Hyaluronic Acid-Conjugated with Hyperbranched Chlorin e6 Using Disulfide Linkage and Its Nanophotosensitizer for Enhanced Photodynamic Therapy of Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
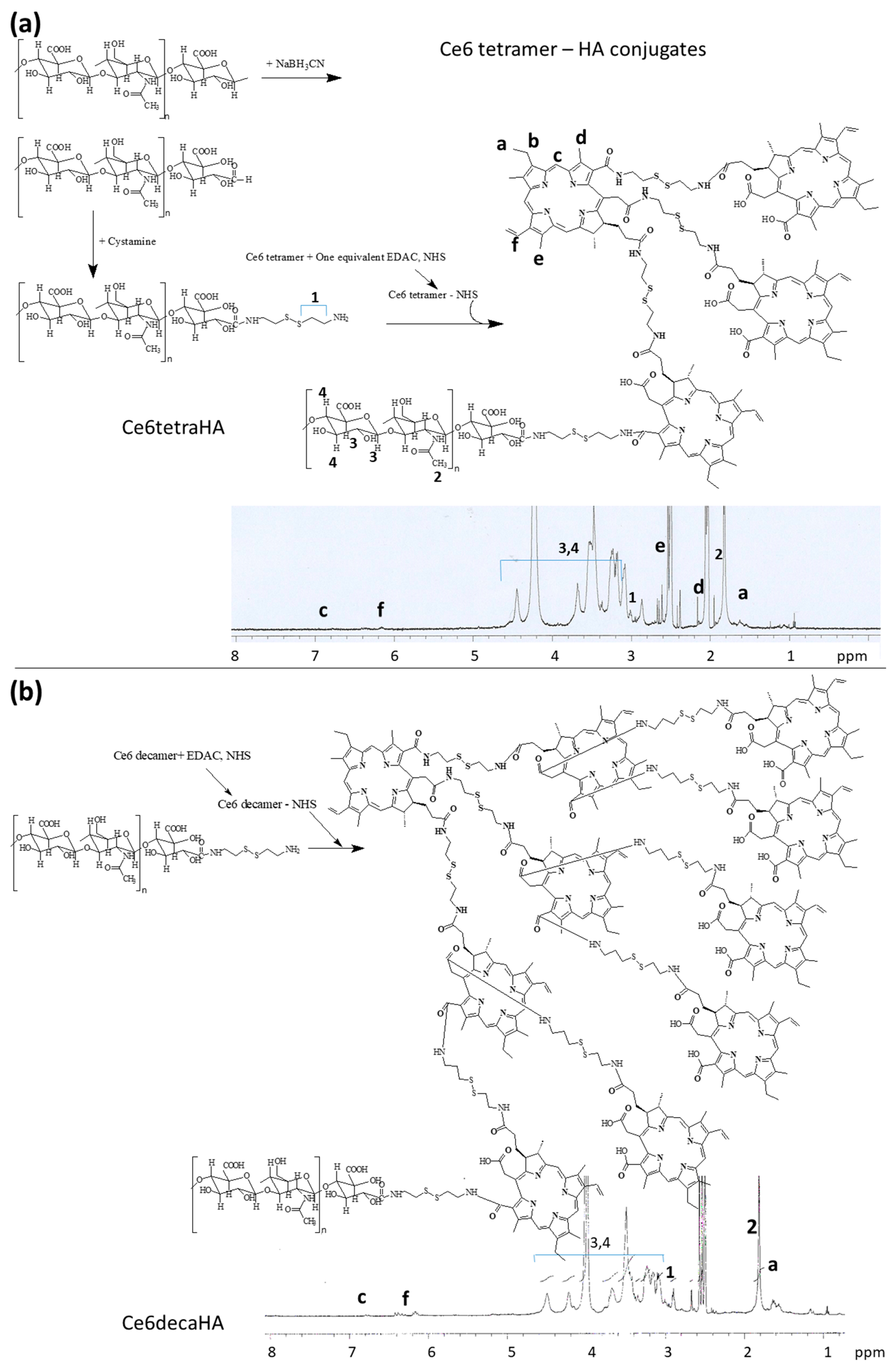
2.2. Synthesis of Ce6tetraHA Conjugates
2.3. Preparation of Ce6tetraHA/Ce6decaHA Nanophotosensitizers
2.4. Characterization of Ce6tetraHA or Ce6decaHA Conjugates and Nanophotosensitizers
2.5. Fluorescence Emission Scan of Nanophotosensitizers
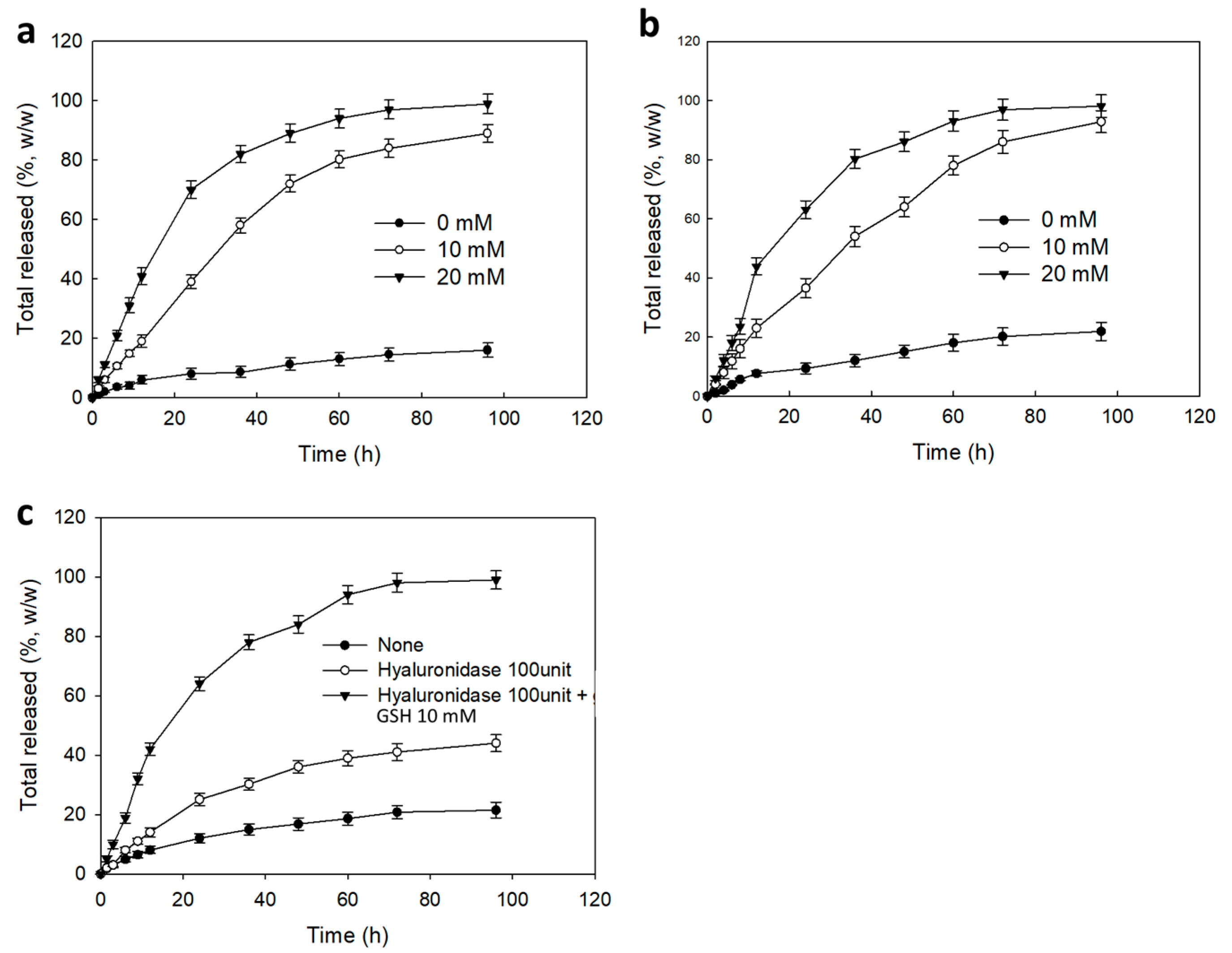
2.6. Ce6 Release from Nanophotosensitizer
2.7. Cell Culture
2.8. PDT Treatment
2.9. Intracellular Ce6 Uptake of Nanophotosensitizers
2.10. Fluorescence Observation of Cells
2.11. ROS Generation
2.12. In vivo Fluorescence Imaging Study Using Animal Tumor Xenograft
2.13. Statistical Analysis
3. Results
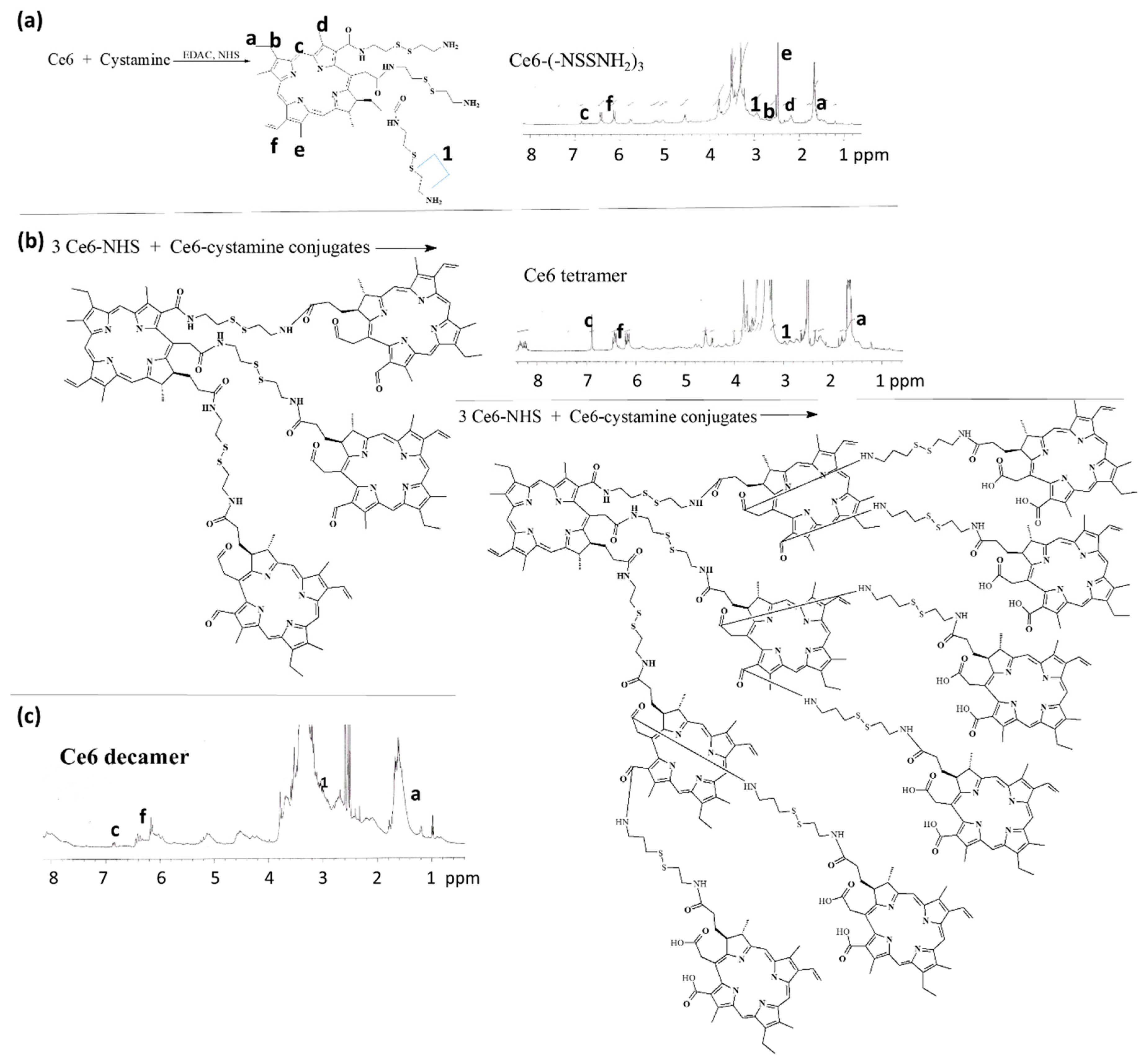
3.1. Synthesis of Ce6tetraHA or Ce6decaHA Conjugates
3.2. Preparation and Characterization of Ce6tetraHA Nanophotosensitizer
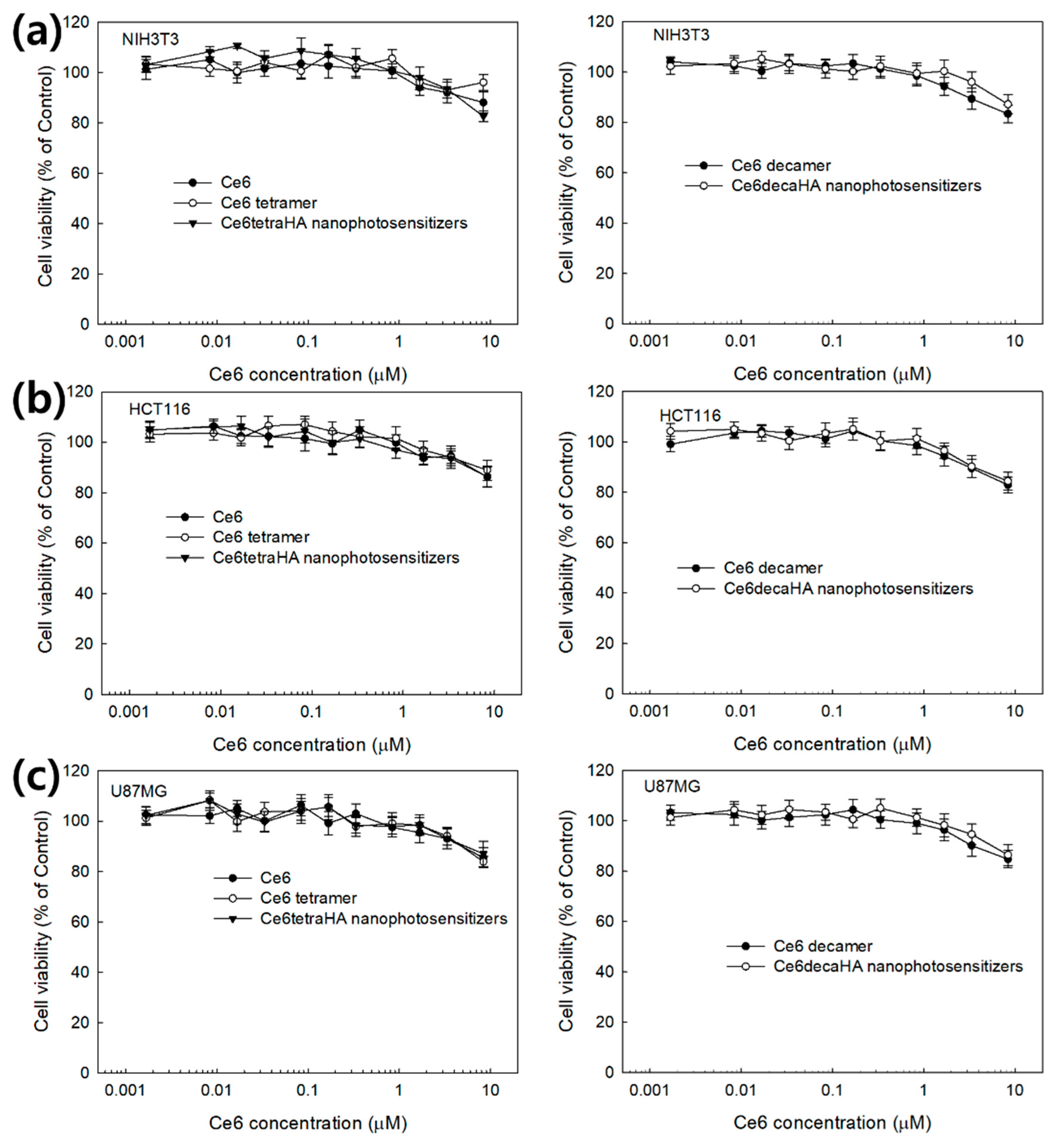
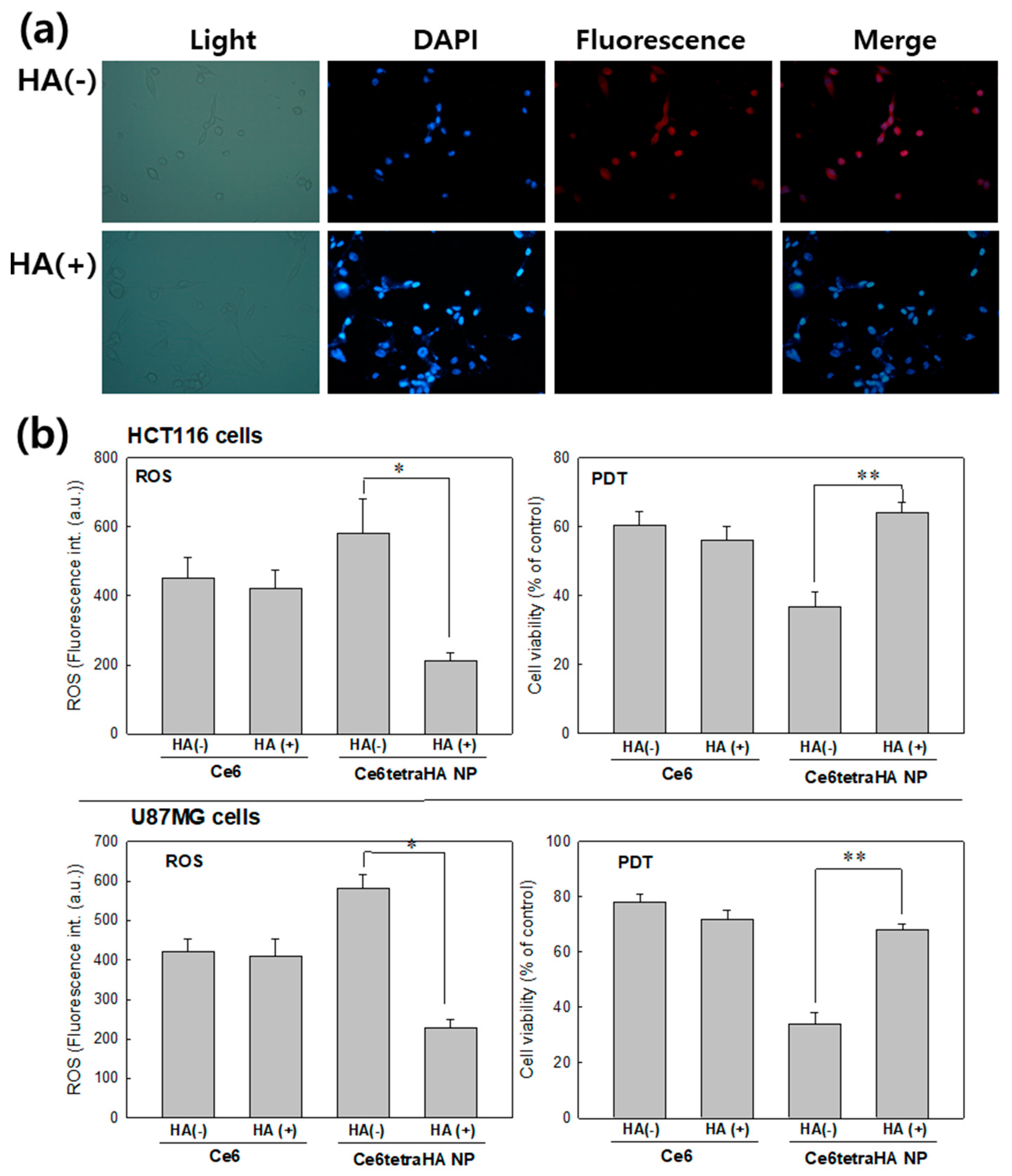
3.3. PDT Efficacy of Nanophotosensitizers at In Vitro Cell Culture
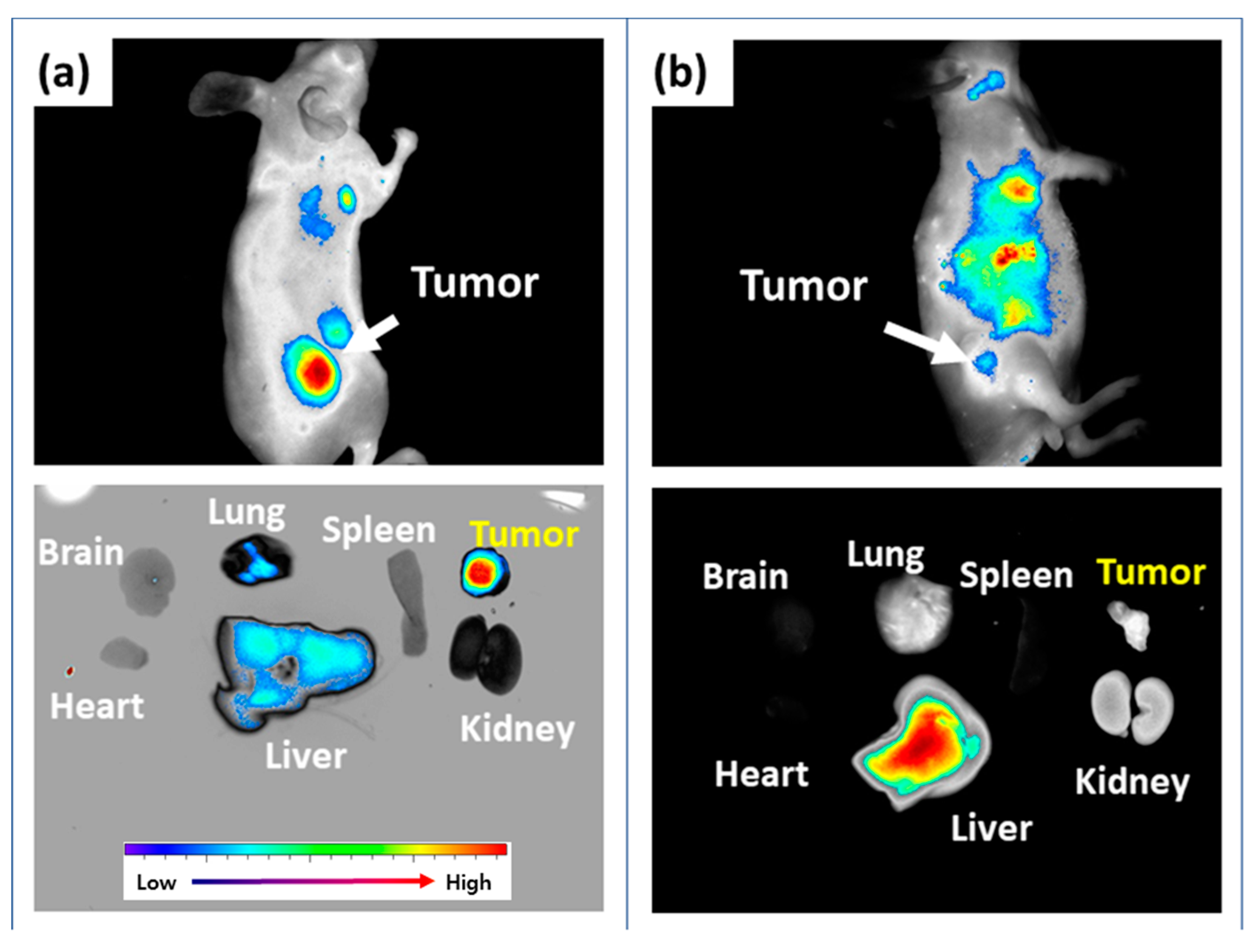
3.4. In Vivo Animal Imaging Study Using Mouse Tumor Xenograft Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kawczyk-Krupka, A.; Bugaj, A.M.; Latos, W.; Zaremba, K.; Wawrzyniec, K.; Sieroń, A. Photodynamic therapy in colorectal cancer treatment: The state of the art in clinical trials. Photodiagn. Photodyn. Ther. 2015, 12, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Messmann, H.; Holstege, A.; Szeimies, R.M.; Lock, G.; Bown, S.G.; Schölmerich, J. Photodynamic therapy: A safe and effective treatment for tumor overgrowth in patients with oesophageal cancer and metal stents. Endoscopy 1995, 27, 629. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Kasai, H.; Horimatsu, T.; Yoshimura, K.; Teramukai, S.; Morita, S.; Tada, H.; Yamamoto, Y.; Kataoka, H.; Kakushima, N.; et al. A multicenter phase II study of salvage photodynamic therapy using talaporfin sodium (ME2906) and a diode laser (PNL6405EPG) for local failure after chemoradiotherapy or radiotherapy for esophageal cancer. Oncotarget 2017, 8, 22135–22144. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.W.; Kim, C.H.; Lee, H.M.; Kim, D.H.; Kwak, T.W.; Chung, K.D.; Jeong, Y.I.; Kang, D.H. Aminolevulinic acid derivatives-based photodynamic therapy in human intra- and extrahepatic cholangiocarcinoma cells. Eur. J. Pharm. Biopharm. 2013, 85, 503–510. [Google Scholar] [CrossRef]
- Zenkevich, E.; Sagun, E.; Knyukshto, V.; Shulga, A.; Mironov, A.; Efremova, O.; Bonnett, R.; Songca, S.P.; Kassem, M. Photophysical and photochemical properties of potential porphyrin and chlorin photosensitizers for PDT. J. Photochem. Photobiol. B Biol. 1996, 33, 171–180. [Google Scholar] [CrossRef]
- Kim, C.H.; Chung, C.W.; Choi, K.H.; Yoo, J.J.; Kim, D.H.; Jeong, Y.I.; Kang, D.H. Effect of 5-aminolevulinic acid-based photodynamic therapy via reactive oxygen species in human cholangiocarcinoma cells. Int. J. Nanomed. 2011, 6, 1357–1363. [Google Scholar] [Green Version]
- Ishizumi, T.; Aizawa, K.; Tsuchida, T.; Okunaka, T.; Kato, H. Spectrometric characteristics and tumor-affinity of a novel photosensitizer: Mono-l-aspartyl aurochlorin e6 (Au-NPe6). Photodiagn. Photodyn. Ther. 2004, 1, 295–301. [Google Scholar] [CrossRef]
- Kataoka, H.; Nishie, H.; Hayashi, N.; Tanaka, M.; Nomoto, A.; Yano, S.; Joh, T. New photodynamic therapy with next-generation photosensitizers. Ann. Transl. Med. 2017, 5, 183. [Google Scholar] [CrossRef]
- Osaki, T.; Hibino, S.; Yokoe, I.; Yamaguchi, H.; Nomoto, A.; Yano, S.; Mikata, Y.; Tanaka, M.; Kataoka, H.; Okamoto, Y. A Basic Study of Photodynamic Therapy with Glucose-Conjugated Chlorin e6 Using Mammary Carcinoma Xenografts. Cancers 2019, 11, 636. [Google Scholar] [CrossRef]
