Preparation of γ-Divinyl-3-Aminopropyltriethoxysilane Modified Lignin and Its Application in Flame Retardant Poly(lactic acid)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.2.1. Preparation of Modified Lignin by Silane Coupling Agent
2.2.2. Preparation of PLA composites
2.3. Characterization
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. Flame Retardancy and Combustion Behavior
2.3.4. Characterization of Residual Chars after Limiting Oxygen Index Test
2.3.5. Mechanical Properties
3. Results and Discussion
3.1. Characterization of Lig and CLig
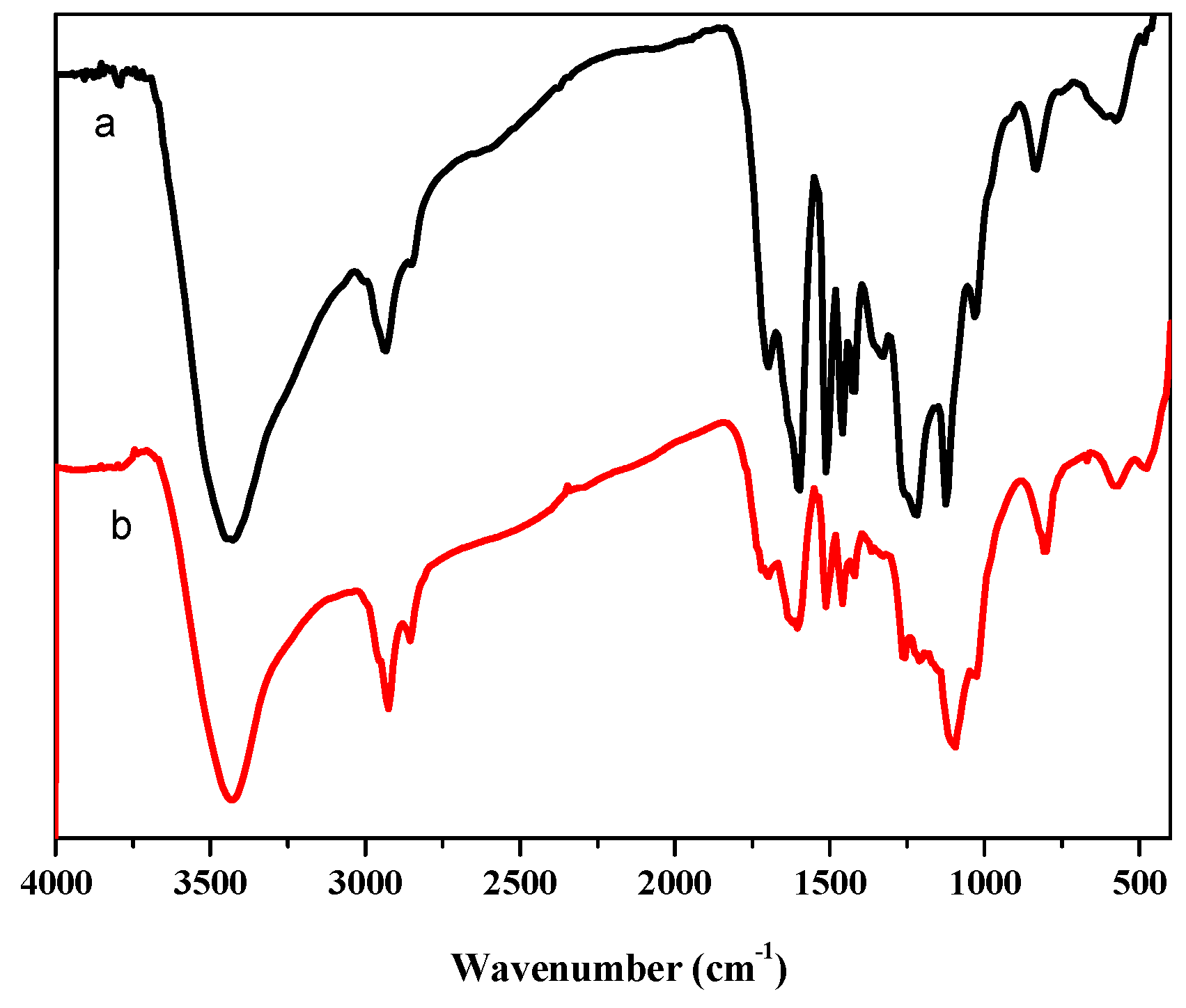
3.1.1. FTIR Analysis
3.1.2. X-ray Fluorescence Spectroscopy Analysis
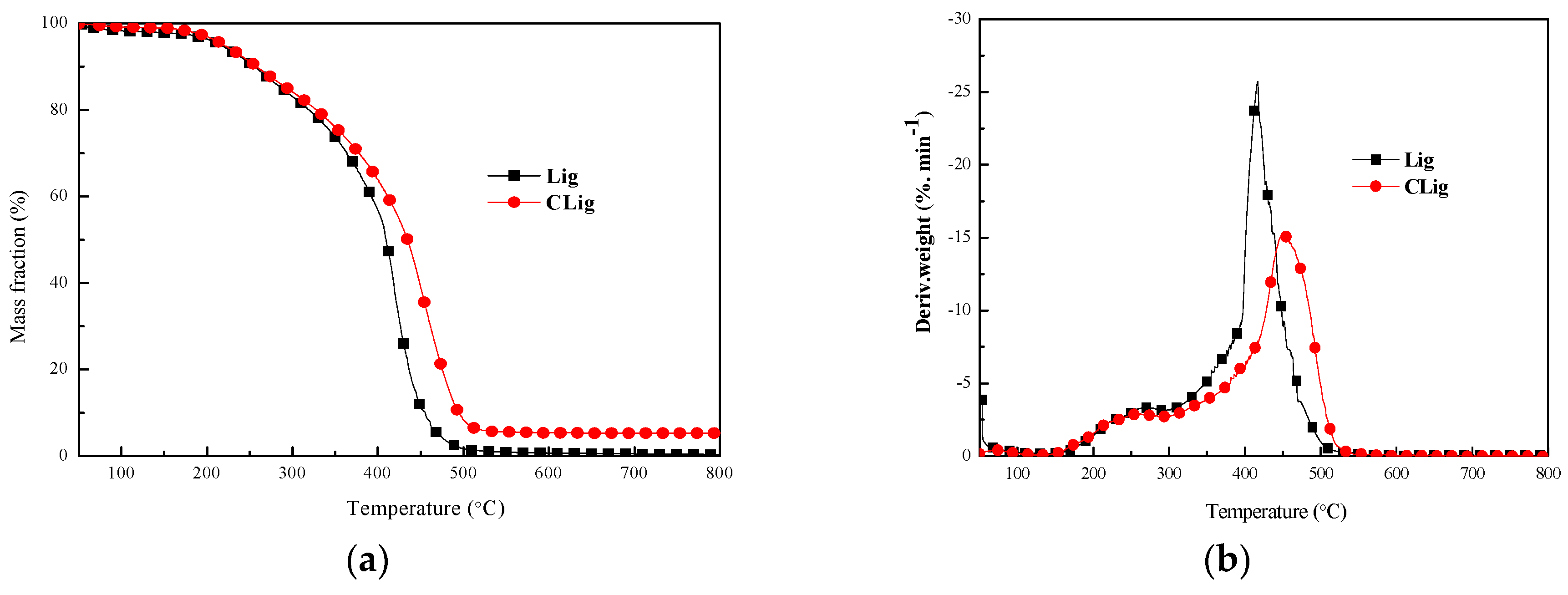
3.1.3. Thermal Stability
3.2. Flame Retardant Properties of PLA Composites
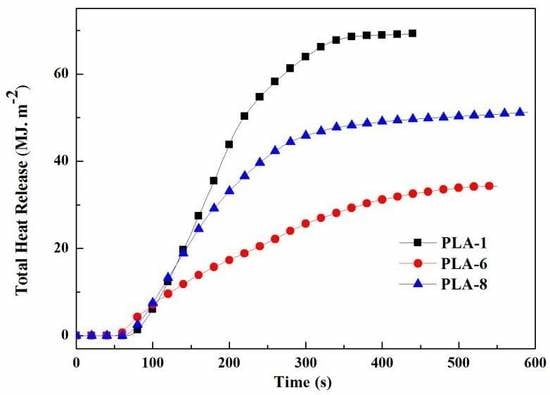
3.3. Combustion Behavior Analysis of PLA Composites
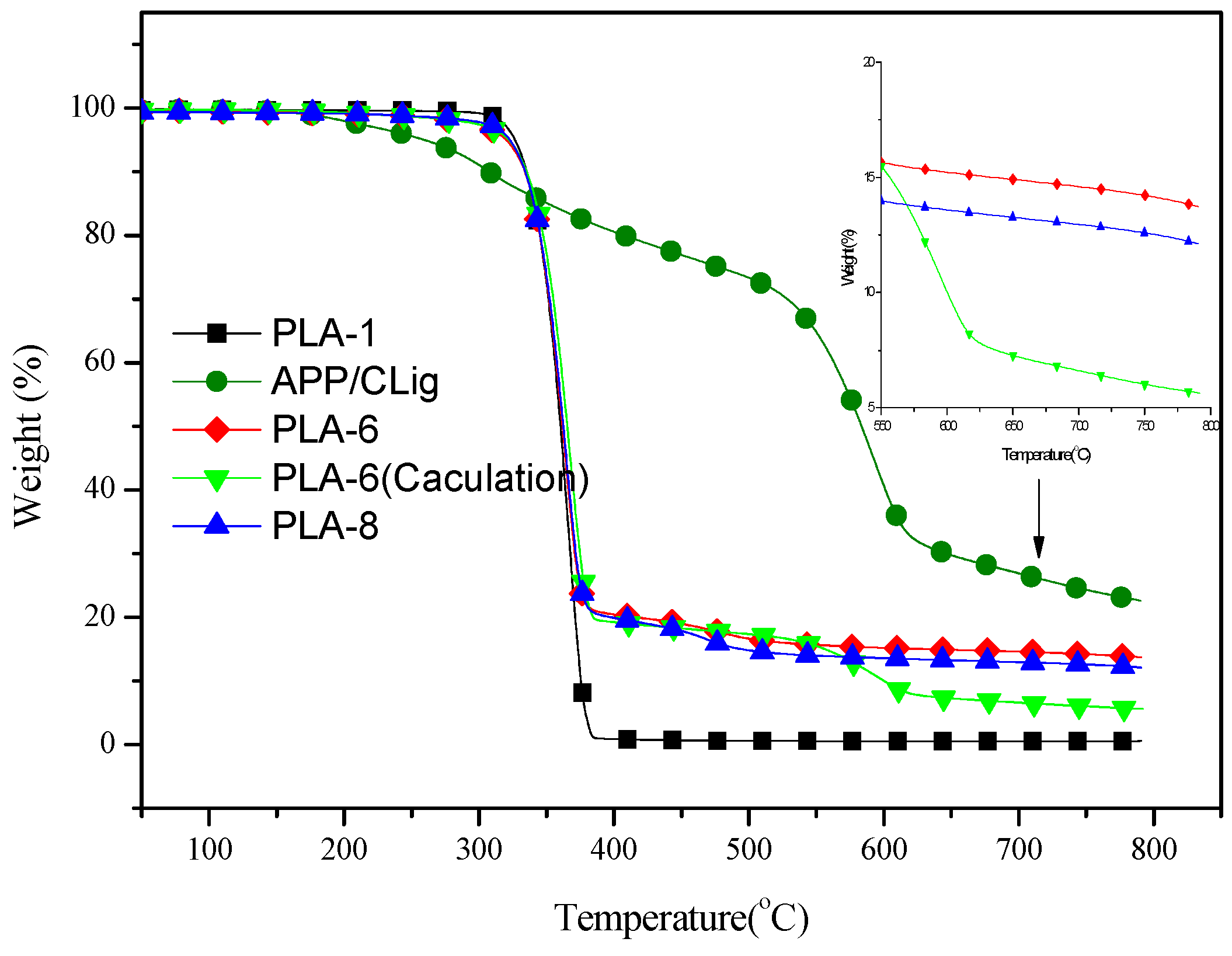
3.4. Thermal Stability of Flame Retardant PLA Composites
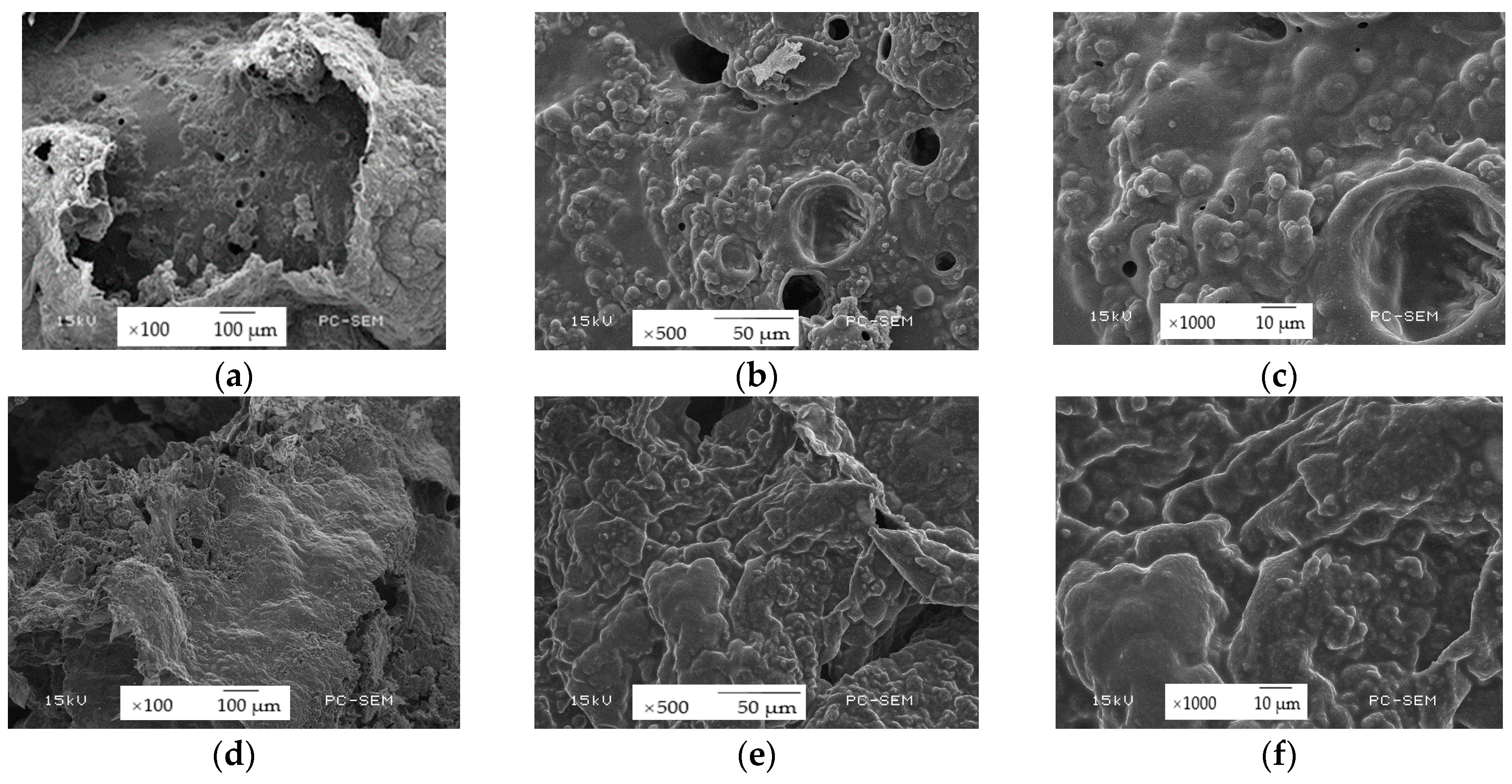
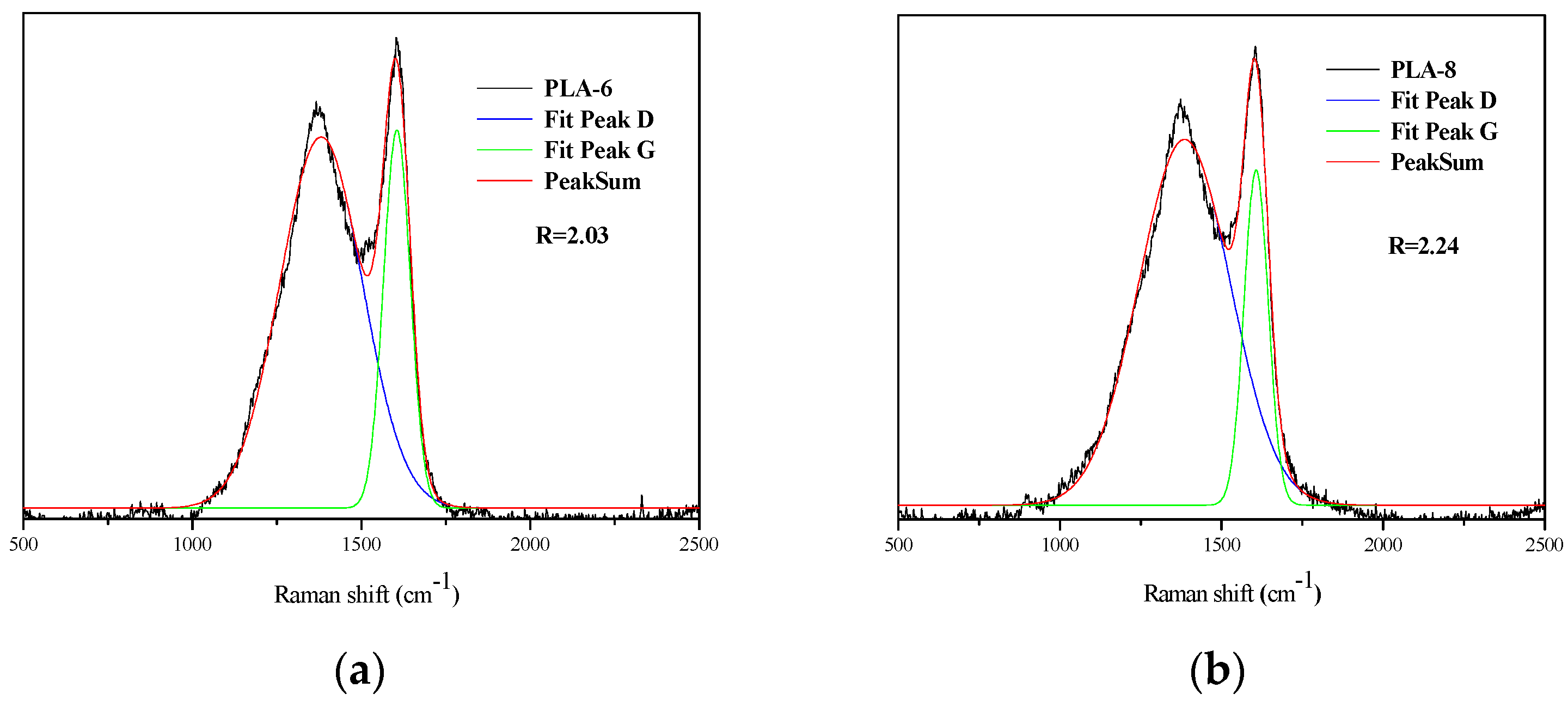
3.5. Structure and Morphology of Char Residues
3.6. Mechanical Properties of PLA Composites
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Feng, C.; Liang, M.; Jiang, J.; Huang, J.; Liu, H. Flame retardant properties and mechanism ofan efficient intumescent flame retardant PLA composites. Polym. Adv. Technol. 2016, 27, 693–700. [Google Scholar] [CrossRef]
