Drug Release of Hybrid Materials Containing Fe(II)Citrate Synthesized by Sol-Gel Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ferrous Citrate Preparation
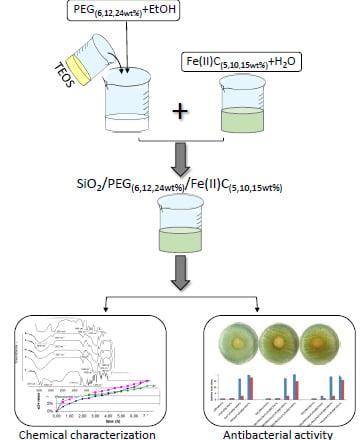
2.2. Sol-Gel Synthesis of the Hybrid Materials
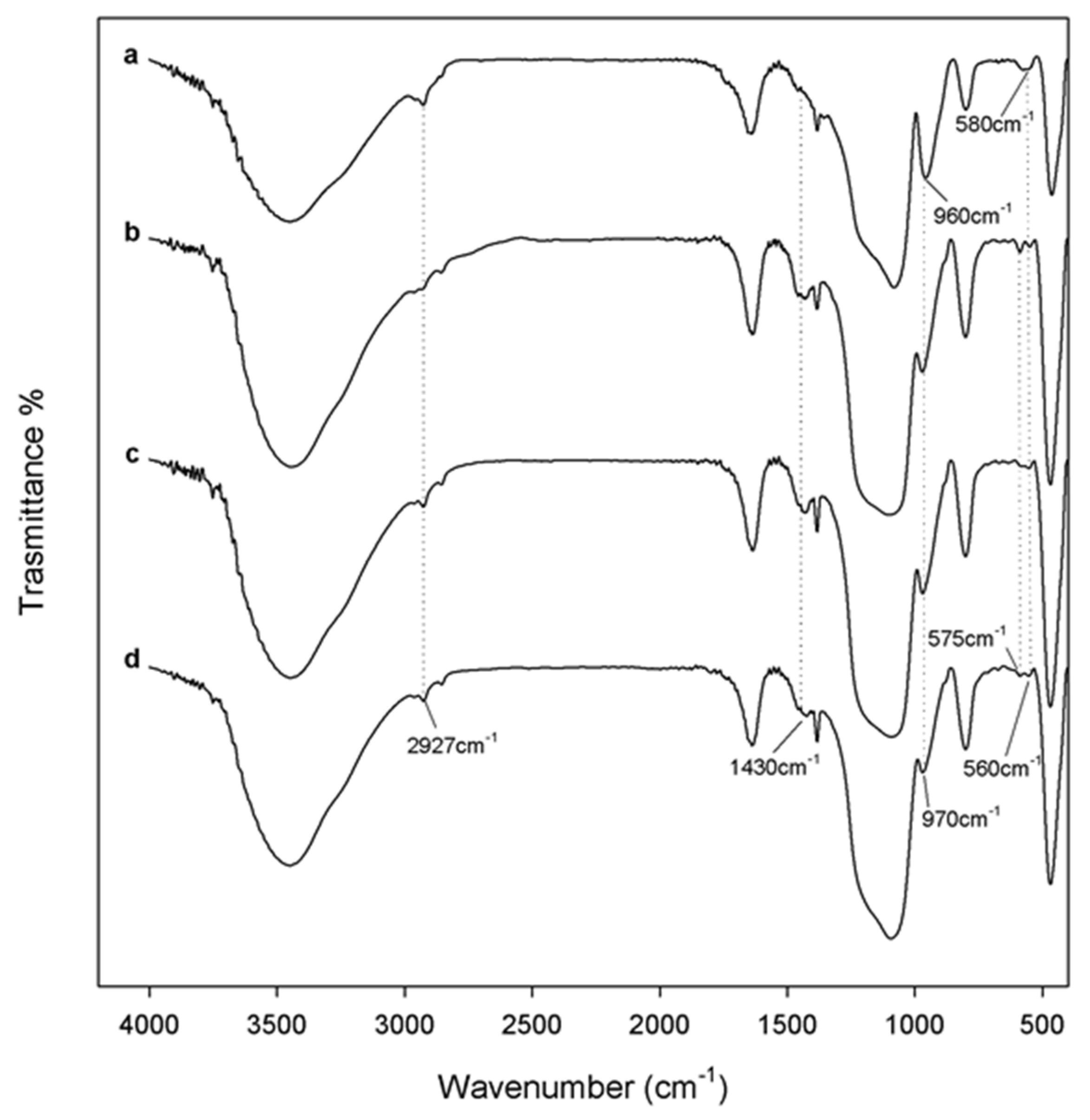
