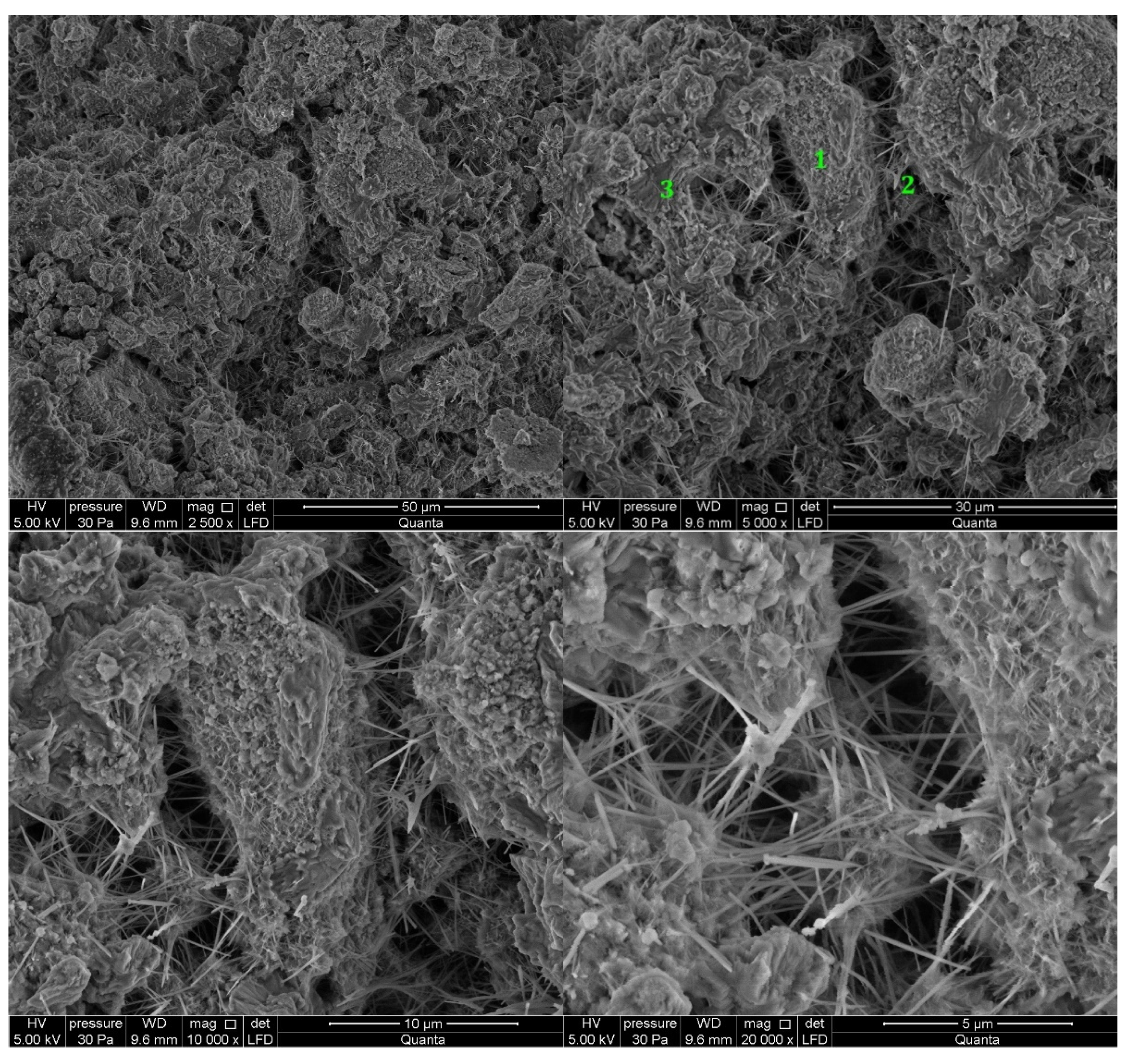
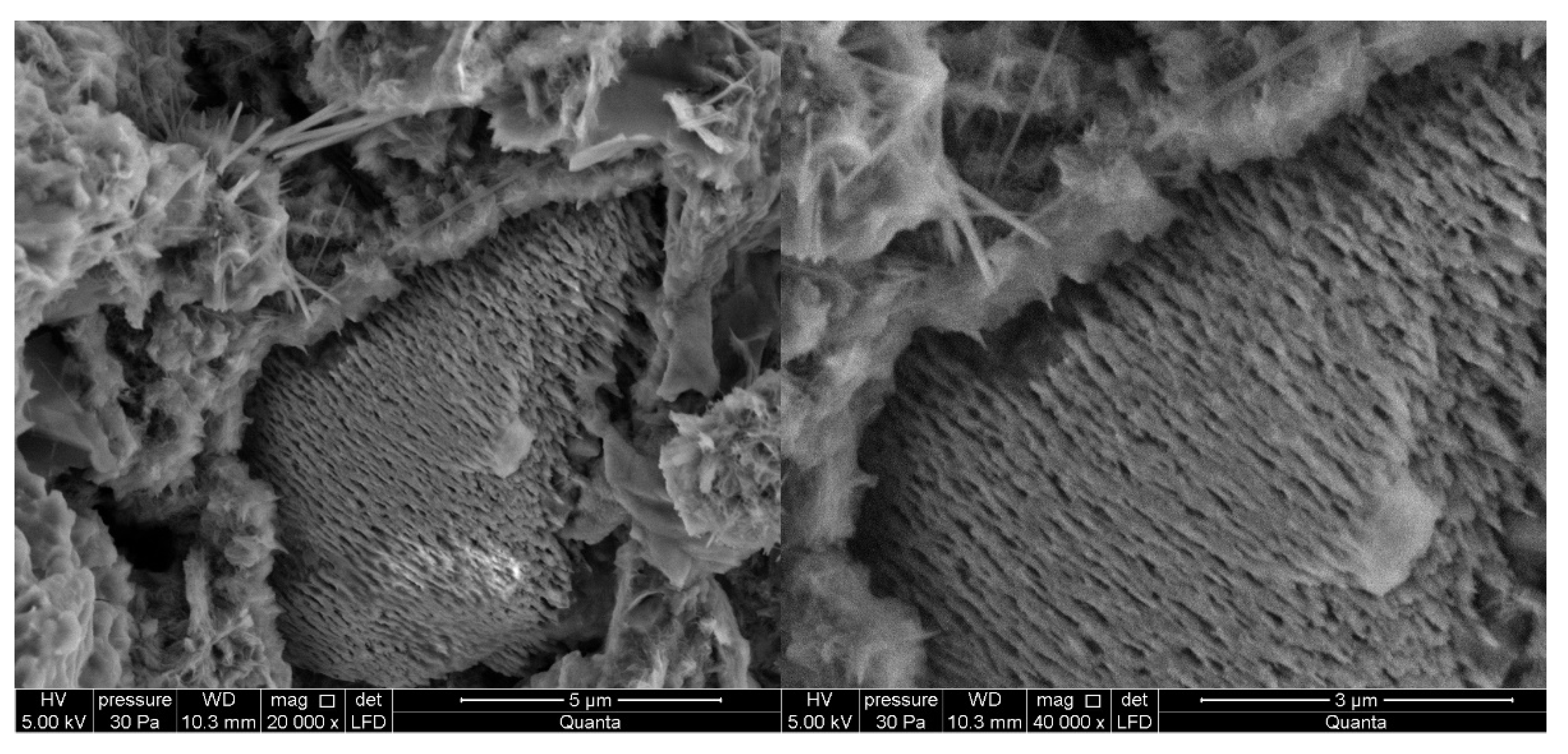
3.2.1. Non-Modified Cement Pastes
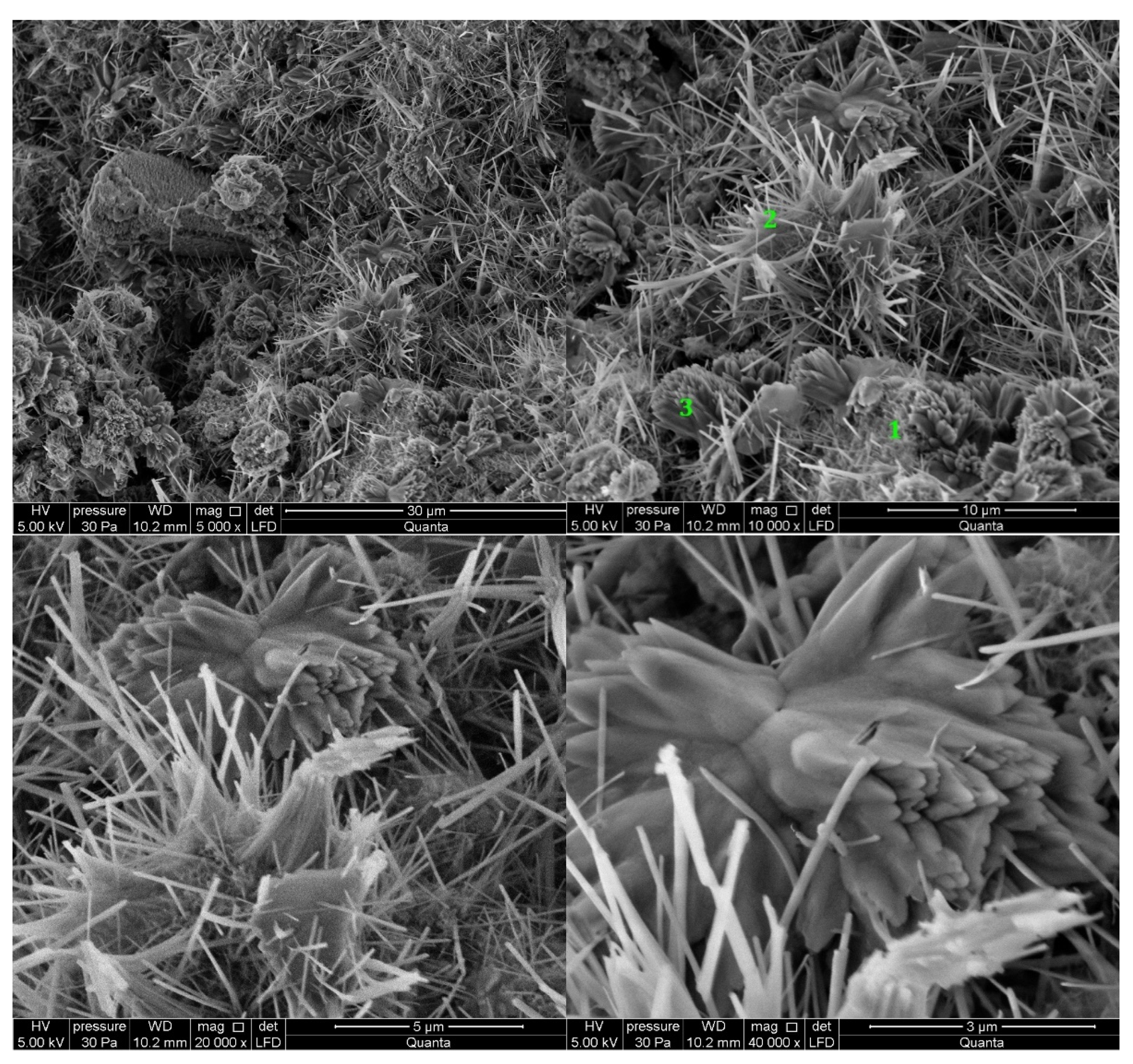
3.2.2. Cement Pastes Modified with Crystallization Seeds (CS)
3.2.3. Cement Pastes Modified with Calcium Nitrate (CN)
3.2.4. Cement Pastes Modified with Sodium Hydroxide (NaOH)
3.2.5. Cement Pastes Modified with Cement Kiln Dust (CKD)
3.3. X-ray Diffraction (XRD) Analysis
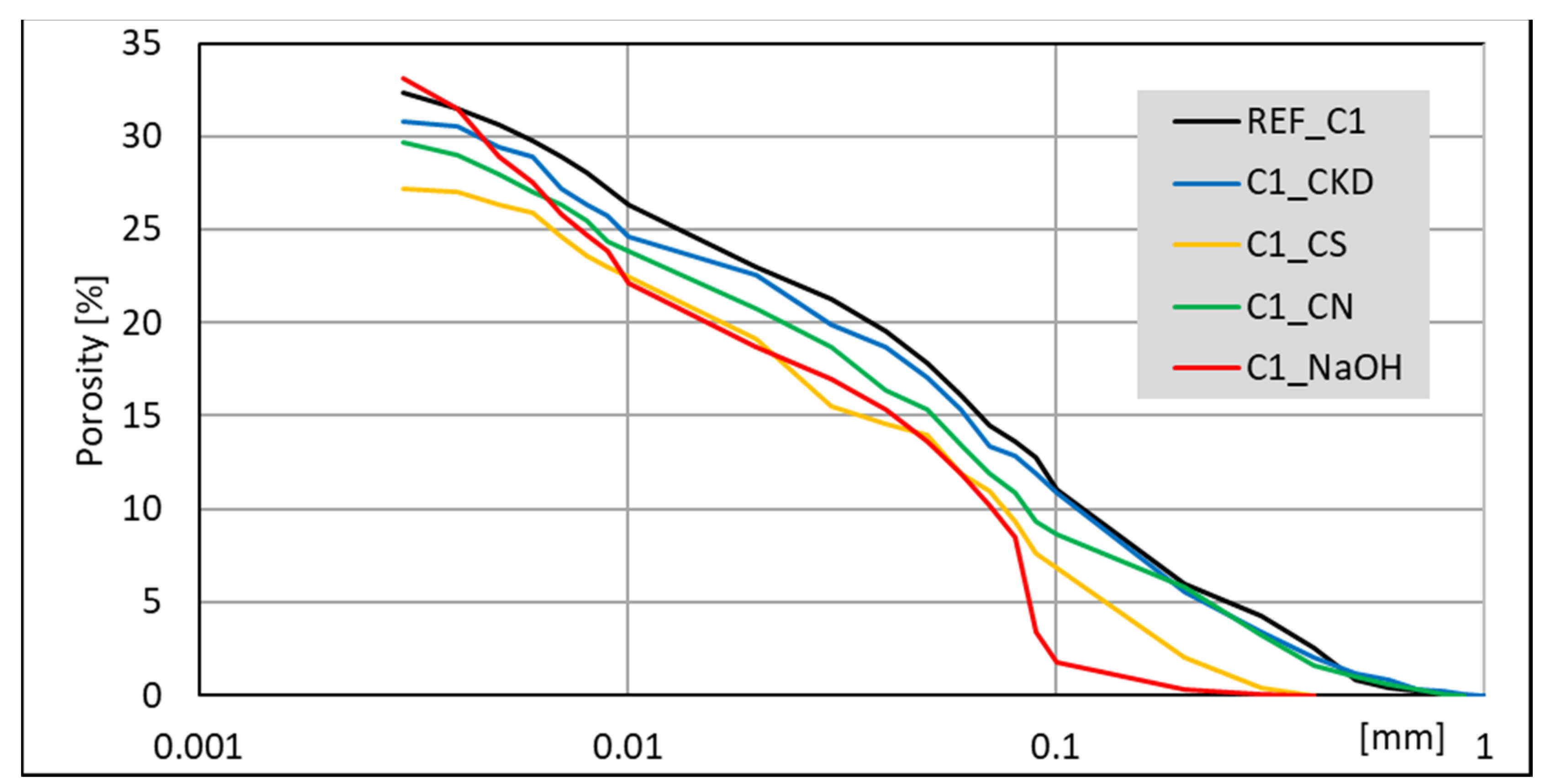
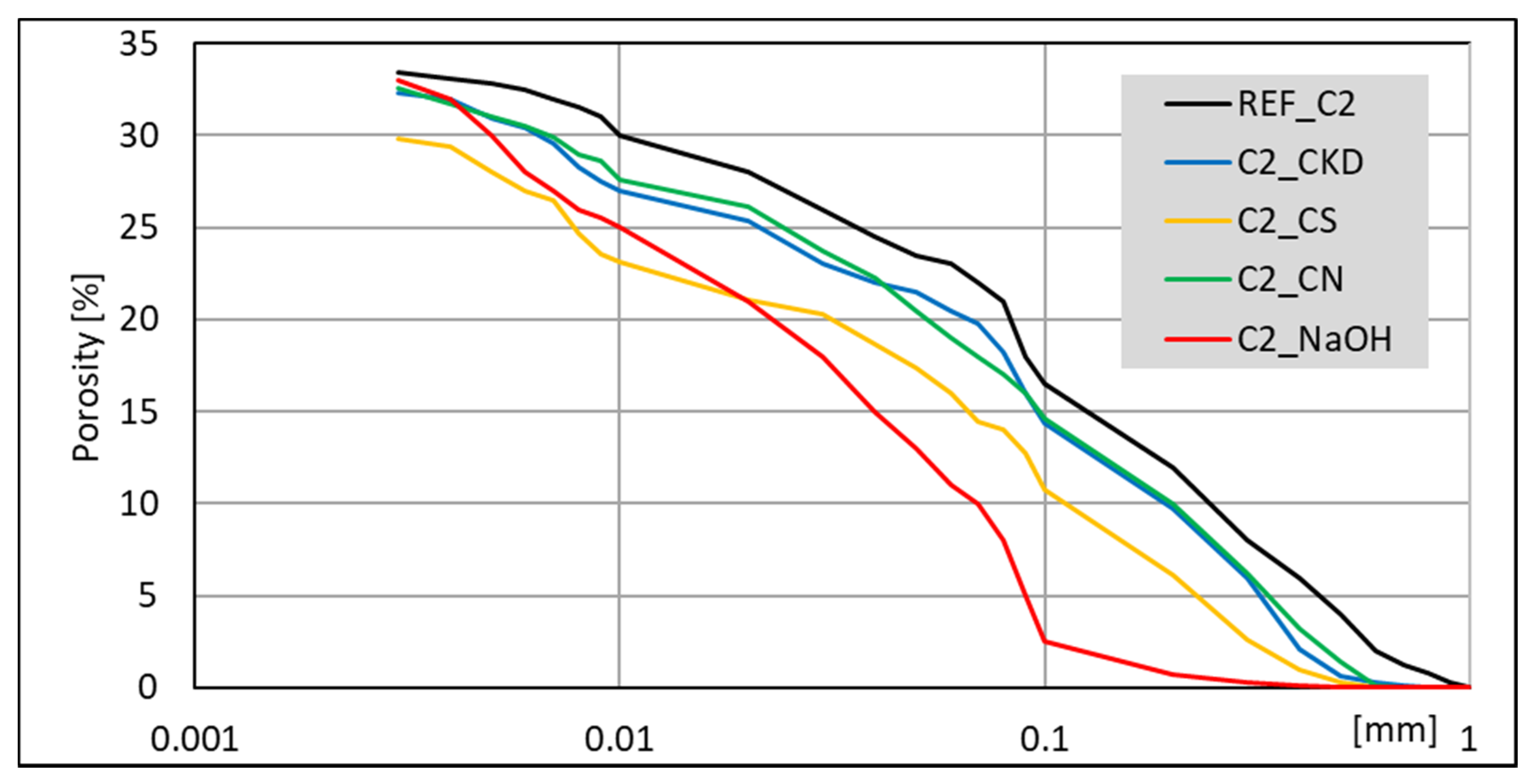
3.4. Mercury Intrusion Porosimetry (MIP) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Pizoń, J. Long-term Compressive Strength of Mortars Modified with Hardening Accelerating Admixtures. Procedia Eng. 2017, 195, 205–211. [Google Scholar] [CrossRef]
- Pizon, J.; Lazniewska-Piekarczyk, B. Comparison of Efficiency of Accelerating Admixtures for Concrete Using Multiple-Criteria Decision Analysis (MCDA). IOP Conf. Ser. Mater. Sci. Eng. 2019, 471, 032043. [Google Scholar] [CrossRef]
- Pizon, J.; Lazniewska-Piekarczyk, B. Microstructure of High C3A Portland Slag Cement Pastes, Modified with Accelerating Admixtures for Concrete. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Prague, Czech Republic, 17–21 June 2019; Volume 603. [Google Scholar]
- Garside, M. Cement Production Globally and in the U.S. from 2010 to 2019. 2020. Available online: https://www.statista.com/statistics/219343/cement-production-worldwide/ (accessed on 12 July 2021).
- Andrew, R.M. Global CO2 Emissions from Cement Production, 1928–2018: Antroposhere—Energy and Emissions. Earth Syst. Sci. Data 2019, 11, 1675–1710. [Google Scholar] [CrossRef] [Green Version]
- Al-Tahan, M.A.; Shatat, M.R.; Abdallah, M.H.; Taher, M.A. Influence of the thermally and hot water treatment of cement kiln dust on the Physico-chemical features of Portland cement. J. Build. Eng. 2021, 38, 102170. [Google Scholar] [CrossRef]
- Colangelo, F.; Farina, I.; Travaglioni, M.; Salzano, C.; Cioffi, R.; Petrillo, A. Innovative Materials in Italy for Eco-Friendly and Sustainable Buildings. Materials 2021, 14, 2048. [Google Scholar] [CrossRef] [PubMed]
- Neville, A.M.; Neville, A.M. Properties of Concrete; Pearson Education Limited; Harlow: London, UK, 1995; ISBN 9780273755807. [Google Scholar]
- Ramachandran, V.S. (Ed.) Concrete Admixtures Handbook: Properties, Science and Technology; Noyes Publications: Park Ridge, IL, USA, 1995. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry; Academic Press: Cambridge, UK, 1990. [Google Scholar]
- Merzouki, T.; Bouasker, M.; Khalifa, N.E.H.; Mounanga, P. Contribution to the modeling of hydration and chemical shrinkage of slag-blended cement at early age. Constr. Build. Mater. 2013, 44, 368–380. [Google Scholar] [CrossRef]