- Wertz, J.; Mauldin, T.; Boday, D. Polylactic acid with improved heat deflection temperatures and self-healing properties for durable goodsapplications. ACS Appl. Mater. Interfaces 2014, 6, 18511–18516. [Google Scholar] [CrossRef] [PubMed]
- Sypaseuth, F.; Gallo, E.; Çiftci, S.; Schartel, B. Polylactic acid biocomposites: Approaches to a completely green flame retarded polymer. e-Polymers 2017, 17, 449–462. [Google Scholar] [CrossRef]
- Blanco, I. End-life prediction of commercial pla used for food packaging through short term TGA experiments: Real chance or low reliability. Chin. J. Polym. Sci. 2014, 32, 681–689. [Google Scholar] [CrossRef]
- Byun, Y.; Rodriguez, K.; Han, J.; Kim, Y. Improved thermal stability of polylactic acid (PLA) composite film via PLA–β-cyclodextrin-inclusion complex systems. Int. J. Biol. Macromol. 2015, 81, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Molde, J.; Soares, R.; Kohn, J. Designing biomaterials for 3D printing. ACS Biomater Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicala, G.; Giordano, D.; Tosto, C.; Filippone, G.; Recca, A.; Blanco, I. Polylactide (PLA) filaments a biobased solution for additive manufacturing: Correlating rheology and thermomechanical properties with printing quality. Materials 2018, 11, 1191. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; He, S.; Zuo, X.; Xue, Y.; Chen, Z.; Chang, C.; Weil, E.; Rafailovich, M. Incorporation of cellulose with adsorbed phosphates into poly(lactic acid) for enhanced mechanical and flame retardant properties. Polym. Degrad. Stab. 2017, 144, 24–32. [Google Scholar] [CrossRef]