- Chung, C.W.; Kim, C.H.; Choi, K.H.; Yoo, J.J.; Kim, D.H.; Chung, K.D.; Jeong, Y.I.; Kang, D.H. Effect of surfactant on 5-aminolevulinic acid uptake and PpIX generation in human cholangiocarcinoma cell. Eur. J. Pharm. Biopharm. 2012, 80, 453–458. [Google Scholar] [CrossRef]
- Wulf, H.C. The background and philosophy behind daylight photodynamic therapy. G. Ital. Dermatol. Venereol. 2018, 153, 776–782. [Google Scholar] [CrossRef]
- Yano, T.; Hatogai, K.; Morimoto, H.; Yoda, Y.; Kaneko, K. Photodynamic therapy for esophageal cancer. Ann. Transl. Med. 2014, 2, 29. [Google Scholar]
- Chin, W.W.; Lau, W.K.; Heng, P.W.; Bhuvaneswari, R.; Olivo, M. Fluorescence imaging and phototoxicity effects of new formulation of chlorin e6-polyvinylpyrrolidone. J. Photochem. Photobiol. B 2006, 84, 103–110. [Google Scholar] [CrossRef]
- Bastien, E.; Schneider, R.; Hackbarth, S.; Dumas, D.; Jasniewski, J.; Röder, B.; Bezdetnaya, L.; Lassalle, H.P. PAMAM G4.5-chlorin e6 dendrimeric nanoparticles for enhanced photodynamic effects. Photochem. Photobiol. Sci. 2015, 14, 2203–2212. [Google Scholar] [CrossRef]
- Ryu, J.H.; Jeong, Y.I.; Kim, H.Y.; Son, G.M.; Lee, H.L.; Chung, C.W.; Chu, C.W.; Kang, D.H. Enhanced Photosensing and Photodynamic Treatment of Colon Cancer Cells Using Methoxy Poly (ethylene glycol)-Conjugated Chlorin e6. J. Nanosci. Nanotechnol. 2018, 18, 1131–1136. [Google Scholar] [CrossRef]
- Zhang, G.D.; Harada, A.; Nishiyama, N.; Jiang, D.L.; Koyama, H.; Aida, T.; Kataoka, K. Polyion complex micelles entrapping cationic dendrimer porphyrin: Effective photosensitizer for photodynamic therapy of cancer. J. Control. Release 2003, 93, 141–150. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, J.H.; Zhao, L.P.; Fan, G.L.; Zheng, R.R.; Qiu, X.Z.; Yu, X.Y.; Li, S.Y.; Zhang, X.Z. Chimeric peptide engineered exosomes for dual-stage light guided plasma membrane and nucleus targeted photodynamic therapy. Biomaterials 2019, 211, 14–24. [Google Scholar] [CrossRef]