2.3. Fourier Transform Infrared (FTIR) Analysis of the Hybrid Materials
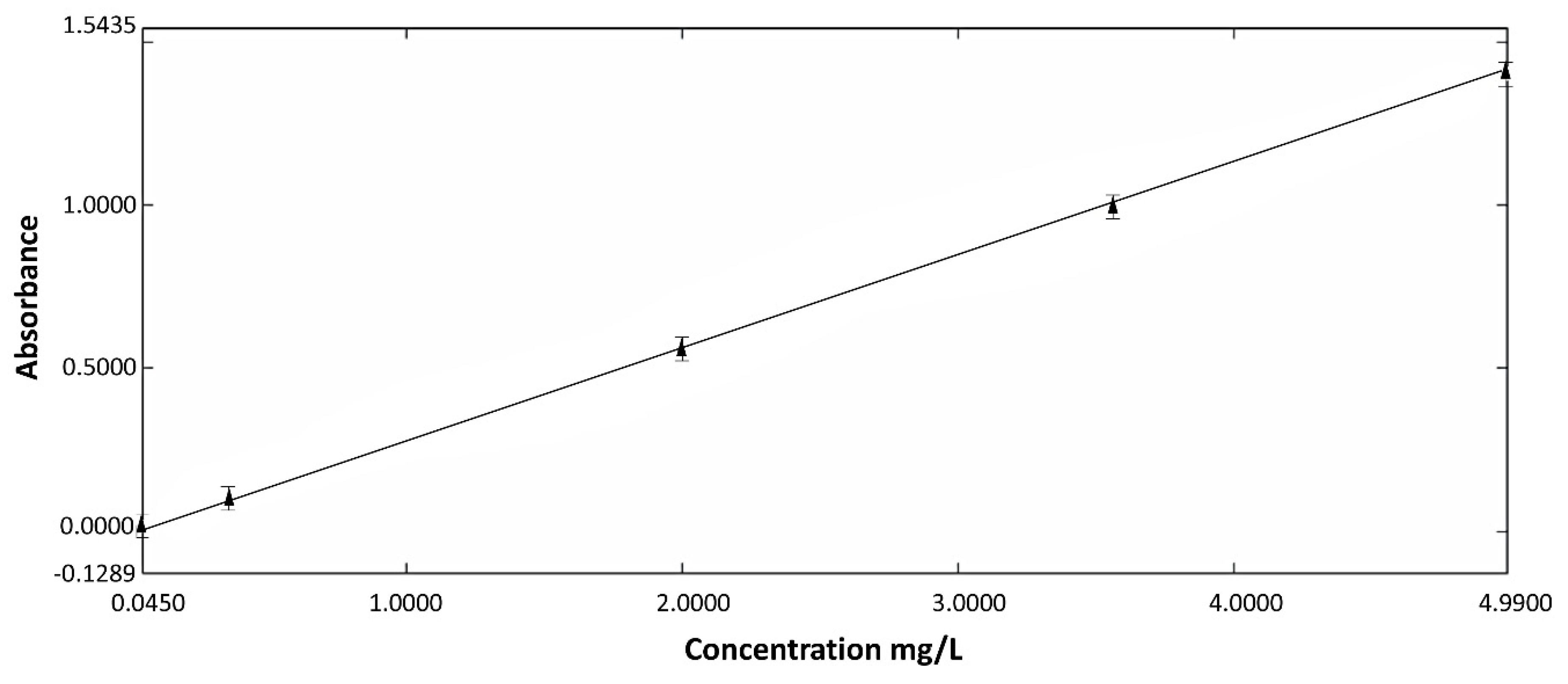
2.4. Study of In Vitro Release
