A Vitronectin-Derived Bioactive Peptide Improves Bone Healing Capacity of SLA Titanium Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Peptides, and Reagents
2.2. Disc Preparation and Surface Characterization
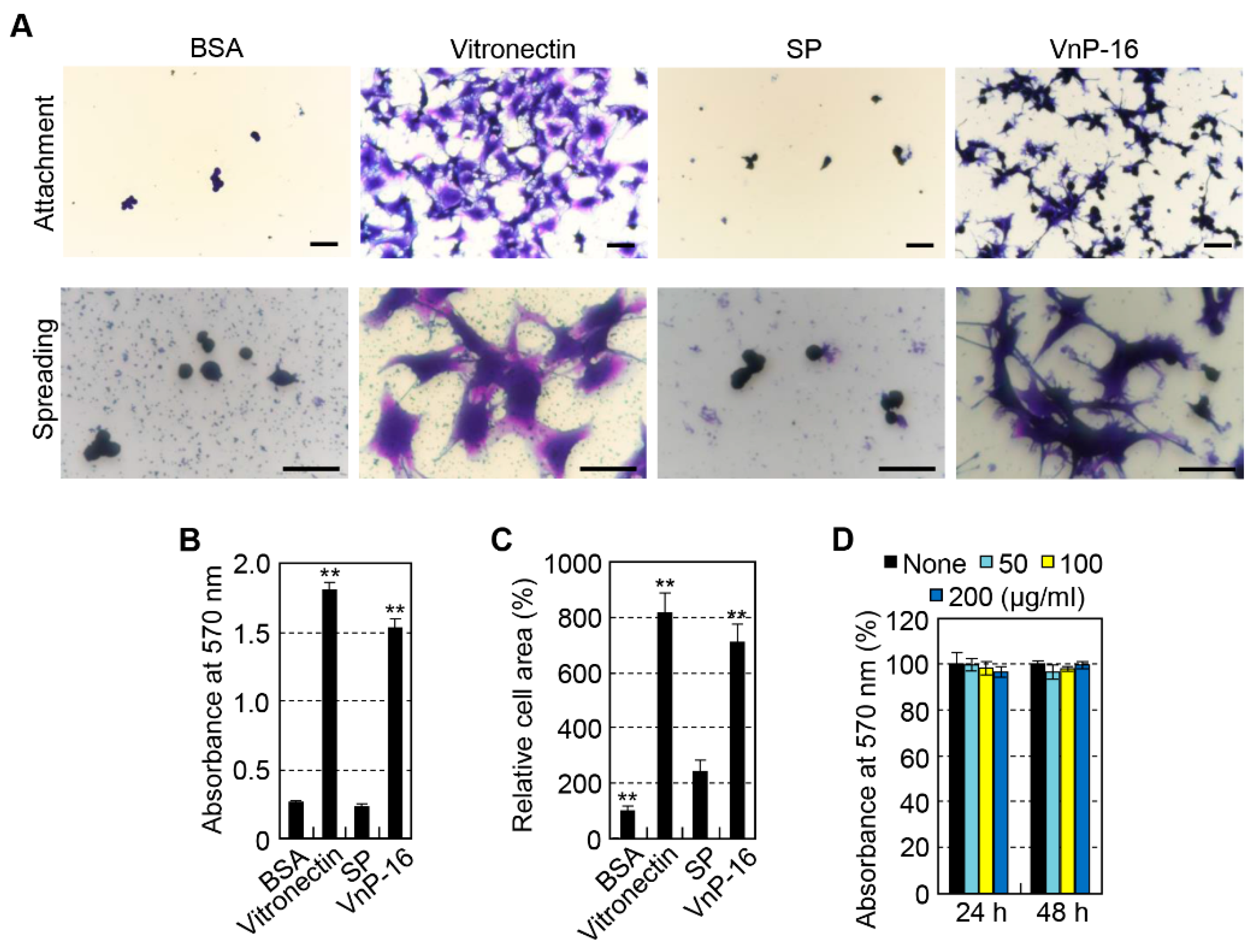
2.3. Cell Attachment and Spreading Assays
2.4. Migration Assay
2.5. Cell Viability Assay
2.6. In Vivo Experiment
2.7. Light Microscopic Evaluation
2.8. Statistics
3. Results
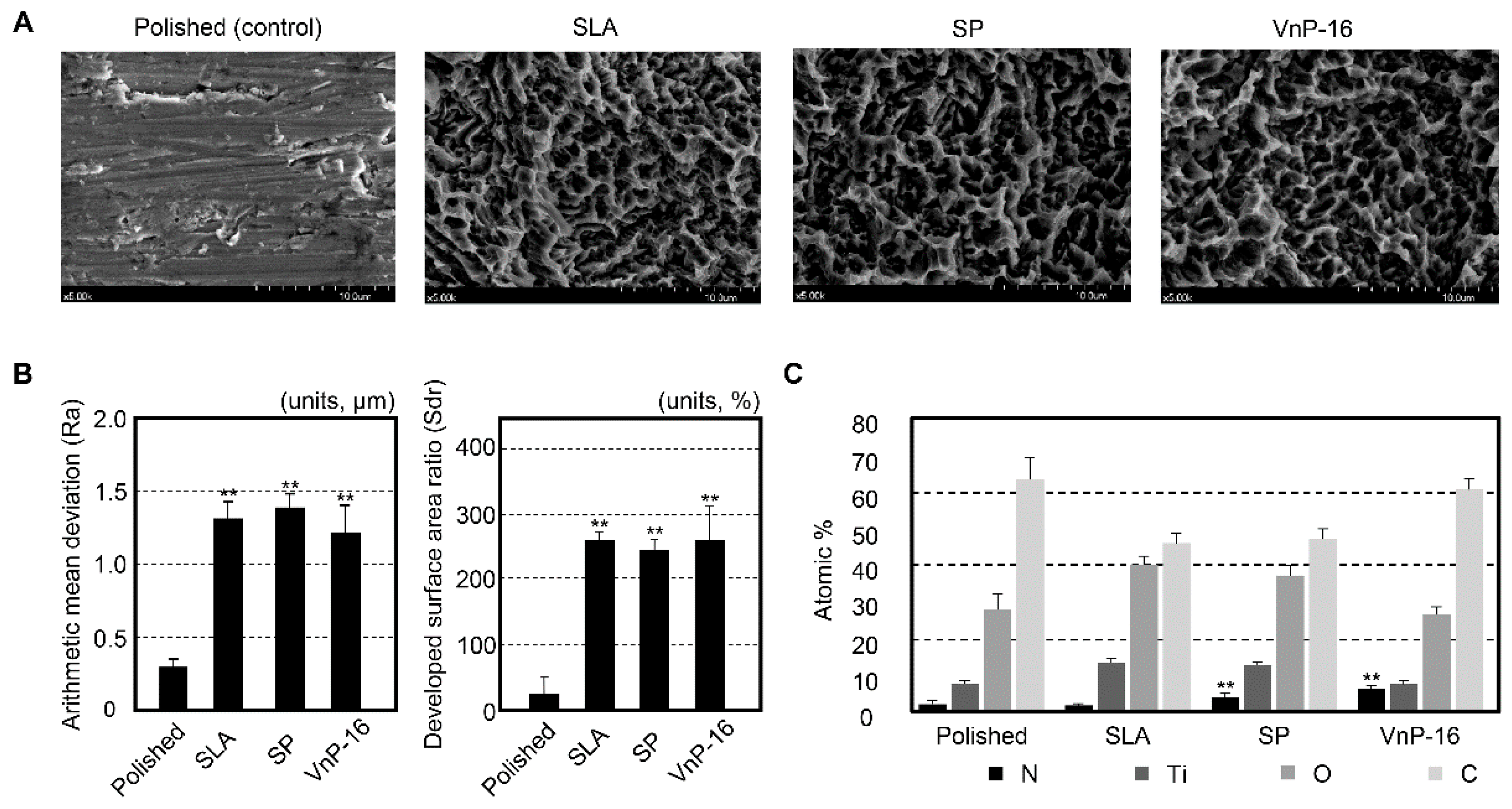
3.1. Surface Characteristics
3.2. Effects of VnP-16 Peptide on Cellular Responses of Human Osteoblast-Like Cells
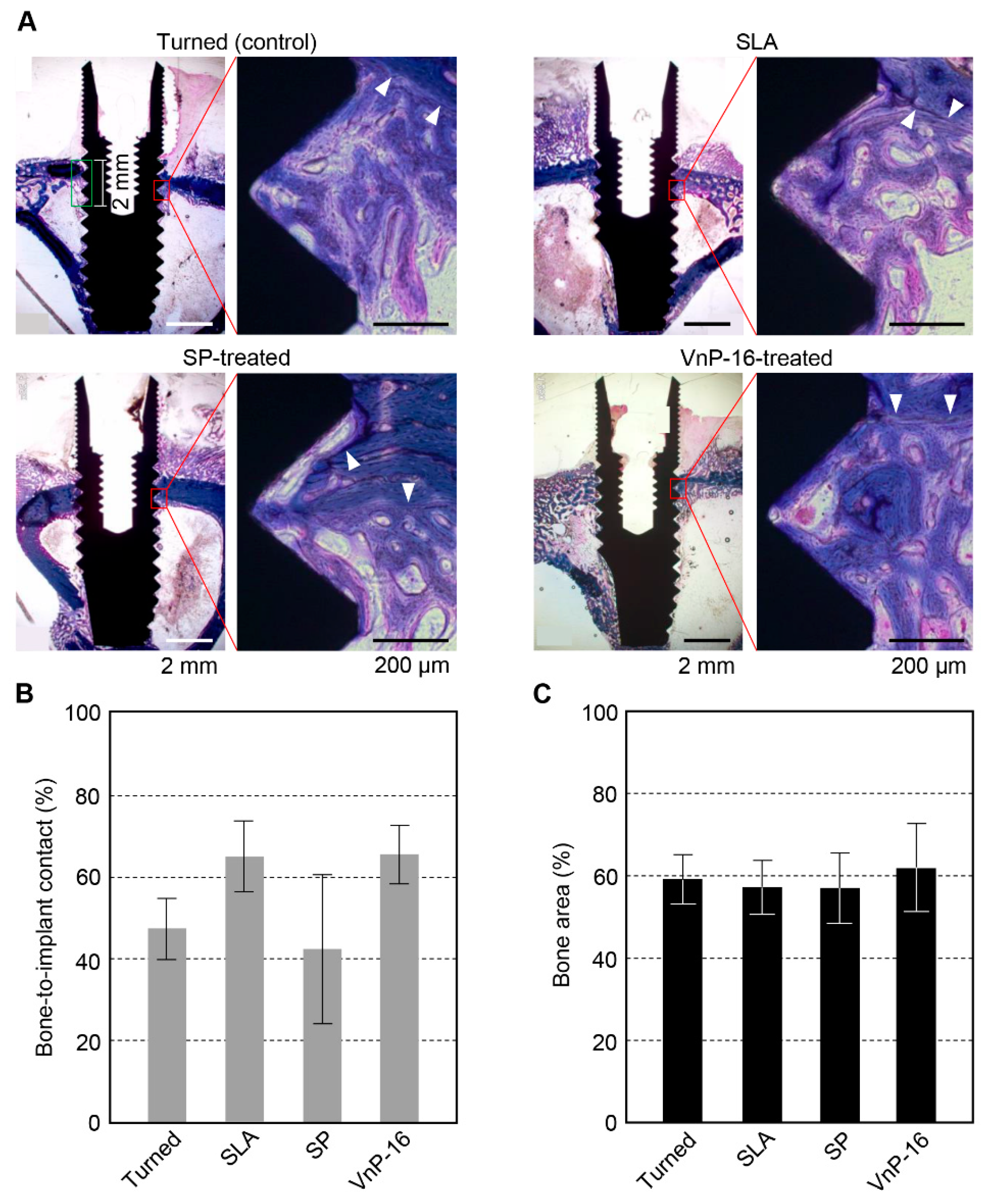
3.3. Histomorphometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Ravanetti, F.; Gazza, F.; D’Arrigo, D.; Graiani, G.; Zamuner, A.; Zedda, M.; Manfredi, E.; Dettin, M.; Cacchioli, A. Enhancement of peri-implant bone osteogenic activity induced by a peptidomimetic functionalization of titanium. Ann. Anat. 2018, 218, 165–174. [Google Scholar] [CrossRef]
- Kang, H.K.; Kim, O.B.; Min, S.K.; Jung, S.Y.; Jang, D.H.; Kwon, T.K.; Min, B.M.; Yeo, I.S. The effect of the DLTIDDSYWYRI motif of the human laminin alpha2 chain on implant osseointegration. Biomaterials 2013, 34, 4027–4037. [Google Scholar] [CrossRef]
- Shekaran, A.; Garcia, A.J. Extracellular matrix-mimetic adhesive biomaterials for bone repair. J. Biomed. Mater. Res. A 2011, 96, 261–272. [Google Scholar] [CrossRef]
- Yeo, I.S.; Min, S.K.; Kang, H.K.; Kwon, T.K.; Jung, S.Y.; Min, B.M. Identification of a bioactive core sequence from human laminin and its applicability to tissue engineering. Biomaterials 2015, 73, 96–109. [Google Scholar] [CrossRef]
- Boron, W.F.; Boulpaep, E.L. Medical Physiology E-Book; Elsevier Health Sciences: Kidlington, Oxford, UK, 2016. [Google Scholar]
