Fabrication of UV Laser-Induced Porous Graphene Patterns with Nanospheres and Their Optical and Electrical Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laser System
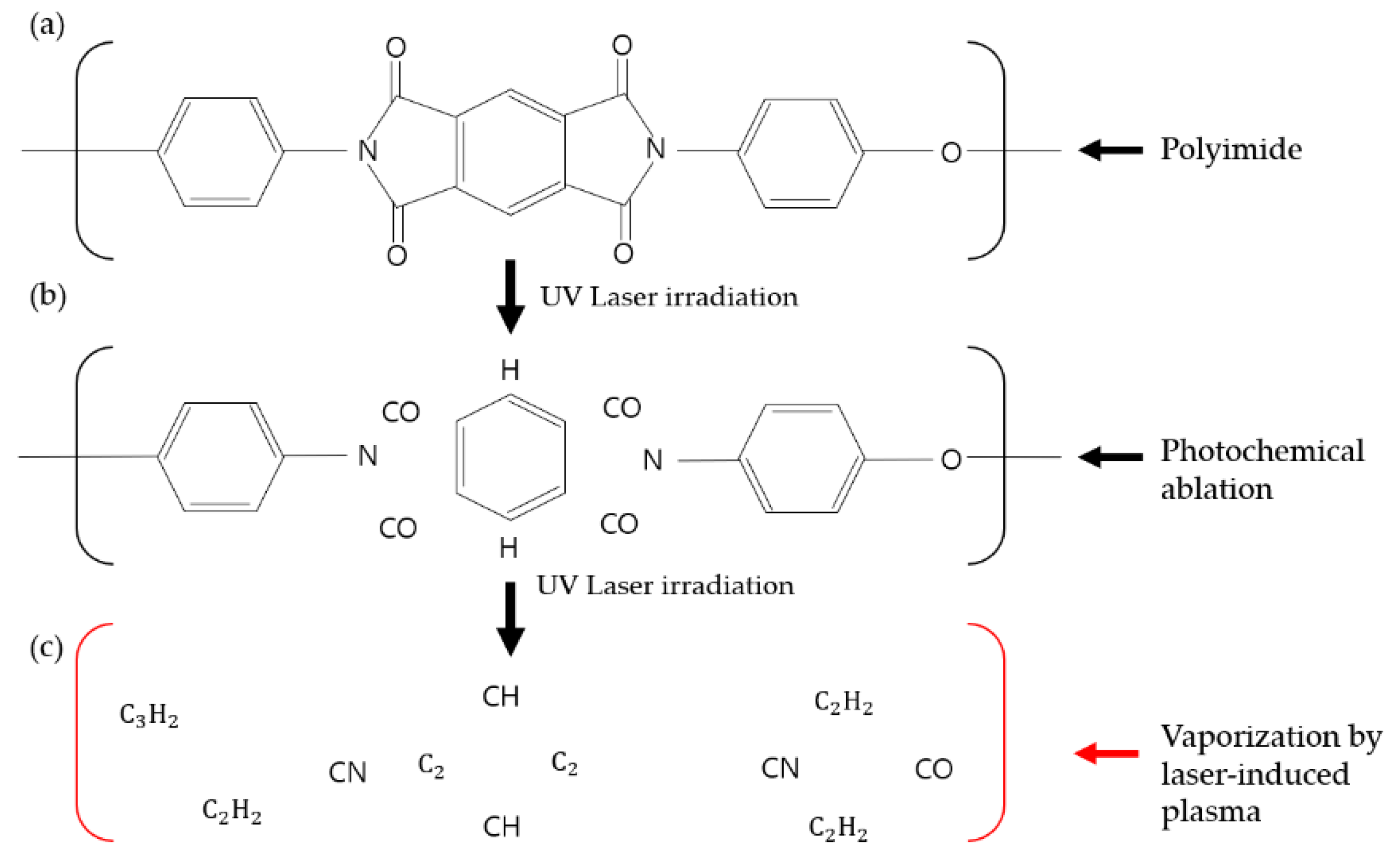
2.2. Principle of Photochemical Ablation of PI, Using a 355 nm Pulsed Laser
3. Results and Discussion
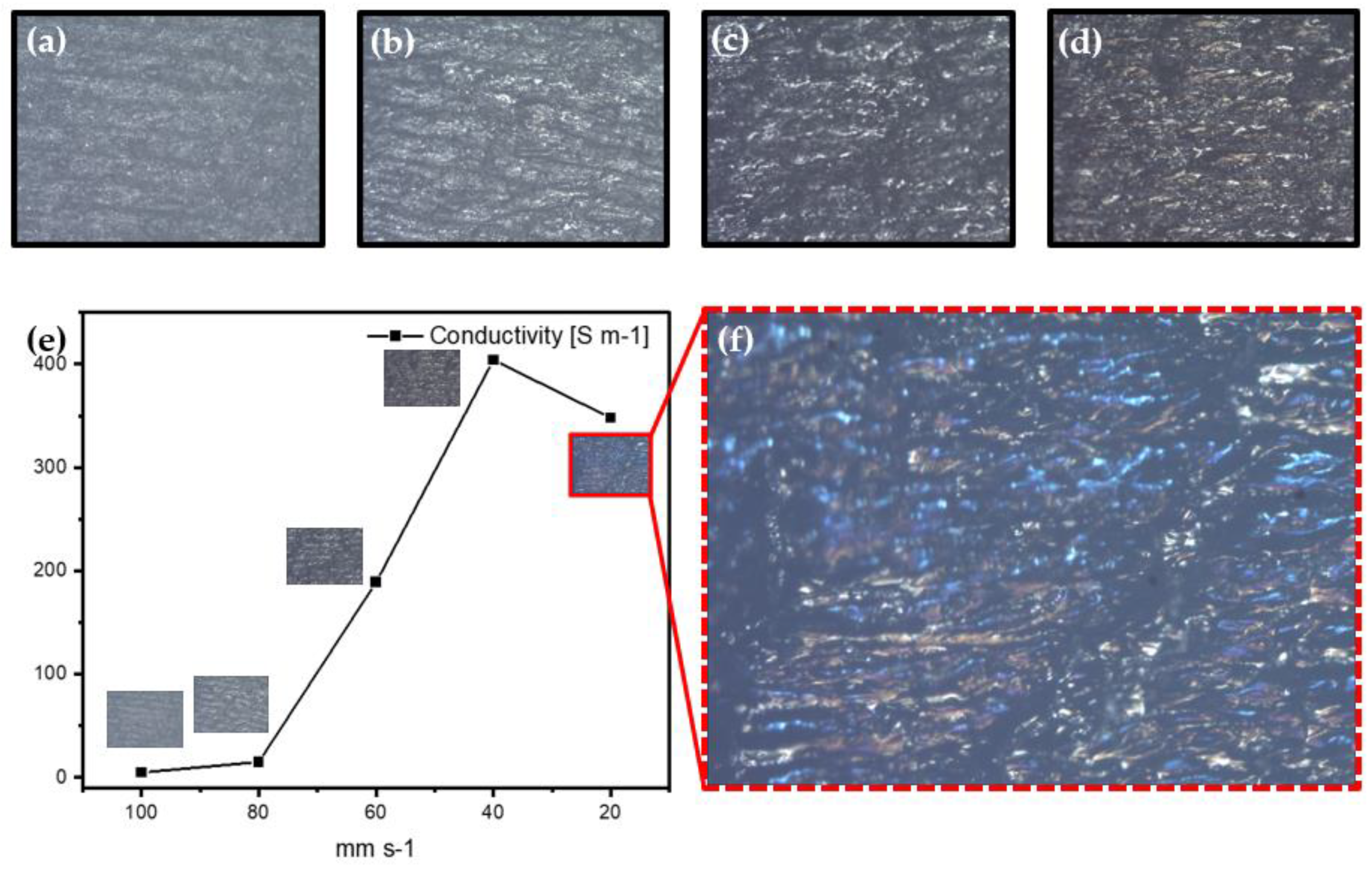
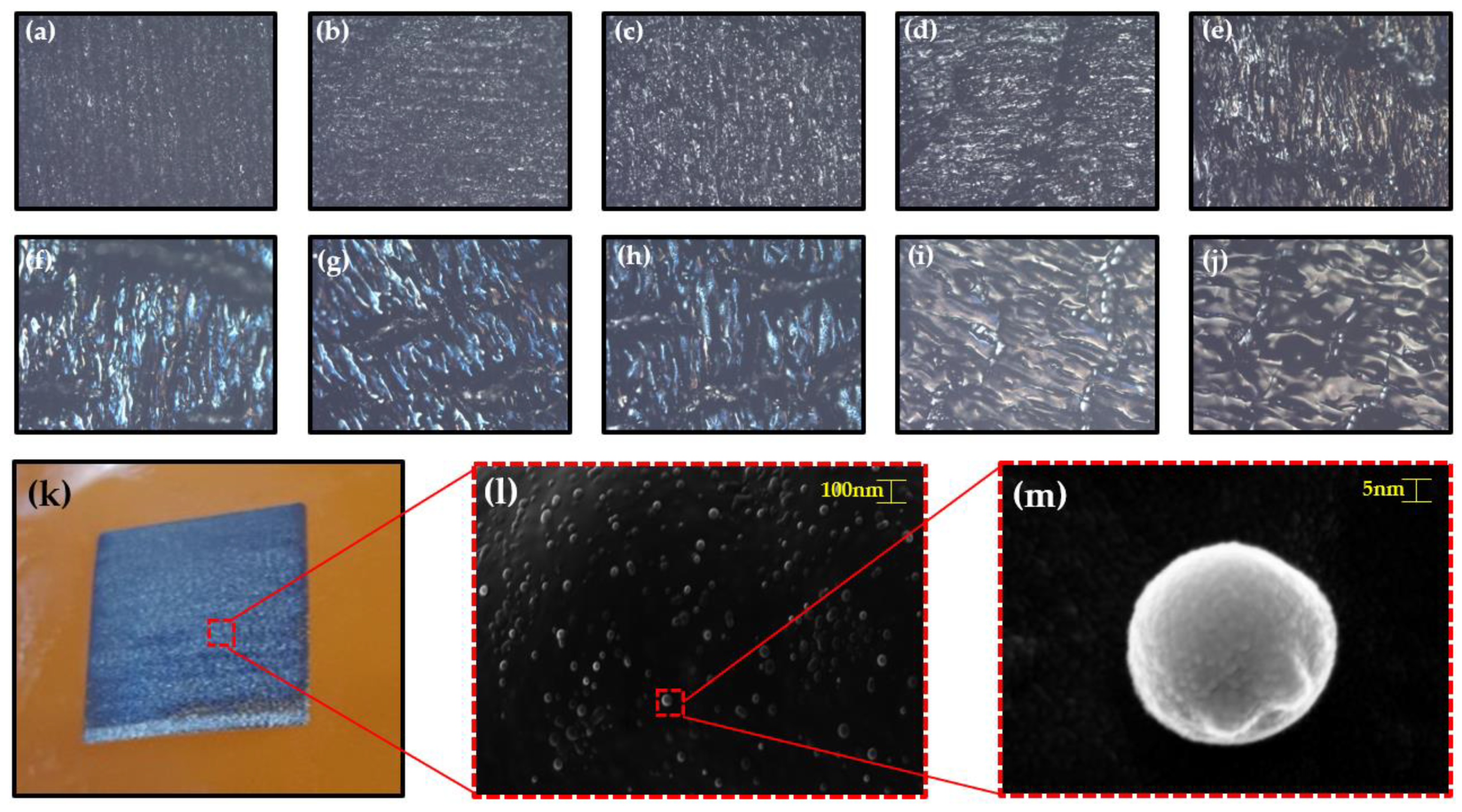
3.1. Morphological Characterization
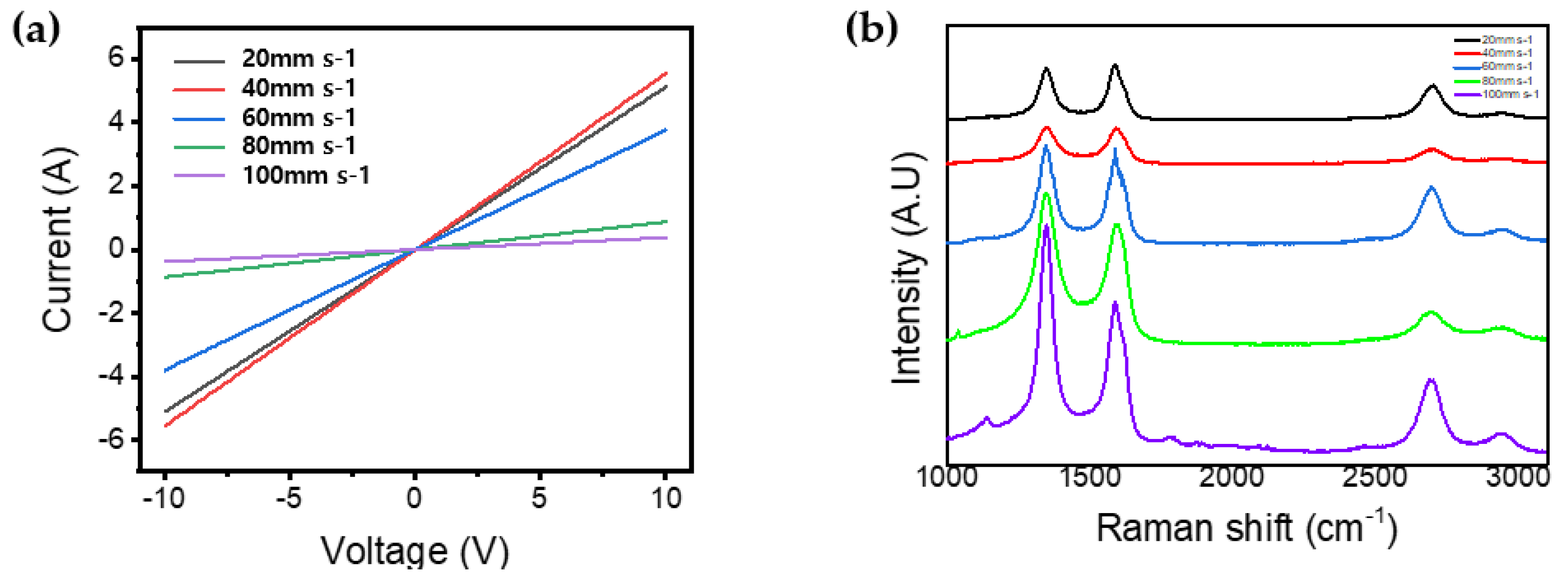
3.2. Electrical and Optical Characterizations
3.3. Application Structural Color Based on Nanospheres Fabricated by Controlling Focal Plane
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wan, X.; Huang, Y.; Chen, Y. Focusing on energy and optoelectronic applications: A journey for graphene and graphene oxide at large scale. Acc. Chem. Res. 2012, 45, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wu, H. Graphene-based nanomaterials: Synthesis, properties, and optical and optoelectronic applications. Adv. Funct. Mater. 2013, 23, 1984–1997. [Google Scholar] [CrossRef]
- Bablich, A.; Kataria, S.; Lemme, M.C. Graphene and two-dimensional materials for optoelectronic applications. Electronics 2016, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Aumanen, J.; Johansson, A.; Koivistoinen, J.; Myllyperkiö, P.; Pettersson, M. Patterning and tuning of electrical and optical properties of graphene by laser induced two-photon oxidation. Nanoscale 2015, 7, 2851–2855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, F.; Fang, D.; Xu, Z. Mechanical and thermal transport properties of graphene with defects. Appl. Phys. Lett. 2011, 99. [Google Scholar] [CrossRef]