- Shubbar, A.A.; Jafer, H.; Abdulredha, M.; Al-Khafaji, Z.S.; Nasr, M.S.; Al Masoodi, Z.; Sadique, M. Properties of cement mortar incorporated high volume fraction of GGBFS and CKD from 1 day to 550 days. J. Build. Eng. 2020, 30, 101327. [Google Scholar] [CrossRef]
- Escalante, J.; Gómez, L.; Johal, K.; Mendoza, G.; Mancha, H.; Méndez, J. Reactivity of blast-furnace slag in Portland cement blends hydrated under different conditions. Cem. Concr. Res. 2001, 31, 1403–1409. [Google Scholar] [CrossRef]
- Pal, S.; Mukherjee, A.; Pathak, S. Investigation of hydraulic activity of ground granulated blast furnace slag in concrete. Cem. Concr. Res. 2003, 33, 1481–1486. [Google Scholar] [CrossRef]
- Topçu, I. High-volume ground granulated blast furnace slag (GGBFS) concrete. In Eco-Efficient Concrete; Woodhead Publishing: Sawston, UK, 2013; pp. 218–240. [Google Scholar]
- Bobrowski, A.; Gawlicki, M.; Łagosz, A.; Łoj, G.; Nocuń-Wczelik, W. Cement: Metody Badań: Wybrane Kierunki Stosowania; Nocuń-Wczelik, W., Ed.; Wydawnictwo AGH: Kraków, Poland, 2010. [Google Scholar]
- Ortega, J.; Albaladejo, A.; Pastor, J.; Sánchez, I.; Climent, M. Influence of using slag cement on the microstructure and durability related properties of cement grouts for micropiles. Constr. Build. Mater. 2013, 38, 84–93. [Google Scholar] [CrossRef]
- Harrisson, A.M.; Winter, N.B.; Taylor, H.F.W. Microstructure and Microchemistry of Slag Cement Pastes. MRS Proc. 1986, 86, 199–208. [Google Scholar] [CrossRef]
- Divsholi, B.S.; Lim, T.Y.D.; Teng, S. Durability Properties and Microstructure of Ground Granulated Blast Furnace Slag Cement Concrete. Int. J. Concr. Struct. Mater. 2014, 8, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Deja, J. Skład Fazowy Zaczynów Żużlowych Aktywowanych Alkaliami. Cem. Wapno Beton 2005, 3, 127–137. [Google Scholar]
- Bhanumathidas, N.; Kalidas, N. Dual Role of Gypsum: Set Retarder and Strength Accelerator. Indian Concr. J. 2004, 2, 170–173. [Google Scholar]
- Kim, J.; Honda, D.; Choi, H.; Hama, Y. Investigation of the Relationship between Compressive Strength and Hydrate Formation Behavior of Low-Temperature Cured Cement upon Addition of a Nitrite-Based Accelerator. Materials 2019, 12, 3936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.; Lee, J.; Choi, H. Effects of Accelerators and Retarders in Early Strength Development of Concrete Based on Low-Temperature-Cured Ordinary Portland and Calcium Sulfoaluminate Cement Blends. Materials 2020, 13, 1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, W.; Thiel, C.; Duran, F.; Gehlen, C. Effect of Calcium Nitrate on the Freeze-Thaw-Resistance of Concrete. In Proceedings of the 2nd International Congress on Concrete Durability, New Delhi, India, 4–6 December 2014. [Google Scholar]