- Guo, Y.; Chang, C.; Halada, G.; Cuiffo, M.; Xue, Y.; Zuo, X.; Pack, S.; Zhang, L.; He, S.; Weil, E.; et al. Engineering flame retardant biodegradable polymer nanocomposites and their application in 3D printing. Polym. Degrad. Stab. 2017, 137, 205–215. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.; Qiu, Y.; Li, L.; Qian, L.; Xin, F. Terminal group effects of phosphazene-triazine bi-group flame retardant additives in flame retardant polylactic acid composites. Polym. Degrad. Stab. 2017, 140, 166–175. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Feng, J.; Su, X.; Zhu, J. An intumescent flame retardant system using beta-cyclodextrin as a carbon source in polylactic acid (PLA). Polym. Adv. Technol. 2011, 22, 1115–1122. [Google Scholar] [CrossRef]
- Ansari, H.; Shabanian, M.; Khonakdar, H.A. Using a β-cyclodextrin functional Fe3O4 as a reinforcement of PLA: Synthesis, thermal and combustion properties. Polym. Plast. Technol. Eng. 2017, 12, 1366–1373. [Google Scholar] [CrossRef]
- Reti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability properties of intumescent PLA including starch and lignin. Polym. Adv. Technol. 2008, 19, 628–635. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xuan, S.; Xing, W.; Bai, Z.; Lu, H. Flame retardancy and thermal degradation of intumescent flame retardant poly(lactic acid)/starch biocomposites. Polym. Plast. Technol. Eng. 2010, 50, 713–720. [Google Scholar] [CrossRef]
