Hydroxyapatite Block Produced by Sponge Replica Method: Mechanical, Clinical and Histologic Observations
Abstract
:1. Introduction
2. Materials and Methods
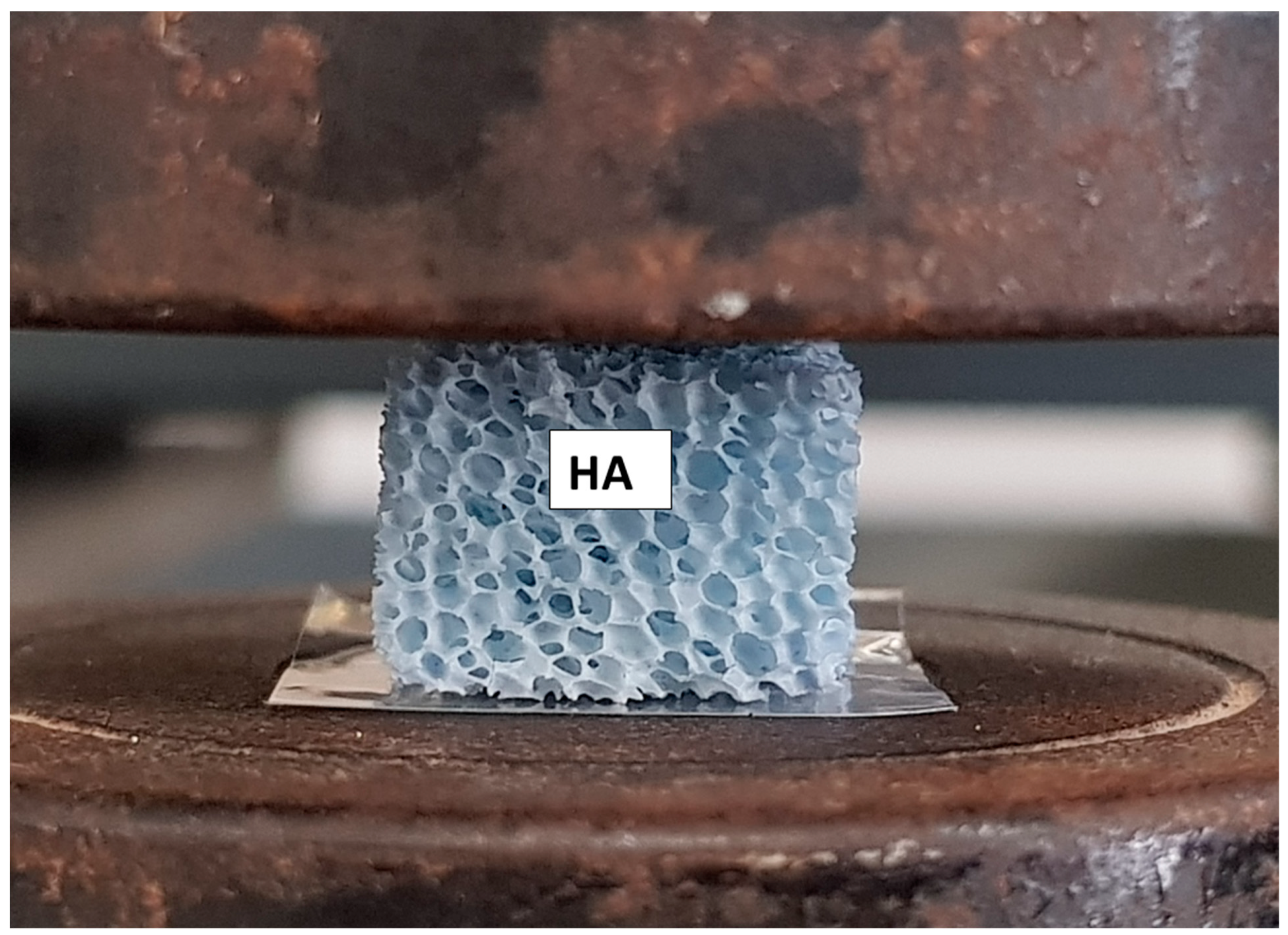
2.1. Fabrication of HA Scaffold
2.2. Packaging and Sterilization
2.3. Device Production Quality Assessment
3. Characterization
3.1. In Vivo Experiment
- 1.