- Justnes, H. Calcium Nitrate as Multifunctional Concrete Admixture. Concr. Plant Int. 2014, 3, 60–64. [Google Scholar]
- Li, Q.; Wang, Y.; Geng, G.; Chen, H.; Hou, P.; Cheng, X.; Monteiro, P.J.M.; Huang, S.; Kim, J.H. Microstructural Study of Hydration of C3S in the Presence of Calcium Nitrate Using Scanning Transmission X-Ray Microscopy (STXM). Available online: https://www.hindawi.com/journals/jnm/2020/8419130/ (accessed on 16 February 2021).
- Balonis, M.; Mędala, M.; Glasser, F.P. Influence of calcium nitrate and nitrite on the constitution of AFm and AFt cement hydrates. Adv. Cem. Res. 2011, 23, 129–143. [Google Scholar] [CrossRef]
- Kunal; Siddique, R.; Rajor, A. Use of cement kiln dust in cement concrete and its leachate characteristics. Resour. Conserv. Recycl. 2012, 61, 59–68. [Google Scholar] [CrossRef]
- Konstantinos, S.; Georg, A. On the Solubilities of Anhydrite and Gypsum. In Constitutive Modeling of Geomaterials: Advances and New Applications; Springer: Berlin/Heidelberg, Germany, 2013; pp. 333–339. [Google Scholar]
- Mackie, A.; Boilard, S.; Walsh, M.; Lake, C. Physicochemical characterization of cement kiln dust for potential reuse in acidic wastewater treatment. J. Hazard. Mater. 2010, 173, 283–291. [Google Scholar] [CrossRef]
- Sreekrishnavilasam, A.; Rahardja, S.; Kmetz, R.; Santagata, M. Soil treatment using fresh and landfilled cement kiln dust. Constr. Build. Mater. 2007, 21, 318–327. [Google Scholar] [CrossRef]
- Batis, G.; Rakanta, E.; Sideri, E.; Chaniotakis, F.; Papageorgiou, A. Advantages of Simultaneous Use of Cement Kiln Dust and Blast Furnace Slag. In Proceedings of the International Conference on Challenges of Concrete Construction, Dundee, Scotland, UK, 9–11 September 2002. [Google Scholar]
- Detwiler, R.J.; Rachel, J.; Javed, I.; Bhatty, M.S.Y.; Bhattacharja, S. Supplementary Cementing Materials for Use in Blended Cements; Skokie, I., Ed.; Portland Cement Association: Chicago, IL, USA, 1996. [Google Scholar]
- Alnahhal, W.; Taha, R.; Al-Nasseri, H.; Nishad, S. Effect of Using Cement Kiln Dust as a Nano-Material on the Strength of Cement Mortars. KSCE J. Civ. Eng. 2017, 22, 1361–1368. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Heikal, M.; Mohammed, M.S.; El-Aleem, S.A.; Hassan, H.S.; García, S.V.; Alomayri, T. Sustainable disposal of cement kiln dust in the production of cementitious materials. J. Clean. Prod. 2019, 232, 1218–1229. [Google Scholar] [CrossRef]