- Cherny, R.C.; Honan, M.A.; Thiagarajan, P. Site-directed mutagenesis of the arginine-glycine-aspartic acid in vitronectin abolishes cell adhesion. J. Biol. Chem. 1993, 268, 9725–9729. [Google Scholar]
- Shin, T.M.; Isas, J.M.; Hsieh, C.L.; Kayed, R.; Glabe, C.G.; Langen, R.; Chen, J. Formation of soluble amyloid oligomers and amyloid fibrils by the multifunctional protein vitronectin. Mol. Neurodegener. 2008, 3, 16. [Google Scholar] [CrossRef]
- Min, S.K.; Kang, H.K.; Jung, S.Y.; Jang, D.H.; Min, B.M. A vitronectin-derived peptide reverses ovariectomy-induced bone loss via regulation of osteoblast and osteoclast differentiation. Cell Death Differ. 2018, 25, 268–281. [Google Scholar] [CrossRef]
- Yeo, I.S. Surface modification of dental biomaterials for controlling bone response. In Bone Response to Dental Implant Materials; Elsevier: Kidlington, Oxford, UK, 2017; pp. 43–46. [Google Scholar]
- Howlett, C.R.; Evans, M.D.; Walsh, W.R.; Johnson, G.; Steele, J.G. Mechanism of initial attachment of cells derived from human bone to commonly used prosthetic materials during cell culture. Biomaterials 1994, 15, 213–222. [Google Scholar] [CrossRef]
- Rivera-Chacon, D.M.; Alvarado-Velez, M.; Acevedo-Morantes, C.Y.; Singh, S.P.; Gultepe, E.; Nagesha, D.; Sridhar, S.; Ramirez-Vick, J.E. Fibronectin and vitronectin promote human fetal osteoblast cell attachment and proliferation on nanoporous titanium surfaces. J. Biomed. Nanotechnol. 2013, 9, 1092–1097. [Google Scholar] [CrossRef]
- Scotchford, C.A.; Ball, M.; Winkelmann, M.; Voros, J.; Csucs, C.; Brunette, D.M.; Danuser, G.; Textor, M. Chemically patterned, metal-oxide-based surfaces produced by photolithographic techniques for studying protein- and cell-interactions. II: Protein adsorption and early cell interactions. Biomaterials 2003, 24, 1147–1158. [Google Scholar] [CrossRef]