- fully or partially edentulous/unilateral or bilateral loss of maxillary premolar with residual height of the alveolar ridge between 3 and 4 mm.
- 2.
- severe illnesses or uncontrolled diabetes;
- 3.
- neck and head radiation therapy;
- 4.
- radiotherapy or chemotherapy;
- 5.
- presence of a residual root, sinus pathology, periodontal disease;
- 6.
- tabagism.
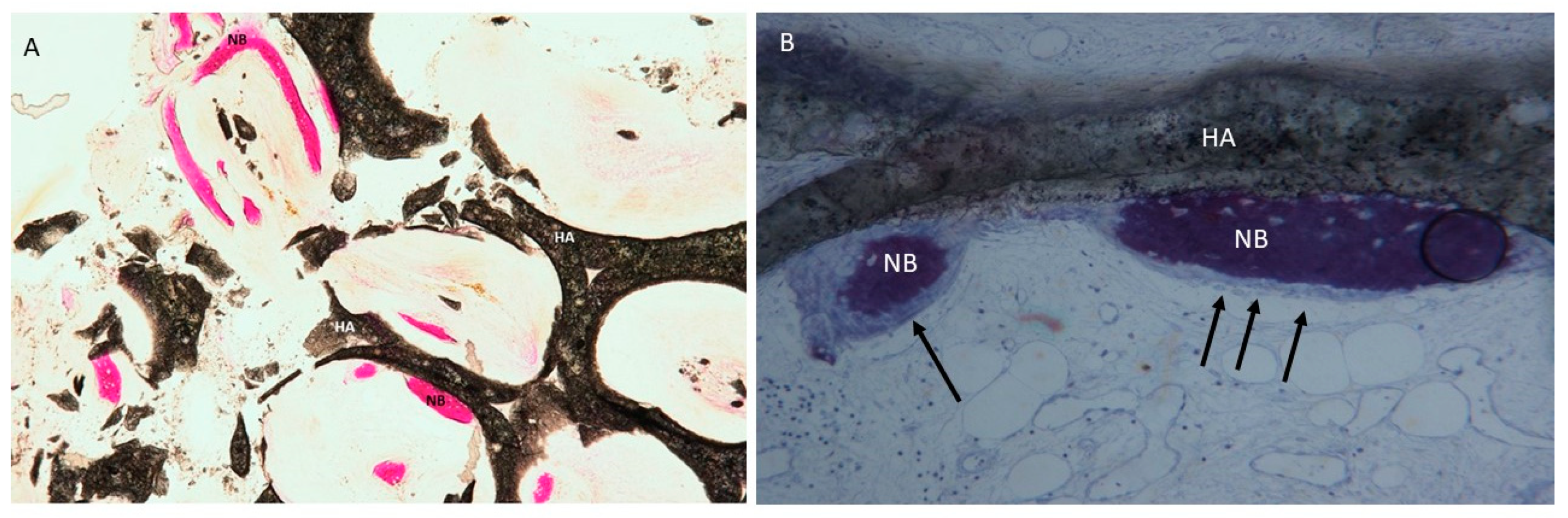
3.2. Processing of Specimens
4. Results
4.1. Scaffold Properties
4.2. Clinical and Histological
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tatum, H. Maxillary and sinus implant reconstructions. Dent. Clin. N. Am. 1986, 30, 207–229. [Google Scholar] [PubMed]
- Scarano, A.; Perrotti, V.; Carinci, F.; Shibli, J.A. Removal of a migrated dental implant from the maxillary sinus after 7 years: A case report. Oral Maxillofac. Surg. 2011, 15, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Piattelli, M.; Scarano, A.; Iezzi, G.; Piattelli, A. Maxillary sinus augmentation with a synthetic cell-binding peptide: Histological and histomorphometrical results in humans. J. Oral Implantol. 2004, 30, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Boyne, P.J.; James, R.A. Grafting of the maxillary sinus floor with autogenous marrow and bone. J. Oral Surg. 1980, 38, 613–616. [Google Scholar] [PubMed]
- Carinci, F.; Piattelli, A.; Degidi, M.; Palmieri, A.; Perrotti, V.; Scapoli, L.; Martinelli, M.; Zuccarino, L.; Pezzetti, F. Effects of demineralized freeze-dried bone allograft on gene expression of osteoblastlike MG63 cells. Int. J. Periodontics Restor. Dent. 2007, 27, 596–601. [Google Scholar]
- Froum, S.J.; Tarnow, D.P.; Wallace, S.S.; Rohrer, M.D.; Cho, S.C. Sinus floor elevation using anorganic bovine bone matrix (OsteoGraf/N) with and without autogenous bone: A clinical, histologic, radiographic, and histomorphometric analysis--Part 2 of an ongoing prospective study. Int. J. Periodontics Restor. Dent. 1998, 18, 528–543. [Google Scholar]
- Scarano, A.; Iezzi, G.; Petrone, G.; Orsini, G.; Degidi, M.; Strocchi, R.; Piattelli, A. Cortical bone regeneration with a synthetic cell-binding peptide: A histologic and histomorphometric pilot study. Implant. Dent. 2003, 12, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Scarano, A.; Perrotti, V.; Iezzi, G.; Piattelli, A. Maxillary sinus augmentation with a porous synthetic hydroxyapatite and bovine-derived hydroxyapatite: A comparative clinical and histologic study. Int. J. Oral Maxillofac. Implants 2007, 22, 980–986. [Google Scholar]