- Michałowski, S.; Hebda, E.; Pielichowski, K. Thermal stability and flammability of polyurethane foams chemically reinforced with POSS. J. Therm. Anal. Calorim. 2017, 130, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Lucie, C.; Fouad, L.; Mario, A.; Richel, A.; Brohez, S.; Delvosalle, C.; Dubois, P. Phosphorus and nitrogen derivatization as efficient route for improvement of lignin flame retardant action in PLA. Eur. Polym. J. 2016, 84, 652–667. [Google Scholar]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Evtuguin, D.; Cordeiro, N.; Silvestre, A.J.D. Structural characterization of stalk lignin from banana plant. Ind. Crops Prod. 2009, 29, 86–95. [Google Scholar] [CrossRef]
- Xing, W.; Yuan, H.; Zhang, P.; Yang, H.; Song, L.; Hu, Y. Functionalized lignin for halogen-free flame retardant rigid polyurethane foam: Preparation, thermal stability, fire performance and mechanical properties. J. Polym. Res. 2013, 20, 1–12. [Google Scholar] [CrossRef]
- Lv, P.; Wang, Z.; Hu, K.; Fan, W. Flammability and thermal degradation of flame retarded PP composites containing melamine phosphate and pentaerythritol derivatives. Polym. Degrad. Stab. 2005, 90, 523–534. [Google Scholar] [CrossRef]
- Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Fierro, V.; Celzard, A. PLA with intumescent system containing lignin and ammonium polyphosphate for flame retardant textile. Polymers 2016, 8, 331. [Google Scholar] [CrossRef]