- Jeong, Y.I.; Cha, B.; Lee, H.L.; Song, Y.H.; Jung, Y.H.; Kwak, T.W.; Choi, C.; Jeong, G.W.; Nah, J.W.; Kang, D.H. Simple nanophotosensitizer fabrication using water-soluble chitosan for photodynamic therapy in gastrointestinal cancer cells. Int. J. Pharm. 2017, 532, 194–203. [Google Scholar] [CrossRef]
- Hu, Y.; Masamune, K. Flexible laser endoscope for minimally invasive photodynamic diagnosis (PDD) and therapy (PDT) toward efficient tumor removal. Opt. Express 2017, 25, 16795–16812. [Google Scholar] [CrossRef]
- Muhanna, N.; Cui, L.; Chan, H.; Burgess, L.; Jin, C.S.; MacDonald, T.D.; Huynh, E.; Wang, F.; Chen, J.; Irish, J.C.; et al. Multimodal image-guided surgical and photodynamic interventions in head and neck cancer: From primary tumor to metastatic drainage. Clin. Cancer Res. 2016, 22, 961–970. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Li, H.; Zeng, Y.P.; Hao, Y.H.; Liu, C.; Liu, J.; Wang, W.D.; Li, R. Photosensitizer-loaded branched polyethylenimine-PEGylated ceria nanoparticles for imaging-guided synchronous photochemotherapy. ACS Appl. Mater. Interfaces 2015, 7, 24218–24228. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Koo, H.; Choi, K.Y.; Lee, S.J.; Kim, K.; Kwon, I.C.; Leary, J.F.; Park, K.; Yuk, S.H.; Park, J.H.; et al. Tumor-targeting hyaluronic acid nanoparticles for photodynamic imaging and therapy. Biomaterials 2012, 33, 3980–3989. [Google Scholar] [CrossRef]
- Gao, S.; Wang, J.; Tian, R.; Wang, G.; Zhang, L.; Li, Y.; Li, L.; Ma, Q.; Zhu, L. Construction and Evaluation of a Targeted Hyaluronic Acid Nanoparticle/Photosensitizer Complex for Cancer Photodynamic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 32509–32519. [Google Scholar] [CrossRef]