- Lundgren, S.; Andersson, S.; Gualini, F.; Sennerby, L. Bone reformation with sinus membrane elevation: A new surgical technique for maxillary sinus floor augmentation. Clin. Implant. Dent. Relat. Res. 2004, 6, 165–173. [Google Scholar] [CrossRef]
- Hallman, M.; Sennerby, L.; Lundgren, S. A clinical and histologic evaluation of implant integration in the posterior maxilla after sinus floor augmentation with autogenous bone, bovine hydroxyapatite, or a 20:80 mixture. Int. J. Oral Maxillofac. Implants 2002, 17, 635–643. [Google Scholar]
- Samartzis, D.; Shen, F.H.; Goldberg, E.J.; An, H.S. Is autograft the gold standard in achieving radiographic fusion in one-level anterior cervical discectomy and fusion with rigid anterior plate fixation? Spine 2005, 30, 1756–1761. [Google Scholar] [CrossRef] [PubMed]
- Gervaso, F.; Scalera, F.; Kunjalukkal Padmanabhan, S.; Sannino, A.; Licciulli, A. High-Performance hydroxyapatite scaffolds for bone tissue engineering applications. Int. J. Appl. Ceram. Technol. 2012, 9, 507–516. [Google Scholar] [CrossRef]
- Fürst, G.; Gruber, R.; Tangl, S.; Zechner, W.; Haas, R.; Mailath, G.; Sanroman, F.; Watzek, G. Sinus grafting with autogenous platelet-rich plasma and bovine hydroxyapatite. A histomorphometric study in minipigs. Clin. Oral Implants Res. 2003, 14, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Piattelli, A.; Perrotti, V.; Iezzi, G. Dense hydroxyapatite inserted into postextraction sockets: A histologic and histomorphometric 20-year case report. J. Periodontol. 2008, 79, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Figliuzzi, M.; Mangano, F.G.; Fortunato, L.; De Fazio, R.; Macchi, A.; Iezzi, G.; Piattelli, A.; Mangano, C. Vertical ridge augmentation of the atrophic posterior mandible with custom-made, computer-aided design/computer-aided manufacturing porous hydroxyapatite scaffolds. J. Craniofac. Surg. 2013, 24, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, E.H. Chemical disinfection and antisepsis in the hospital. J. Hosp. Res. 1972, 9, 5–31. [Google Scholar]