- Van De Heede, P.; de Belie, N. Environmental impact and life cycle assessment (LCA) of traditional and ‘green’ concretes: Literature review and theoretical calculations. Cem. Concr. Compos. 2012, 34, 431–442. [Google Scholar] [CrossRef]
- Aimi Noorliyana, H.; Kamarudin, H.; Begum, N.; Abdullah, M.M.A.B.; Abdul Razak, K.; Ekaputri, J. Effect of Sodium Hydroxide (NaOH) Concentration on Compressive Strength of Alkali-Activated Slag (AAS) Mortars. Appl. Mech. Mater. 2015, 754–755, 300–304. [Google Scholar] [CrossRef]
- Mohamed, O.A. A Review of Durability and Strength Characteristics of Alkali-Activated Slag Concrete. Materials 2019, 12, 1198. [Google Scholar] [CrossRef] [Green Version]
- Łukowski, P. Domieszki Do Zapraw i Betonów; Polski Cement: Kraków, Poland, 2003. [Google Scholar]
- Wu, J.; Ding, Q.; Yang, W.; Wang, L.; Wang, H. Influence of Submicron Fibrillated Cellulose Fibers from Cotton on Hydration and Microstructure of Portland Cement Paste. Molecules 2021, 26, 5831. [Google Scholar] [CrossRef]
- Thomas, J.; Jennings, H.M.; Chen, J.J. Influence of Nucleation Seeding on the Hydration Mechanisms of Tricalcium Silicate and Cement. J. Phys. Chem. C 2009, 113, 4327–4334. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Ji, X.; Jiang, Y.; Pan, T. Effect of C-S-H Nucleating Agent on Cement Hydration. Appl. Sci. 2021, 11, 6638. [Google Scholar] [CrossRef]
- Diandian, Z. Microstructure and Hydration of Cement-Based Materials Incorporating Calcined Clay and Calcium-Silicate-Hydrate (C-S-H) Seed. Ph. D. Thesis, University of Calgary, Calgary, AB, Canada, July 2020. [Google Scholar]
- Hewlett, P.; Liska, M. Lea’s Chemistry of Cement and Concrete; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 97808-1007730. [Google Scholar]
- Wyrzykowski, M.; Assmann, A.; Hesse, C.; Lura, P. Microstructure development and autogenous shrinkage of mortars with C-S-H seeding and internal curing. Cem. Concr. Res. 2020, 129, 105967. [Google Scholar] [CrossRef]
- Kutschera, M.; Nicoleau, L.; Bräu, M. Nano-optimized Construction Materials by Nano-seeding and Crystallization Control. In Nanotechnology in Civil Infrastructure; Springer: Berlin/Heidelberg, Germany, 2011; pp. 175–205. [Google Scholar] [CrossRef]
- Szostak, B.; Golewski, G.L. Improvement of Strength Parameters of Cement Matrix with the Addition of Siliceous Fly Ash by Using Nanometric C-S-H Seeds. Energies 2020, 13, 6734. [Google Scholar] [CrossRef]
- Łukowski, P. Modyfikacja Materiałowa Betonu; Polski Cement: Krakow, Poland, 2016. [Google Scholar]