- Fang, H.; Declerck, Y.A. Targeting the tumor microenvironment: From understanding pathways to effective clinical trials. Cancer Res. 2013, 73, 4965–4977. [Google Scholar] [CrossRef]
- Anari, F.; Ramamurthy, C.; Zibelman, M. Impact of tumor microenvironment composition on therapeutic responses and clinical outcomes in cancer. Future Oncol. 2018, 14, 1409–1421. [Google Scholar] [CrossRef]
- Wang, T.; Yu, X.; Han, L.; Liu, T.; Liu, Y.; Zhang, N. Tumor microenvironment dual-responsive core-shell nanoparticles with hyaluronic acid-shield for efficient co-delivery of doxorubicin and plasmid DNA. Int. J. Nanomed. 2017, 12, 4773–4788. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef]
- Huang, W.C.; Chen, S.H.; Chiang, W.H.; Huang, C.W.; Lo, C.L.; Chern, C.S.; Chiu, H.C. Tumor microenvironment-responsive nanoparticle delivery of chemotherapy for enhanced selective cellular uptake and transportation within tumor. Biomacromolecules 2016, 17, 3883–3892. [Google Scholar] [CrossRef]
- Park, H.K.; Lee, S.J.; Oh, J.S.; Lee, S.G.; Jeong, Y.I.; Lee, H.C. Smart nanoparticles based on hyaluronic acid for redox-responsive and CD44 receptor-mediated targeting of tumor. Nanoscale Res. Lett. 2015, 10, 981. [Google Scholar] [CrossRef]
- Lee, S.J.; Jeong, Y.I. Hybrid nanoparticles based on chlorin e6conjugated hyaluronic acid/poly(l-histidine) copolymer for theranostic application to tumors. J. Mater. Chem. B 2018, 6, 2851–2859. [Google Scholar] [CrossRef]
- Zeng, Y.; Ma, J.; Zhan, Y.; Xu, X.; Zeng, Q.; Liang, J.; Chen, X. Hypoxia-activated prodrugs and redox-responsive nanocarriers. Int. J. Nanomed. 2018, 13, 6551–6574. [Google Scholar] [CrossRef]
- Yang, S.; Gao, H. Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol. Res. 2017, 126, 97–108. [Google Scholar] [CrossRef]