- Cacchioli, A.; Ravanetti, F.; Bagno, A.; Dettin, M.; Gabbi, C. Human Vitronectin-Derived Peptide Covalently Grafted onto Titanium Surface Improves Osteogenic Activity: A Pilot In Vivo Study on Rabbits. Tissue Eng. Part. A 2009, 15, 2917–2926. [Google Scholar] [CrossRef]
- Petrie, T.A.; Raynor, J.E.; Dumbauld, D.W.; Lee, T.T.; Jagtap, S.; Templeman, K.L.; Collard, D.M.; Garcia, A.J. Multivalent integrin-specific ligands enhance tissue healing and biomaterial integration. Sci. Transl. Med. 2010, 2, 45ra60. [Google Scholar] [CrossRef]
- Rezania, A.; Healy, K.E. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnol. Prog. 1999, 15, 19–32. [Google Scholar] [CrossRef]
- Vukicevic, S.; Luyten, F.P.; Kleinman, H.K.; Reddi, A.H. Differentiation of canalicular cell processes in bone cells by basement membrane matrix components: Regulation by discrete domains of laminin. Cell 1990, 63, 437–445. [Google Scholar] [CrossRef]
- Martin, L.J.; Akhavan, B.; Bilek, M.M.M. Electric fields control the orientation of peptides irreversibly immobilized on radical-functionalized surfaces. Nat. Commun. 2018, 9, 357. [Google Scholar] [CrossRef]
- Stewart, C.; Akhavan, B.; Wise, S.G.; Bilek, M.M. A review of biomimetic surface functionalization for bone-integrating orthopedic implants: Mechanisms, current approaches, and future directions. Prog. Mater. Sci. 2019, 106, 100588. [Google Scholar] [CrossRef]
- Pagel, M.; Hassert, R.; John, T.; Braun, K.; Wiessler, M.; Abel, B.; Beck-Sickinger, A.G. Multifunctional Coating Improves Cell Adhesion on Titanium by using Cooperatively Acting Peptides. Angew Chem. Int. Ed. Engl. 2016, 55, 4826–4830. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kang, S.H.; Kim, H.Y.; Yeo, I.L. Control Variable Implants Improve Interpretation of Surface Modification and Implant Design Effects on Early Bone Responses: An In Vivo Study. Int J. Oral Maxillofac Implant. 2018, 33, 1033–1040. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implant. 2010, 25, 63–74. [Google Scholar]