- Onghena, S.; Grunewald, S.; de Schutter, G. Strength Development of Concrete: Balancing Production Requirements and Ecological Impact. In Proceedings of the II International Conference on Concrete Sustainability, Madrin, Spain, 13–15 June 2016; pp. 1065–1076. [Google Scholar]
- John, E.; Epping, J.D.; Stephan, D. The influence of the chemical and physical properties of C-S-H seeds on their potential to accelerate cement hydration. Constr. Build. Mater. 2019, 228, 116723. [Google Scholar] [CrossRef]
- Gallé, C. Effect of drying on cement-based materials pore structure as identified by mercury intrusion porosimetry: A comparative study between oven-, vacuum-, and freeze-drying. Cem. Concr. Res. 2001, 31, 1467–1477. [Google Scholar] [CrossRef]
- Tracz, T. Open porosity of cement pastes and their gas permeability. Bull. Pol. Acad. Sci. Tech. Sci. 2016, 64, 775–783. [Google Scholar] [CrossRef]
- Chikh, N.; Zouaoui, M.C.; Aggoun, S.; Duval, R. Effects of calcium nitrate and triisopropanolamine on the setting and strength evolution of Portland cement pastes. Mater. Struct. 2007, 41, 31–36. [Google Scholar] [CrossRef]
- Heikal, M. Effect of calcium formate as an accelerator on the physicochemical and mechanical properties of pozzolanic cement pastes. Cem. Concr. Res. 2004, 34, 1051–1056. [Google Scholar] [CrossRef]
- Justnes, H.; Nygaard, E.C. Technical calcium nitrate as set accelerator for cement at low temperatures. Cem. Concr. Res. 1995, 25, 1766–1774. [Google Scholar] [CrossRef]
- Dittmar, S.; Hauck, H.; Konig, R. C-S-H: A State-of-the-Art Concept to Accelerate Concrete Hardening: Part I. BFT Int. 2013, 1, 44–51. [Google Scholar]
- John, E.; Matschei, T.; Stephan, D. Nucleation seeding with calcium silicate hydrate—A review. Cem. Concr. Res. 2018, 113, 74–85. [Google Scholar] [CrossRef]
- Hubner, M.; Jennings, H.; Thomas, J. Influence of Nucleation Seeding on the Compressive Strength of Ordinary Portland Cement and Alkali Activated Blast-Furnace Slag; Infrastructure Technology Institute Year 2 Final Report; Civil and Environmental Engineering, Northwestern University Technological Institute: Evanston, IL, USA, 2009. [Google Scholar]
- Wells, L.; Taylor, K. Hydration of magnesia in dolomitic hydrated limes and putties. J. Res. Natl. Inst. Stand. Technol. 1937, 19, 215. [Google Scholar] [CrossRef]
- Qian, C.; Yi, H.; Du, W. Bacteria fixing CO2 to enhance the volume stability of ground steel slag powder as a component of cement-based materials aiming at clean production. J. Clean. Prod. 2021, 314, 127821. [Google Scholar] [CrossRef]
- Vola, G.; Sarandrea, L.; Mazzieri, M.; Bresciani, P.; Ardit, M.; Cruciani, G. Reactivity and Overburning Tendency of Quicklime Burnt at High Temperature. ZKG 2019, 10, 20–31. [Google Scholar]