- Chu, C.W.; Ryu, J.H.; Jeong, Y.I.; Kwak, T.W.; Lee, H.L.; Kim, H.Y.; Son, G.M.; Kim, H.W.; Kang, D.H. Redox-responsive nanophotosensitizer composed of chlorin e6-conjugated dextran for photodynamic treatment of colon cancer cells. J. Nanomater. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef] [Green Version]
- Nanashima, A.; Abo, T.; Nonaka, T.; Nonaka, Y.; Morisaki, T.; Uehara, R.; Ohnita, K.; Fukuda, D.; Murakami, G.; Tou, K.; et al. Photodynamic therapy using talaporfin sodium (Laserphyrin®) for bile duct carcinoma: A preliminary clinical trial. Anticancer Res. 2012, 32, 4931–4938. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [Green Version]
- Zoepf, T.; Jakobs, R.; Rosenbaum, A.; Apel, D.; Arnold, J.C.; Riemann, J.F. Photodynamic therapy with 5-aminolevulinic acid is not effective in bile duct cancer. Gastrointest. Endosc. 2001, 54, 763–766. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef]
- Lamch, Ł.; Pucek, A.; Kulbacka, J.; Chudy, M.; Jastrzębska, E.; Tokarska, K.; Bułka, M.; Brzózka, Z.; Wilk, K.A. Recent progress in the engineering of multifunctional colloidal nanoparticles for enhanced photodynamic therapy and bioimaging. Adv. Colloid Interface Sci. 2018, 261, 62–81. [Google Scholar] [CrossRef]
- Zottel, A.; Videtič Paska, A.; Jovčevska, I. Nanotechnology Meets Oncology: Nanomaterials in Brain Cancer Research, Diagnosis and Therapy. Materials 2019, 12, 1588. [Google Scholar] [CrossRef]
- Tang, W.; Fan, W.; Lau, J.; Deng, L.; Shen, Z.; Chen, X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 2019, 48, 2967–3014. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Ciofani, G. Nanostructured carriers as innovative tools for cancer diagnosis and therapy. APL Bioeng. 2019, 3, 011502. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Li, L.; Su, X.T.; Wang, K.; Lu, Z.J.; Yuan, C.Z.; Feng, J.B.; Yan, S.; Kong, B.H.; Song, K. Development and evaluation of novel tumor-targeting paclitaxel-loaded nano-carriers for ovarian cancer treatment: In vitro and in vivo. J. Exp. Clin. Cancer Res. 2018, 37, 29. [Google Scholar] [CrossRef]