- Wang, F.; Wang, K.; Zheng, B.; Dong, X.; Mei, X.; Lv, J.; Duan, W.; Wang, W. Laser-induced graphene: Preparation, functionalization and applications. Mater. Technol. 2018, 33, 340–356. [Google Scholar] [CrossRef]
- Tao, L.Q.; Tian, H.; Liu, Y.; Ju, Z.Y.; Pang, Y.; Chen, Y.Q.; Wang, D.Y.; Tian, X.G.; Yan, J.C.; Deng, N.Q.; et al. An intelligent artificial throat with sound-sensing ability based on laser induced graphene. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.Y.; Ma, Y.W.; Lee, J.U.; Je, G.J.; Shin, B.S. Flexible and highly sensitive strain sensor based on laser-induced graphene pattern fabricated by 355 nm pulsed laser. Sensors 2019, 19, 4867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobinger, M.R.; Romero, F.J.; Salinas-Castillo, A.; Becherer, M.; Lugli, P.; Morales, D.P.; Rodríguez, N.; Rivadeneyra, A. Flexible and robust laser-induced graphene heaters photothermally scribed on bare polyimide substrates. Carbon N. Y. 2019, 144, 116–126. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Peng, Z.; Li, Y.; Gao, C.; Ji, Y.; Ye, R.; Kim, N.D.; Zhong, Q.; Yang, Y.; et al. High-Performance Pseudocapacitive Microsupercapacitors from Laser-Induced Graphene. Adv. Mater. 2016, 28, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, M.; Li, Y.; Tour, J.M. In Situ Synthesis of Efficient Water Oxidation Catalysts in Laser-Induced Graphene. ACS Energy Lett. 2018, 3, 677–683. [Google Scholar] [CrossRef]
- Ma, Y.W.; Jeong, M.Y.; Lee, S.M.; Shin, B.S. Fabrication of a high-density nano-porous structure on polyimide by using ultraviolet laser irradiation. J. Korean Phys. Soc. 2016, 68, 668–673. [Google Scholar] [CrossRef]