- Kim, S.; Choi, J.Y.; Jung, S.Y.; Kang, H.K.; Min, B.M.; Yeo, I.L. A laminin-derived functional peptide, PPFEGCIWN, promotes bone formation on sandblasted, large-grit, acid-etched titanium implant surfaces. Int. J. Oral Maxillofac. Implant. 2019, 34, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Min, S.K.; Kim, H.; Kang, H.K.; Jung, S.Y.; Lee, S.H.; Choi, Y.; Roh, S.; Jeong, D.; Min, B.M. Vacuolar-type H+-ATPase-mediated acidosis promotes in vitro osteoclastogenesis via modulation of cell migration. Int. J. Mol. Med. 2007, 19, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 2012, 20, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage-Schliff (sawing and grinding) technique. J. Oral Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef]
- Gruber, H.E. Adaptations of Goldner’s Masson trichrome stain for the study of undecalcified plastic embedded bone. Biotech. Histochem. 1992, 67, 30–34. [Google Scholar] [CrossRef]
- Buser, D.; Janner, S.F.; Wittneben, J.G.; Bragger, U.; Ramseier, C.A.; Salvi, G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant. Dent. Relat Res. 2012, 14, 839–851. [Google Scholar] [CrossRef]
- Van Velzen, F.J.; Ofec, R.; Schulten, E.A.; Ten Bruggenkate, C.M. 10-year survival rate and the incidence of peri-implant disease of 374 titanium dental implants with a SLA surface: A prospective cohort study in 177 fully and partially edentulous patients. Clin. Oral Implant. Res. 2015, 26, 1121–1128. [Google Scholar] [CrossRef]
- Tsolaki, I.N.; Madianos, P.N.; Vrotsos, J.A. Outcomes of dental implants in osteoporotic patients. A literature review. J. Prosthodont. 2009, 18, 309–323. [Google Scholar] [CrossRef]