- Martí Coma-Cros, E.; Lancelot, A.; San Anselmo, M.; Neves Borgheti-Cardoso, L.; Valle-Delgado, J.J.; Serrano, J.L.; Fernàndez-Busquets, X.; Sierra, T. Micelle carriers based on dendritic macromolecules containing bis-MPA and glycine for antimalarial drug delivery. Biomater. Sci. 2019, 7, 1661–1674. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Jeong, Y.I.; Park, H.K.; Kang, D.H.; Oh, J.S.; Lee, S.G.; Lee, H.C. Enzyme-responsive doxorubicin release from dendrimer nanoparticles for anticancer drug delivery. Int. J. Nanomed. 2015, 10, 5489–5503. [Google Scholar]
- Lyulin, S.V.; Vattulainen, I.; Gurtovenko, A.A. Complexes comprised of charged dendrimers, linear polyelectrolytes, and counterions: Insight through coarse-grained molecular dynamics simulations. Macromolecules 2008, 41, 4961–4968. [Google Scholar] [CrossRef]
- Kadhim, A.; McKenzie, L.K.; Bryant, H.E.; Twyman, L.J. Synthesis and Aggregation of a Porphyrin-Cored Hyperbranched Polyglycidol and Its Application as a Macromolecular Photosensitizer for Photodynamic Therapy. Mol. Pharm. 2019, 16, 1132–1139. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.E. Advances in redox-responsive drug delivery systems of tumor microenvironment. J. Nanobiotechnol. 2018, 16, 74. [Google Scholar] [CrossRef]
- Uthaman, S.; Huh, K.M.; Park, I.K. Tumor microenvironment-responsive nanoparticles for cancer theragnostic applications. Biomater. Res. 2018, 22, 22. [Google Scholar] [CrossRef]