- Ma, Y.W.; Oh, J.Y.; Ahn, S.; Shin, B.S. A method for fabricating a micro-structured surface of polyimide with open and closed pores. J. Korean Phys. Soc. 2016, 69, 386–389. [Google Scholar] [CrossRef]
- Shin, B.S.; Oh, J.Y.; Sohn, H. Theoretical and experimental investigations into laser ablation of polyimide and copper films with 355-nm Nd:YVO4 laser. J. Mater. Process. Technol. 2007, 187–188, 260–263. [Google Scholar] [CrossRef]
- Zhu, S.E.; Yuan, S.; Janssen, G.C.A.M. Optical transmittance of multilayer graphene. EPL 2014, 108, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ma, F.; Liang, W.; Wang, R.; Sun, M. Optical, photonic and optoelectronic properties of graphene, h-NB and their hybrid materials. Nanophotonics 2017, 6, 943–976. [Google Scholar] [CrossRef]
- Jung, I.; Rhyee, J.S.; Son, J.Y.; Ruoff, R.S.; Rhee, K.Y. Colors of graphene and graphene-oxide multilayers on various substrates. Nanotechnology 2012, 23. [Google Scholar] [CrossRef]
- Gong, T.; Zhang, X.; Fu, Y.; Zhou, G.; Chi, H.; Li, T. A facile fabrication of colorimetric graphene oxide reflecting films for ultrasensitive optical gas sensing. Sens. Actuators B Chem. 2018, 261, 83–90. [Google Scholar] [CrossRef]
- Stavenga, D.G. Thin film and multilayer optics cause structural colors of many insects and birds. Mater. Today Proc. 2014, 1, 109–121. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Dong, Q.; Shi, L.; Yin, H.W.; Liu, X.H.; Zi, J. Color production in blue and green feather barbs of the rosy-faced lovebird. Mater. Today Proc. 2014, 1, 130–137. [Google Scholar] [CrossRef]