- Zhang, R.; Xiao, X.; Tai, Q.; Huang, H.; Hu, Y. Modification of lignin and its application as char agent in intumescent flame-retardant poly(lactic acid). Polym. Eng. Sci. 2012, 10, 2620–2626. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, R.; Tai, A. The preparation of lignin based hybrids and its application in flame retardant poly(lactic acid) composites. In Proceedings of the National Conference on Flame Retardancy, Huangshan, China, 15 May 2013; China Flame Retardant Society: Beijing, China, 2013; pp. 90–99. [Google Scholar]
- Yu, Y.; Fu, S.; Song, P.; Luo, X.; Jin, Y.; Lu, F.; Wu, Q.; Ye, J. Functionalized lignin by grafting phosphorus-nitrogen improves the thermal stability and flame retardancy of polypropylene. Polym. Degrad. Stab. 2012, 97, 541–546. [Google Scholar] [CrossRef]
- Li, Y.; Guo, R.; Fan, J. Synthesis and characterization of novel organic-silicon flame retardant. J. Shaanxi Univ. Sci. Technol. 2015, 33, 90–94. [Google Scholar]
- Deng, S.; Wang, L.; Yang, J.; Cao, Z.; Wang, Y. Flame-retardant and smoke-suppressed silicone foams with chitosan-based nanocoatings. Ind. Eng. Chem. Res. 2016, 55, 7239–7248. [Google Scholar] [CrossRef]
- Liu, L.; Qian, M.; Huang, G.; Huang, G.; Yu, Y.; Fu, S. Fabrication of Green Lignin-based flame retardants for enhancing the thermal and fire retardancy properties of polypropylene/wood composites. ACS Sustain. Chem. Eng. 2016, 4, 2422–2431. [Google Scholar] [CrossRef]
- Feng, C.; Li, Z.; Liang, M.; Huang, J.; Liu, H. Preparation and characterization of a novel oligomeric charring agent and its application in halogen-free flame retardant polypropylene. J. Anal. Appl. Pyrolysis 2015, 111, 238–246. [Google Scholar] [CrossRef]