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione levels in human tumors. Biomarkers 2012, 17, 671–691. [Google Scholar] [CrossRef]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhou, Y.; Shi, G.; Sang, X.; Ni, C. Preparations of hyperbranched polymer nano micelles and the pH/redox controlled drug release behaviors. Mater. Sci. Eng. C 2017, 79, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Afify, A.; Purnell, P.; Nguyen, L. Role of CD44s and CD44v6 on human breast cancer cell adhesion, migration, and invasion. Exp. Mol. Pathol. 2009, 86, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Platt, V.M.; Szoka, F.C., Jr. Anticancer therapeutics: Targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol. Pharm. 2008, 5, 474–486. [Google Scholar] [CrossRef] [PubMed]




| Types of Nanophotosensitizers | Ce6 Contents (%, w/w) | Particle Size (nm) a |
|---|---|---|
| Ce6tetraHA NP | 22.1 | 82.6 ± 5.6 |
| Ce6decaHA NP | 39.8 | 181.4 ± 12.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.; Jung, S.; Kim, D.M.; Lim, S.-H.; Shim, Y.H.; Kwon, H.; Kim, D.H.; Lee, C.-M.; Kim, B.H.; Jeong, Y.-I. Hyaluronic Acid-Conjugated with Hyperbranched Chlorin e6 Using Disulfide Linkage and Its Nanophotosensitizer for Enhanced Photodynamic Therapy of Cancer Cells. Materials 2019, 12, 3080. https://doi.org/10.3390/ma12193080
Jung S, Jung S, Kim DM, Lim S-H, Shim YH, Kwon H, Kim DH, Lee C-M, Kim BH, Jeong Y-I. Hyaluronic Acid-Conjugated with Hyperbranched Chlorin e6 Using Disulfide Linkage and Its Nanophotosensitizer for Enhanced Photodynamic Therapy of Cancer Cells. Materials. 2019; 12(19):3080. https://doi.org/10.3390/ma12193080
Chicago/Turabian StyleJung, Shin, Seunggon Jung, Doo Man Kim, Sa-Hoe Lim, Yong Ho Shim, Hanjin Kwon, Do Hoon Kim, Chang-Min Lee, Byung Hoon Kim, and Young-Il Jeong. 2019. "Hyaluronic Acid-Conjugated with Hyperbranched Chlorin e6 Using Disulfide Linkage and Its Nanophotosensitizer for Enhanced Photodynamic Therapy of Cancer Cells" Materials 12, no. 19: 3080. https://doi.org/10.3390/ma12193080