- Yoneyama, A.; Choi, H.; Inoue, M.; Kim, J.; Lim, M.; Sudoh, Y. Effect of a Nitrite/Nitrate-Based Accelerator on the Strength Development and Hydrate Formation in Cold-Weather Cementitious Materials. Materials 2021, 14, 1006. [Google Scholar] [CrossRef] [PubMed]
- Mota, B.; Matschei, T.; Scrivener, K. Impact of NaOH and Na2SO4 on the kinetics and microstructural development of white cement hydration. Cem. Concr. Res. 2018, 108, 172–185. [Google Scholar] [CrossRef]
- Adaska, W.S.; Taubert, D.H. Beneficial Uses of Cement Kiln Dust. In Proceedings of the 50th IEEE/PCA Cement Industry Technical Conference, Miami, FL, USA, 19–22 May 2008. [Google Scholar]
- Siddique, R. Cement Kiln Dust. In Waste Materials and By-Products in Concrete; Springer: Berlin/Heidelberg, Germany, 2008; pp. 351–380. [Google Scholar]
- Kalina, L.; Bílek, J.V.; Kiripolský, T.; Novotný, R.; Másilko, J. Cement Kiln By-Pass Dust: An Effective Alkaline Activator for Pozzolanic Materials. Materials 2018, 11, 1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aïtcin, P.-C.; Flatt, R.J. Science and Technology of Concrete Admixtures; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780081006931. [Google Scholar]
- Maddalena, R.; Li, K.; Chater, P.A.; Michalik, S.; Hamilton, A. Direct synthesis of a solid calcium-silicate-hydrate (C-S-H). Constr. Build. Mater. 2019, 223, 554–565. [Google Scholar] [CrossRef]
- Skinner, L.; Chae, S.R.; Benmore, C.; Wenk, H.R.; Monteiro, P.J.M. Nanostructure of Calcium Silicate Hydrates in Cements. Phys. Rev. Lett. 2010, 104, 195502. [Google Scholar] [CrossRef]
- Grangeon, S.; Fernandez-Martinez, A.; Baronnet, A.; Marty, N.; Poulain, A.; Elkaïm, E.; Roosz, C.; Gaboreau, S.; Henocq, P.; Claret, F. Quantitative X-ray pair distribution function analysis of nanocrystalline calcium silicate hydrates: A contribution to the understanding of cement chemistry. J. Appl. Crystallogr. 2017, 50, 14–21. [Google Scholar] [CrossRef]
- Maddalena, R.; Hall, C.; Hamilton, A. Effect of silica particle size on the formation of calcium silicate hydrate [C-S-H] using thermal analysis. Thermochim. Acta 2018, 672, 142–149. [Google Scholar] [CrossRef]
- Richardson, I.; Groves, G.W. The structure of the calcium silicate hydrate phases present in hardened pastes of white Portland cement/blast-furnace slag blends. J. Mater. Sci. 1997, 32, 4793–4802. [Google Scholar] [CrossRef]
- Soyer-Uzun, S.; Chae, S.R.; Benmore, C.J.; Wenk, H.-R.; Monteiro, P.J.M. Compositional Evolution of Calcium Silicate Hydrate (C-S-H) Structures by Total X-Ray Scattering. J. Am. Ceram. Soc. 2011, 95, 793–798. [Google Scholar] [CrossRef]