- Lee, C.H.; Yu, J.; Wang, Y.; Tang, A.Y.L.; Kan, C.W.; Xin, J.H. Effect of graphene oxide inclusion on the optical reflection of a silica photonic crystal film. RSC Adv. 2018, 8, 16593–16602. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Scotti, G.; Sikanen, T.; Jokinen, V.; Fraanssila, S. Laser Direct Writing of Thick Hybrid Polymers for Microfluidic Chips. Micromachines 2014, 5, 472–485. [Google Scholar] [CrossRef]
- Giacomo, A.D.; Dellaglio, M.; Gaudiuso, R.; Amoruso, S.; Pascale, O.D. Effects of the background environment on formation, evolution and emission spectra of laser-induced plasmas. Spectrochim. Acta B 2012, 78, 1–19. [Google Scholar] [CrossRef]
- Abdou, M.S.A.; Orfino, F.P.; Son, Y.; Holdcroft, S. Interaction of Oxygen with Conjugated Polymers: Charge Transfer Complex Formation with Poly(3-alkylthiophenes). J. Am. Chem. Soc. 1997, 119, 4518–4524. [Google Scholar] [CrossRef]
- Frisoli, J.K.; Hefetz, Y.; Deutsch, T.F. Time-resolved UV absorption of polyimide. Appl. Phys. A. 1991, 52, 168–172. [Google Scholar] [CrossRef]
- Qu, C.; Hu, J.; Liu, X.; Li, Z.; Ding, Y. Morphology and Mechanical Properties of Polyimide Films: The Effects of UV Irradiation on Microscale Surface. Materials 2017, 10, 1329. [Google Scholar] [CrossRef] [Green Version]
- Plis, E.A.; Engelhart, D.P.; Cooper, R.; Johnston, W.R.; Reguson, D.; Horrmann, R. Review of Radiation-Induced Effects in Polyimide. Appl. Sci. 2019, 9, 1999. [Google Scholar] [CrossRef] [Green Version]
- Yingling, Y.G.; Garrison, B.J. Photochemical ablation of organic solids. Nucl. Instrum. Methods Phys. Res. B 2003, 202, 188–194. [Google Scholar] [CrossRef]