- Scarano, A.; Lorusso, F.; Arcangelo, M.; D’Arcangelo, C.; Celletti, R.; de Oliveira, P.S. Lateral Sinus Floor Elevation Performed with Trapezoidal and Modified Triangular Flap Designs: A Randomized Pilot Study of Post-Operative Pain Using Thermal Infrared Imaging. Int. J. Environ. Res. Public Health 2018, 15, 1277. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; de Oliveira, P.S.; Traini, T.; Lorusso, F. Sinus Membrane Elevation with Heterologous Cortical Lamina: A Randomized Study of a New Surgical Technique for Maxillary Sinus Floor Augmentation without Bone Graft. Materials (Basel) 2018, 11, 1457. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, A.; Scarano, A.; Quaranta, M. High-precision, cost-effective cutting system for producing thin sections of oral tissues containing dental implants. Biomaterials 1997, 18, 577–579. [Google Scholar] [CrossRef]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Tsuruga, E.; Takita, H.; Itoh, H.; Wakisaka, Y.; Kuboki, Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J. Biochem. 1997, 121, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.L.; Dias, R.O.; Piattelli, A.; Onuma, T.; Gouveia Cardoso, L.A.; Salomão, M.; Scarano, A.; Ayub, E.; Shibli, J.A. Simultaneous sinus membrane elevation and dental implant placement without bone graft: A 6-month follow-up study. J. Periodontol. 2011, 82, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, G.; Piattelli, A.; Giuliani, A.; Mangano, C.; Manzon, L.; Degidi, M.; Iaculli, F.; Scarano, A.; Filippone, A.; Perrotti, V. Molecular, Cellular and Pharmaceutical Aspects of Bone Grafting Materials and Membranes During Maxillary Sinus-lift Procedures. Part 1: A General Overview. Curr. Pharm. Biotechnol. 2017, 18, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Lorusso, F.; Ravera, L.; Mortellaro, C.; Piattelli, A. Bone Regeneration in Iliac Crestal Defects: An Experimental Study on Sheep. Biomed. Res. Int. 2016, 2016, 4086870. [Google Scholar] [CrossRef] [PubMed]
- Autefage, H.; Allen, F.; Tang, H.M.; Kallepitis, C.; Gentleman, E.; Reznikov, N.; Nitiputri, K.; Nommeots-Nomm, A.; O’Donnell, M.D.; Lange, C.; et al. Multiscale analyses reveal native-like lamellar bone repair and near perfect bone-contact with porous strontium-loaded bioactive glass. Biomaterials 2019, 209, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Lombardi, T.; Ottonelli, R.; Berton, F.; Perinetti, G.; Traini, T. New bone formation after transcrestal sinus floor elevation was influenced by sinus cavity dimensions: A prospective histologic and histomorphometric study. Clin. Oral Implants Res. 2018, 29, 465–479. [Google Scholar] [CrossRef]