- Khatib, J.M. Sustainability of Construction Materials; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780081009956. [Google Scholar]
- Siddique, R. Self-Compacting Concrete: Materials, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128173695. [Google Scholar]
- Yao, Y.; Wang, Y.; Wei, Q.; Cui, S.; Hao, L. Effect of the Formation of Amorphous Networks on the Structure and Hydration Characteristics of Granulated Blast Furnace Slag. Materials 2020, 13, 1462. [Google Scholar] [CrossRef] [Green Version]
- Poursaee, A. Corrosion of Steel in Concrete Structures; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 97878242-3812. [Google Scholar]
- Siddique, R.; Cachim, P. Waste and Supplementary Cementitious Materials in Concrete; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780081021569. [Google Scholar]
- Najim, K.; Al-Jumaily, I.; Atea, A.M. Characterization of sustainable high performance/self-compacting concrete produced using CKD as a cement replacement material. Constr. Build. Mater. 2016, 103, 123–129. [Google Scholar] [CrossRef]
- Tomita, Y.; Yoneyama, A.; Choi, H.; Inoue, M.; Kim, J.; Choi, H.; Sudoh, Y. Evaluation of Mechanical and Shrinkage Behavior of Lowered Temperatures Cementitious Mortars Mixed with Nitrite–Nitrate Based Accelerator. Materials 2020, 13, 3686. [Google Scholar] [CrossRef] [PubMed]
- Stefanoni, M.; Angst, U.; Elsener, B. Influence of Calcium Nitrate and Sodium Hydroxide on Carbonation-Induced Steel Corrosion in Concrete. Corrosion 2019, 75, 737–744. [Google Scholar] [CrossRef]












| Sym. | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Cl– | Na2O | K2O | CaOfree | Ign. Loss. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 20.25 | 6.83 | 3.23 | 65.66 | 1.39 | 0.69 | 0.01 | 0.15 | 1.02 | 2.80 | 0.15 |
| C2 | 21.25 | 5.00 | 3.40 | 64.81 | 2.09 | 0.55 | 0.03 | 0.11 | 1.03 | 1.48 | 0.35 |
| GGBFS | 37.35 | 7.30 | 1.22 | 43.90 | 5.73 | 0.62 | 0.03 | 0.55 | 0.56 | - | 0.17 |
| Anhydrite | 0.61 | - | - | 40.16 | 0.40 | 54.83 | - | 0.02 | - | - | 2.71 |
| Phase Composition | Blaine’s Specific Surface Area | ||||||||||
| C2S | C3S | C3A | C4AF | ||||||||
| C1 | 11 | 64 | 14 | 7 | 3000 cm2/g | ||||||
| C2 | 12 | 69 | 4 | 12 | 3000 cm2/g | ||||||
| GGBFS | - | - | - | - | 3900 cm2/g | ||||||
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Cl– | Na2O | K2O | P2O5 | TiO2 | Mn2O3 | SrO | ZnO | Ign. Loss. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18.16 | 4.55 | 2.03 | 57.13 | 1.26 | 1.95 | 1.44 | 0.12 | 2.38 | 0.12 | 0.22 | 0.06 | 0.10 | 0.24 | 11.70 |
| No. | Portland Clinker | GGBFS | Anhydrite | Sand | Water | CKD | Admixture | |||
|---|---|---|---|---|---|---|---|---|---|---|