- Brenna, J.T.; Creasy, W.R.; Volksen, W. Elemental composition of ions associated with low mass carbon clusters generated by UV laser ablation of polyimide. Chem. Phys. Lett. 1989, 163, 499–502. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Fernandes, A.J.S.; Leitao, C.; Deuermeier, J.; Marques, A.C.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-Induced Graphene Strain Sensors Produced by Ultraviolet Irradiation of Polyimide. Adv. Funct. Mater. 2018, 28, 1805271. [Google Scholar] [CrossRef]
- Du, Q.; Ai, J.; Qin, Z.; Liu, J.; Zeng, X. Fabrication of superhydrophobic/superhydrophilic patterns on polyimide surface by ultraviolet laser direct texturing. J. Mater. Process. 2018, 251, 188–196. [Google Scholar] [CrossRef]
- Hou, T.; Bai, S.; Zhou, W.; Hu, A. Laser direct writing of highly conductive circuits on modified polyimide Laser Direct Writing of Highly Conductive Circuits on Modified Polyimide. JLMN 2017, 12, 10–15. [Google Scholar] [CrossRef]
- Gamaly, E.G.; Rode, A.V.; Luther-Davies, B. Laser ablation of carbon at the threshold of plasma formation. Appl. Phys. A. 1999, 69, S121–S127. [Google Scholar] [CrossRef]
- Dong, Y.; Rismiller, S.C.; Lin, J. Molecular dynamic simulation of layered graphene clusters formation from polyimides under extreme conditions. Carbon 2016, 104, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Kappes, R.S.; Schonfeld, F.; Li, C.; Golriz, A.A.; Nagel, M.; Lippert, T.; Butt, H.; Cutmann, J.S. A study of photothermal laser ablation of various polymers on microsecond time scales. SpringPlus 2014, 3, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokobza, L.; Bruneel, J.L.; Couzi, M. Raman spectroscopy as a tool for the analysis of carbon-based materials (highly oriented pyrolitic graphite, multilayer graphene and multiwall carbon nanotubes) and of some of their elastomeric composites. Vib. Spectrosc. 2014, 74, 57–63. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakabson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- Akhavan, O.; Bijanzad, K.; Mirsepah, A. Synthesis of graphene from natural and industrial carbonaceous wastes. RSC Adv. 2014, 4, 20441–20448. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, Z.; Yao, J.; Beitler, E.; Zhu, Y.; Tour, J.M. Growth of graphene from solid carbon sources. Nature 2010, 468, 549–552. [Google Scholar] [CrossRef]
- Silva, D.L.C.E.; Kassab, L.R.P.; Santos, A.D.D. Evaluation of Carbon thin Films Using Raman Spectroscopy. Mater. Res. 2018, 21, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lax, M. Multiple Scattering of Waves. Rev. Mod. Phys. 1951, 23, 287. [Google Scholar] [CrossRef]
- Ingram, A.L.; Parker, A.R. A review of the diversity and evolution of photonic structures in butterflies, incorporating the work of John Huxley (The Natural History Museum, London from 1961 to 1990). Philos. Trans. R. Soc. Lond. Ser. B 2008, 363, 2465–2480. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Parameter | Unit | Value |
|---|---|---|
| Wavelength | nm | 355 |
| Average power | Watt | ~2.5 |
| Pulse duration | ns | 25 |
| Repetition rate | kHz | 30 |
| Mode | - | TEM00 |
| Beam diameter | mm | 1.5 |
| Beam divergence | mard | <0.5 |
| Polymer Bond | C–N | C–H | C≡C | O–O | C=C | C–C | N–N | H–H |