- Cicala, G.; Saccullo, G.; Blanco, I.; Samal, S.; Battiato, S.; Dattilo, S.; Saake, B. Polylactide/lignin blends: Effects of processing conditions on structure and thermo-mechanical properties. J. Therm. Anal. Calorim. 2017, 130, 515–524. [Google Scholar] [CrossRef]
- Jin, X.; Gu, X.; Chen, C.; Tang, W.; Li, H.; Liu, X.; Bourbigot, S.; Zhang, Z.; Sun, J.; Zhang, S. The fire performance of polylactic acid containing a novel intumescent flame retardant and intercalated layered double hydroxides. J. Mater. Sci. 2017, 52, 12235–12250. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, B.; Wang, X.; Chen, L.; Wang, X.; Wang, Y. A flame-retardant-free and thermo-cross-linkable copolyester: Flame-retardant and anti-dripping mode of action. Polymer 2014, 55, 2394–2403. [Google Scholar] [CrossRef]
- Enescu, D.; Frache, D.; Lavaselli, M.; Monticelli, O.; Marino, F. Novel phosphorousnitrogen intumescent flame retardant system. Its effects on flame retardancyand thermal properties of polypropylene. Polym. Degrad. Stab. 2013, 98, 297–305. [Google Scholar] [CrossRef]
- Su, X.; Yi, Y.; Tao, J.; Qi, H.; Li, D. Synergistic effect between a novel triazine charring agent and ammonium polyphosphate on flame retardancy andthermal behavior of polypropylene. Polym. Degrad. Stab. 2014, 105, 12–20. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, Y.; Liu, S.; Chi, Z.; Xu, J. Synergistic effects of 4A zeoliteon the flame retardant properties and thermal stability of a novel halogen-freePP/IFR composite. Polym. Adv. Technol. 2013, 24, 478–486. [Google Scholar] [CrossRef]