- Iezzi, G.; Piattelli, A.; Giuliani, A.; Mangano, C.; Manzon, L.; Degidi, M.; Iaculli, F.; Scarano, A.; Filippone, A.; Perrotti, V. Molecular, Cellular and Pharmaceutical Aspects of Bone Grafting Materials and Membranes During Maxillary Sinus-lift Procedures. Part 2: Detailed characteristics of the materials. Curr. Pharm. Biotechnol. 2017, 18, 33–44. [Google Scholar] [CrossRef]
- D’Alessandro, D.; Perale, G.; Milazzo, M.; Moscato, S.; Stefanini, C.; Pertici, G.; Danti, S. Bovine bone matrix/poly(l-lactic-co-ε-caprolactone)/gelatin hybrid scaffold (SmartBone®) for maxillary sinus augmentation: A histologic study on bone regeneration. Int. J. Pharm. 2017, 523, 534–544. [Google Scholar] [CrossRef]
- Scarano, A.; Quaranta, M.; Piattelli, A. Bone Sectioning Using the Precise 1 Automated Cutting System. In Handbook of Histology Methods for Bone and Cartilage; Springer: Berlin, Germany, 2003; pp. 265–269. [Google Scholar]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 2041731416648810. [Google Scholar] [CrossRef]
- Gogolewski, S.; Mainil-Varlet, P. The effect of thermal treatment on sterility, molecular and mechanical properties of various polylactides. I. Poly(L-lactide). Biomaterials 1996, 17, 523–528. [Google Scholar] [CrossRef]
- Reissmann, D.R.; Poxleitner, P.; Heydecke, G. Location, intensity, and experience of pain after intra-oral versus extra-oral bone graft harvesting for dental implants. J. Dent. 2018, 79, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Kessler, P.; Thorwarth, M.; Bloch-Birkholz, A.; Nkenke, E.; Neukam, F.W. Harvesting of bone from the iliac crest--comparison of the anterior and posterior sites. Br. J. Oral Maxillofac. Surg. 2005, 43, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Lorusso, F.; Staiti, G.; Sinjari, B.; Tampieri, A.; Mortellaro, C. Sinus Augmentation with Biomimetic Nanostructured Matrix: Tomographic, Radiological, Histological and Histomorphometrical Results after 6 Months in Humans. Front. Physiol. 2017, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Ferracini, R.; Martínez Herreros, I.; Russo, A.; Casalini, T.; Rossi, F.; Perale, G. Scaffolds as Structural Tools for Bone-Targeted Drug Delivery. Pharmaceutics 2018, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Civinini, R.; De Biase, P.; Carulli, C.; Matassi, F.; Nistri, L.; Capanna, R.; Innocenti, M. The use of an injectable calcium sulphate/calcium phosphate bioceramic in the treatment of osteonecrosis of the femoral head. Int. Orthop. 