- Choi, S.H.; Jeong, W.S.; Cha, J.Y.; Lee, J.H.; Lee, K.J.; Yu, H.S.; Choi, E.H.; Kim, K.M.; Hwang, C.J. Overcoming the biological aging of titanium using a wet storage method after ultraviolet treatment. Sci. Rep. 2017, 7, 3833. [Google Scholar] [CrossRef]
- Lee, J.B.; Jo, Y.H.; Choi, J.Y.; Seol, Y.J.; Lee, Y.M.; Ku, Y.; Rhyu, I.C.; Yeo, I.L. The Effect of Ultraviolet Photofunctionalization on a Titanium Dental Implant with Machined Surface: An In Vitro and In Vivo Study. Materials 2019, 12, 2078. [Google Scholar] [CrossRef]
- Choi, J.Y.; Park, J.I.; Chae, J.S.; Yeo, I.L. Comparison of micro-computed tomography and histomorphometry in the measurement of bone-implant contact ratios. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 87–95. [Google Scholar] [CrossRef]
- Bernhardt, R.; Kuhlisch, E.; Schulz, M.C.; Eckelt, U.; Stadlinger, B. Comparison of bone-implant contact and bone-implant volume between 2D-histological sections and 3D-SRmicroCT slices. Eur. Cell Mater. 2012, 23, 237–247, discussion 247–248. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, C.-B.; Jung, S.Y.; Park, C.Y.; Kang, H.K.; Yeo, I.-S.L.; Min, B.-M. A Vitronectin-Derived Bioactive Peptide Improves Bone Healing Capacity of SLA Titanium Surfaces. Materials 2019, 12, 3400. https://doi.org/10.3390/ma12203400
Cho C-B, Jung SY, Park CY, Kang HK, Yeo I-SL, Min B-M. A Vitronectin-Derived Bioactive Peptide Improves Bone Healing Capacity of SLA Titanium Surfaces. Materials. 2019; 12(20):3400. https://doi.org/10.3390/ma12203400
Chicago/Turabian StyleCho, Chang-Bin, Sung Youn Jung, Cho Yeon Park, Hyun Ki Kang, In-Sung Luke Yeo, and Byung-Moo Min. 2019. "A Vitronectin-Derived Bioactive Peptide Improves Bone Healing Capacity of SLA Titanium Surfaces" Materials 12, no. 20: 3400. https://doi.org/10.3390/ma12203400