- Zhu, J.; Xue, L.; Wei, W.; Mu, C.; Jiang, M.; Zhou, Z. Modification of lignin with silane coupling agent to improve the interface of poly(L-lactic) Acid/Lignin composites. Bioresources 2015, 10, 4315–4325. [Google Scholar] [CrossRef]

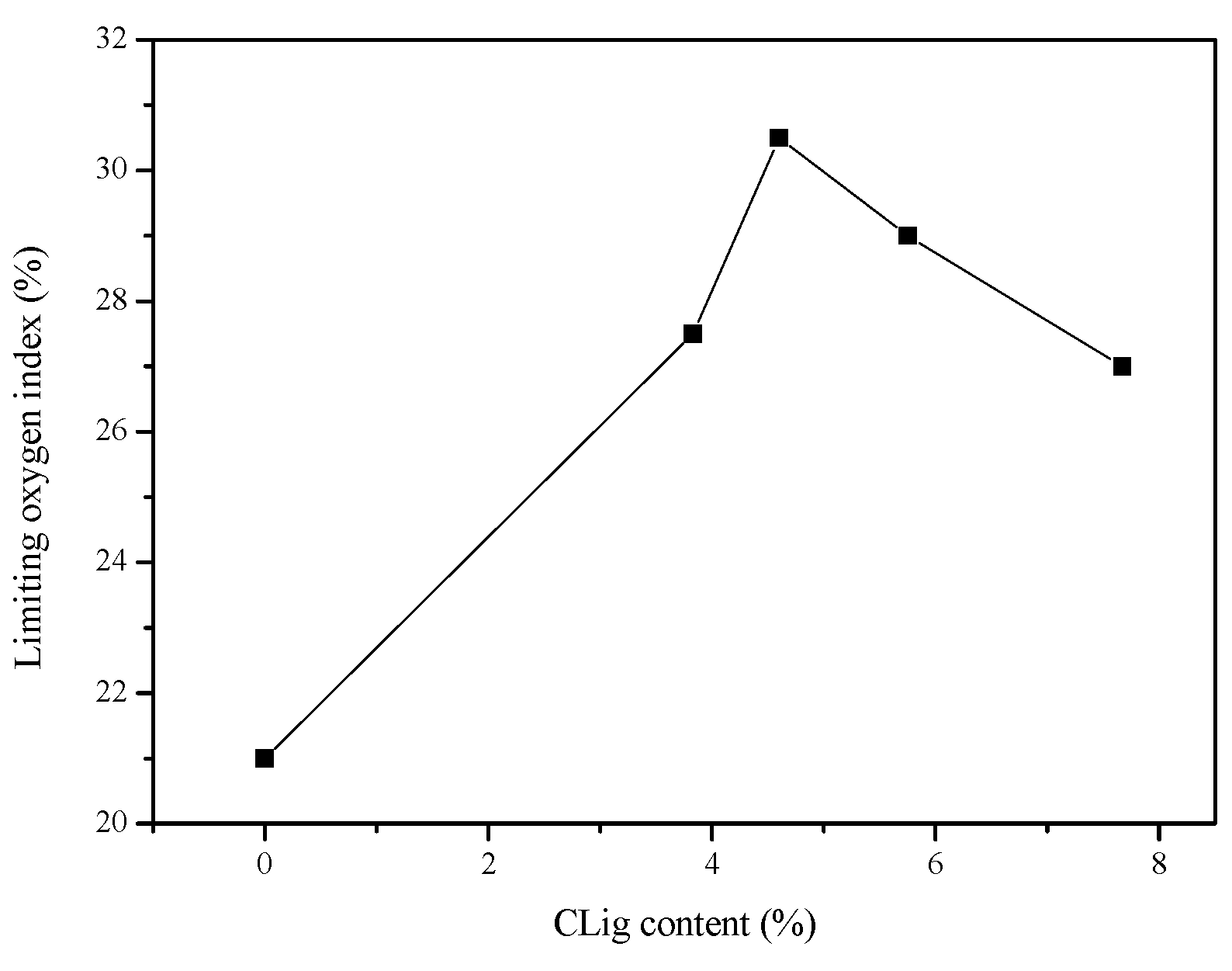

| Sample | Component (wt%) | LOI (%) | UL-94 | |||||
|---|---|---|---|---|---|---|---|---|
| PLA | APP | CLig | Lignin | Ranking | Ignition of the Cotton | t1/t2 (s) | ||
| PLA-1 | 100 | - | - | - | 21 | NR | Yes | >30 |
| PLA-2 | 77 | 23 | - | - | 29 | V-2 | Yes | 11/0 |
| PLA-3 | 77 | - | 23 | - | 22 | NR | Yes | >30 |
| PLA-4 | 77 | 15.33 | 7.67 | - | 27 | V-1 | No | 9/2 |
| PLA-5 | 77 | 17.25 | 5.75 | - | 29 | V0 | No | 1/0 |
| PLA-6 | 77 | 18.40 | 4.60 | - | 30.5 | V0 | No | 0/1 |
| PLA-7 | 77 | 19.17 | 3.83 | - | 27.5 | V2 | Yes | 12/0 |
| PLA-8 | 77 | 18.40 | - | 4.60 | 25 | V0 | No | 2/3 |
| Elements | O/% | Si/% | S/% | Fe/% | Ca/% | Cu/% | |
|---|---|---|---|---|---|---|---|
| Samples | |||||||
| Lig | 91.63 | 2.58 | 4.19 | 1.28 | 0.18 | 0.14 | |
| CLig | 79.85 | 14.33 | 4.07 | 1.33 | 0.22 | 0.20 | |
| Samples | T5% (°C) | T50% (°C) | Tmax (%) | Residual (%) 800 °C | |
|---|---|---|---|---|---|
| Tmax1 | Tmax2 | ||||
| Lig | 214 | 409 | 271 | 417 | 0 |
| CLig | 220 | 435 | 261 | 452 | 5 |
| Sample | TTI (s) | PHRR (kW/m2) | Av-HRR (kW/m2) | TTPH (s) | THR (MJ/m2) | Residual Mass (%) |
|---|---|---|---|---|---|---|
| PLA-1 | 64 | 428.4 | 184.6 | 185 | 69.2 | 0 |
| PLA-6 | 48 | 205.3 | 68.7 | 65 | 34.3 | 40.6 |