| type | [g] | type | [% m.c.] | [g] | ||||||
| 1 | C1 | 285.3 | 153.6 | 11.07 | 1350 | 225 | - | - | - | - |
| 2 | 285.3 | 153.6 | 11.07 | 1350 | 225 | - | CS | 4.0 | 18.0 | |
| 3 | 285.3 | 153.6 | 11.07 | 1350 | 225 | - | CN | 2.0 | 9.0 | |
| 4 | 285.3 | 153.6 | 11.07 | 1350 | 225 | - | NaOH | 2.0 | 9.0 | |
| 5 | 270.9 | 145.8 | 10.8 | 1350 | 225 | 22.5 | - | - | - | |
| 6 | C2 | 284.8 | 153.4 | 11.8 | 1350 | 225 | - | - | - | - |
| 7 | 284.8 | 153.4 | 11.8 | 1350 | 225 | - | CS | 4.0 | 18.0 | |
| 8 | 284.8 | 153.4 | 11.8 | 1350 | 225 | - | CN | 2.0 | 9.0 | |
| 9 | 284.8 | 153.4 | 11.8 | 1350 | 225 | - | NaOH | 2.0 | 9.0 | |
| 10 | 270.4 | 145.6 | 11.5 | 1350 | 225 | 22.5 | - | - | - | |
| No. | Portland Clinker | GGBFS | Anhydrite | Water | Water Demand | CKD | Admixture | |||
|---|---|---|---|---|---|---|---|---|---|---|
| type | [g] | [g] | % | [g] | type | [% m.c.] | [g] | |||
| 1 | C1 | 285.3 | 153.6 | 11.07 | 217 | 43 | - | - | - | - |
| 2 | 285.3 | 153.6 | 11.07 | 218 | 44 | - | CS | 4.0 | 18.0 | |
| 3 | 285.3 | 153.6 | 11.07 | 169 | 34 | - | CN | 2.0 | 9.0 | |
| 4 | 285.3 | 153.6 | 11.07 | 183 | 37 | - | NaOH | 2.0 | 9.0 | |
| 5 | 270.9 | 145.8 | 10.8 | 167 | 33 | 22.5 | - | - | - | |
| 6 | C2 | 284.8 | 153.4 | 11.8 | 149 | 30 | - | - | - | - |
| 7 | 284.8 | 153.4 | 11.8 | 150 | 30 | - | CS | 4.0 | 18.0 | |
| 8 | 284.8 | 153.4 | 11.8 | 150 | 30 | - | CN | 2.0 | 9.0 | |
| 9 | 284.8 | 153.4 | 11.8 | 178 | 36 | - | NaOH | 2.0 | 9.0 | |
| 10 | 270.4 | 145.6 | 11.5 | 152 | 30 | 22.5 | - | - | - | |
| Paste | C1_REF | C1_CS | C1_CN | C1_NaOH | C1_CKD |
|---|---|---|---|---|---|
| Content [%] | |||||
| C3S | 16.1 | 13.3 | 14.6 | 14.1 | 14.9 |
| C2S | 9.6 | 8 | 8.8 | 6.8 | 7.8 |
| C3A | 5.4 | 5.9 | 5.6 | 3.2 | 3.6 |
| C4AF | 2.6 | 2.3 | 2.1 | 2.0 | 2.2 |
| Portlandite | 9.2 | 6.5 | 4.4 | 4.4 | 8.5 |
| Anhydrite, calcite, quartz, ettringite, other | Each < 1.0 | ||||
| Sum of crystalline components | 42.9 | 36.0 | 35.5 | 30.5 | 37.0 |
| Amorphous phase | 55.1 | 62.2 | 62.3 | 68.1 | 61.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizoń, J.; Łaźniewska-Piekarczyk, B. Microstructure of CEM II/B-S Pastes Modified with Set Accelerating Admixtures. Materials 2021, 14, 6300. https://doi.org/10.3390/ma14216300
Pizoń J, Łaźniewska-Piekarczyk B. Microstructure of CEM II/B-S Pastes Modified with Set Accelerating Admixtures. Materials. 2021; 14(21):6300. https://doi.org/10.3390/ma14216300
Chicago/Turabian StylePizoń, Jan, and Beata Łaźniewska-Piekarczyk. 2021. "Microstructure of CEM II/B-S Pastes Modified with Set Accelerating Admixtures" Materials 14, no. 21: 6300. https://doi.org/10.3390/ma14216300