|---|---|---|---|---|---|---|---|---|
| Bond Energy (eV) | 3.04 | 4.30 | 8.44 | 5.12 | 6.40 | 3.62 | 9.76 | 4.48 |
| Laser Speed (mm/s) | Spot Size (μm) | Laser Fluence (mJ/cm2) |
|---|---|---|
| 100 | 35 | 3.77 |
| 80 | 70 | 4.15 |
| 60 | 100 | 4.53 |
| 40 | 120 | 4.90 |
| 20 | 140 | 5.28 |
| Laser Speed (mm/s) | Thickness of 3D Carbon Network (µm) | Total Thickness of Pattern (µm) |
|---|---|---|
| 60 | 30 | 130 |
| 40 | 45 | 125 |
| 20 | 65 | 120 |
| Laser Speed (mm/s) | |
|---|---|
| 100 | 1.42 |
| 80 | 1.21 |
| 60 | 1.05 |
| 40 | 1.03 |
| 20 | 0.94 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-U.; Ma, Y.-W.; Jeong, S.-Y.; Shin, B.-S. Fabrication of UV Laser-Induced Porous Graphene Patterns with Nanospheres and Their Optical and Electrical Characteristics. Materials 2020, 13, 3930. https://doi.org/10.3390/ma13183930
Lee J-U, Ma Y-W, Jeong S-Y, Shin B-S. Fabrication of UV Laser-Induced Porous Graphene Patterns with Nanospheres and Their Optical and Electrical Characteristics. Materials. 2020; 13(18):3930. https://doi.org/10.3390/ma13183930
Chicago/Turabian StyleLee, Jun-Uk, Yong-Won Ma, Sung-Yeob Jeong, and Bo-Sung Shin. 2020. "Fabrication of UV Laser-Induced Porous Graphene Patterns with Nanospheres and Their Optical and Electrical Characteristics" Materials 13, no. 18: 3930. https://doi.org/10.3390/ma13183930
APA StyleLee, J.-U., Ma, Y.-W., Jeong, S.-Y., & Shin, B.-S. (2020). Fabrication of UV Laser-Induced Porous Graphene Patterns with Nanospheres and Their Optical and Electrical Characteristics. Materials, 13(18), 3930. https://doi.org/10.3390/ma13183930