2012, 36, 1583–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massari, L.; Benazzo, F.; Falez, F.; Perugia, D.; Pietrogrande, L.; Setti, S.; Osti, R.; Vaienti, E.; Ruosi, C.; Cadossi, R. Biophysical stimulation of bone and cartilage: State of the art and future perspectives. Int. Orthop. 2019, 43, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Scarano, A.; Iezzi, G.; Orsini, G.; Perrotti, V.; Mangano, F.; Montini, S.; Piccirilli, M.; Piattelli, A. Maxillary sinus augmentation using an engineered porous hydroxyapatite: A clinical, histological, and transmission electron microscopy study in man. J. Oral Implantol. 2006, 32, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Fabrication, Properties and Applications of Dense Hydroxyapatite: A Review. J. Funct. Biomater. 2015, 6, 1099–1140. [Google Scholar] [CrossRef] [Green Version]
- Frame, J.W.; Rout, P.G.; Browne, R.M. Ridge augmentation using solid and porous hydroxylapatite particles with and without autogenous bone or plaster. J. Oral Maxillofac. Surg. 1987, 45, 771–778. [Google Scholar] [CrossRef]
- Turco, G.; Porrelli, D.; Marsich, E.; Vecchies, F.; Lombardi, T.; Stacchi, C.; Di Lenarda, R. Three-Dimensional Bone Substitutes for Oral and Maxillofacial Surgery: Biological and Structural Characterization. J. Funct. Biomater. 2018, 9, 62. [Google Scholar] [CrossRef] [PubMed]


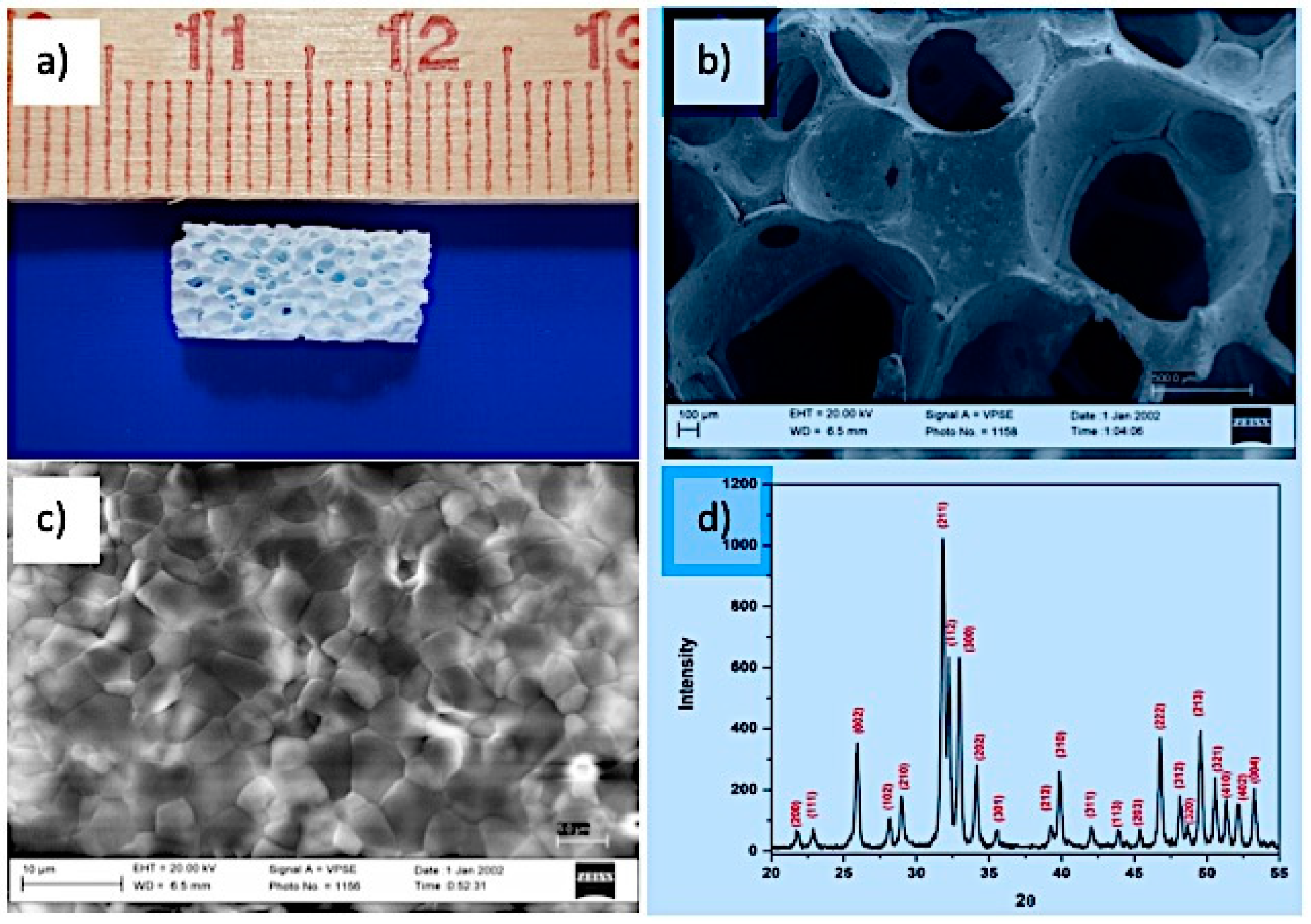

| HA Scaffold Physical Properties | |
|---|---|
| Linear shrinkage (%) | 18 ± 1 |
| Porosity % | 85 ± 3 |
| Pore size (micron) | >300 |
| Compressive strength (MPa) | 0.8 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarano, A.; Lorusso, F.; Santos de Oliveira, P.; Kunjalukkal Padmanabhan, S.; Licciulli, A. Hydroxyapatite Block Produced by Sponge Replica Method: Mechanical, Clinical and Histologic Observations. Materials 2019, 12, 3079. https://doi.org/10.3390/ma12193079
Scarano A, Lorusso F, Santos de Oliveira P, Kunjalukkal Padmanabhan S, Licciulli A. Hydroxyapatite Block Produced by Sponge Replica Method: Mechanical, Clinical and Histologic Observations. Materials. 2019; 12(19):3079. https://doi.org/10.3390/ma12193079
Chicago/Turabian StyleScarano, Antonio, Felice Lorusso, Pablo Santos de Oliveira, Sanosh Kunjalukkal Padmanabhan, and Antonio Licciulli. 2019. "Hydroxyapatite Block Produced by Sponge Replica Method: Mechanical, Clinical and Histologic Observations" Materials 12, no. 19: 3079. https://doi.org/10.3390/ma12193079