| PLA-8 | 58 | 299.4 | 96.7 | 110 | 51.3 | 19.5 |
| Samples | T5% (°C) | Tmax (°C) | Residue (%) | |||||
|---|---|---|---|---|---|---|---|---|
| 600 °C | 700 °C | 800 °C | ||||||
| Exp. | Cal. | Exp. | Cal. | Exp. | Cal. | |||
| APP | 302 | 593 | 33.2 | - | 20.4 | - | 16.3 | - |
| CLig | 199 | 356 | 41.5 | - | 39.3 | - | 38.6 | - |
| APP/CLig(4:1) | 259 | 593 | 40.7 | 34.7 | 26.9 | 24.1 | 22.6 | 20.7 |
| PLA-1 | 327 | 366 | 0.5 | - | 0.5 | - | 0.5 | - |
| PLA-6 | 320 | 375 | 15.2 | 10.0 | 14.6 | 6.6 | 13.7 | 5.6 |
| PLA-8 | 323 | 365 | 13.6 | - | 13.0 | - | 12.1 | - |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| PLA1 | 61.1 ± 3.1 | 5.8 ± 0.2 |
| PLA6 | 41.2 ± 2.1 | 6.6 ± 0.3 |
| PLA8 | 29.5 ± 1.5 | 5.2 ± 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Zong, X.; Wang, N.; Yan, N.; Shan, X.; Li, J. Preparation of γ-Divinyl-3-Aminopropyltriethoxysilane Modified Lignin and Its Application in Flame Retardant Poly(lactic acid). Materials 2018, 11, 1505. https://doi.org/10.3390/ma11091505
Song Y, Zong X, Wang N, Yan N, Shan X, Li J. Preparation of γ-Divinyl-3-Aminopropyltriethoxysilane Modified Lignin and Its Application in Flame Retardant Poly(lactic acid). Materials. 2018; 11(9):1505. https://doi.org/10.3390/ma11091505
Chicago/Turabian StyleSong, Yan, Xu Zong, Nan Wang, Ning Yan, Xueying Shan, and Jinchun Li. 2018. "Preparation of γ-Divinyl-3-Aminopropyltriethoxysilane Modified Lignin and Its Application in Flame Retardant Poly(lactic acid)" Materials 11, no. 9: 1505. https://doi.org/10.3390/ma11091505