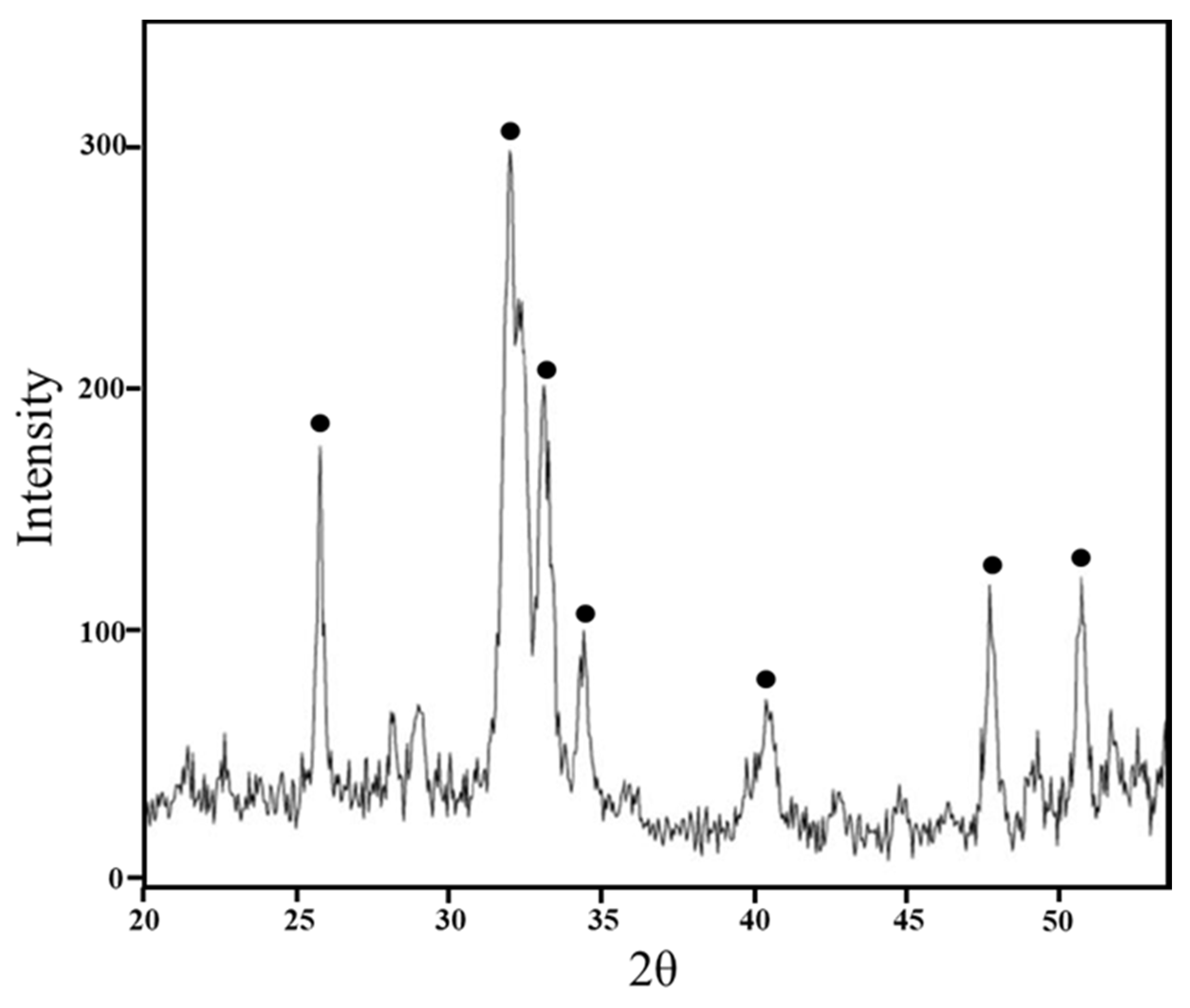
2.5. Bioactivity Test
2.6. Antibacterial Activity
3. Results
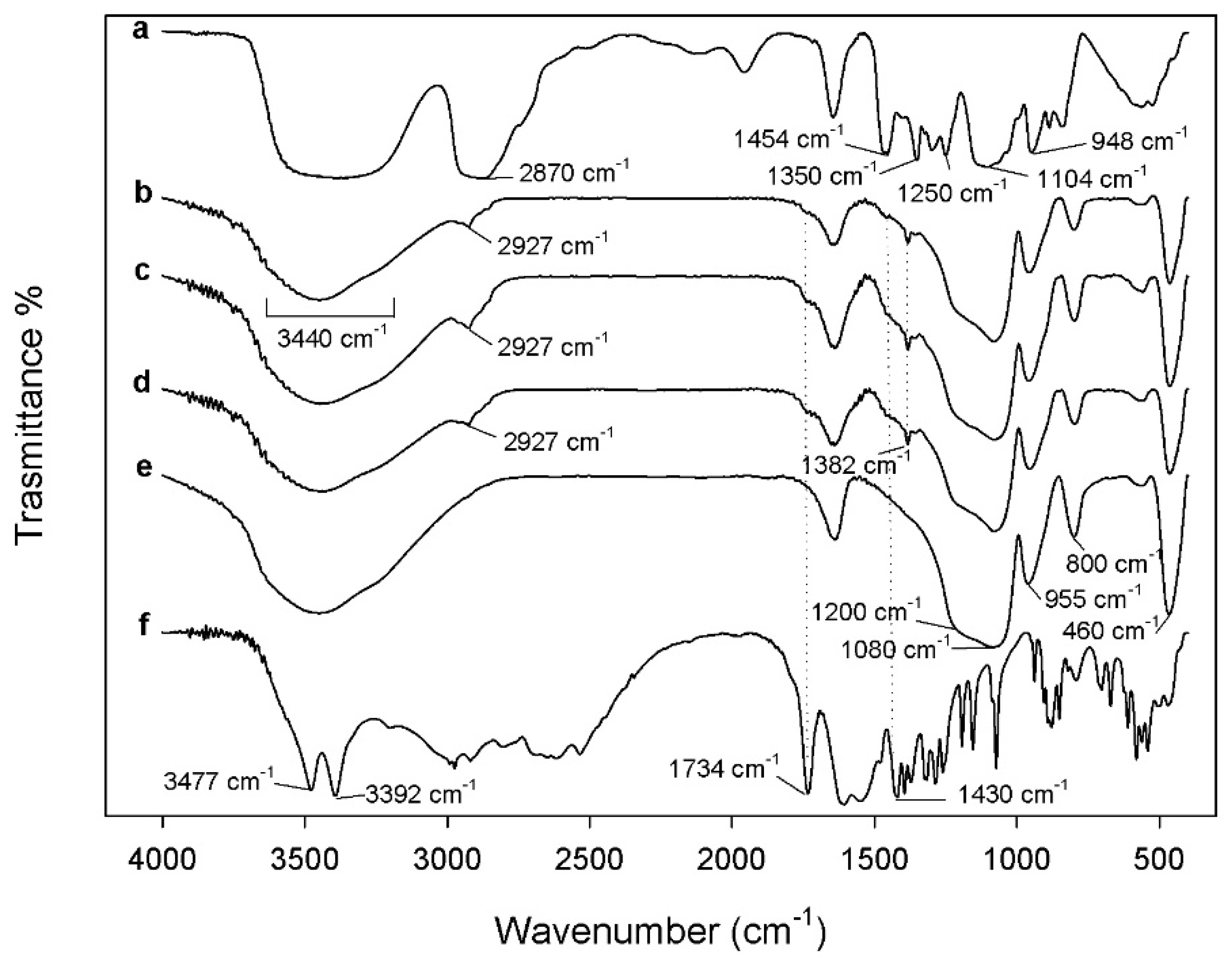
3.1. FTIR Analysis
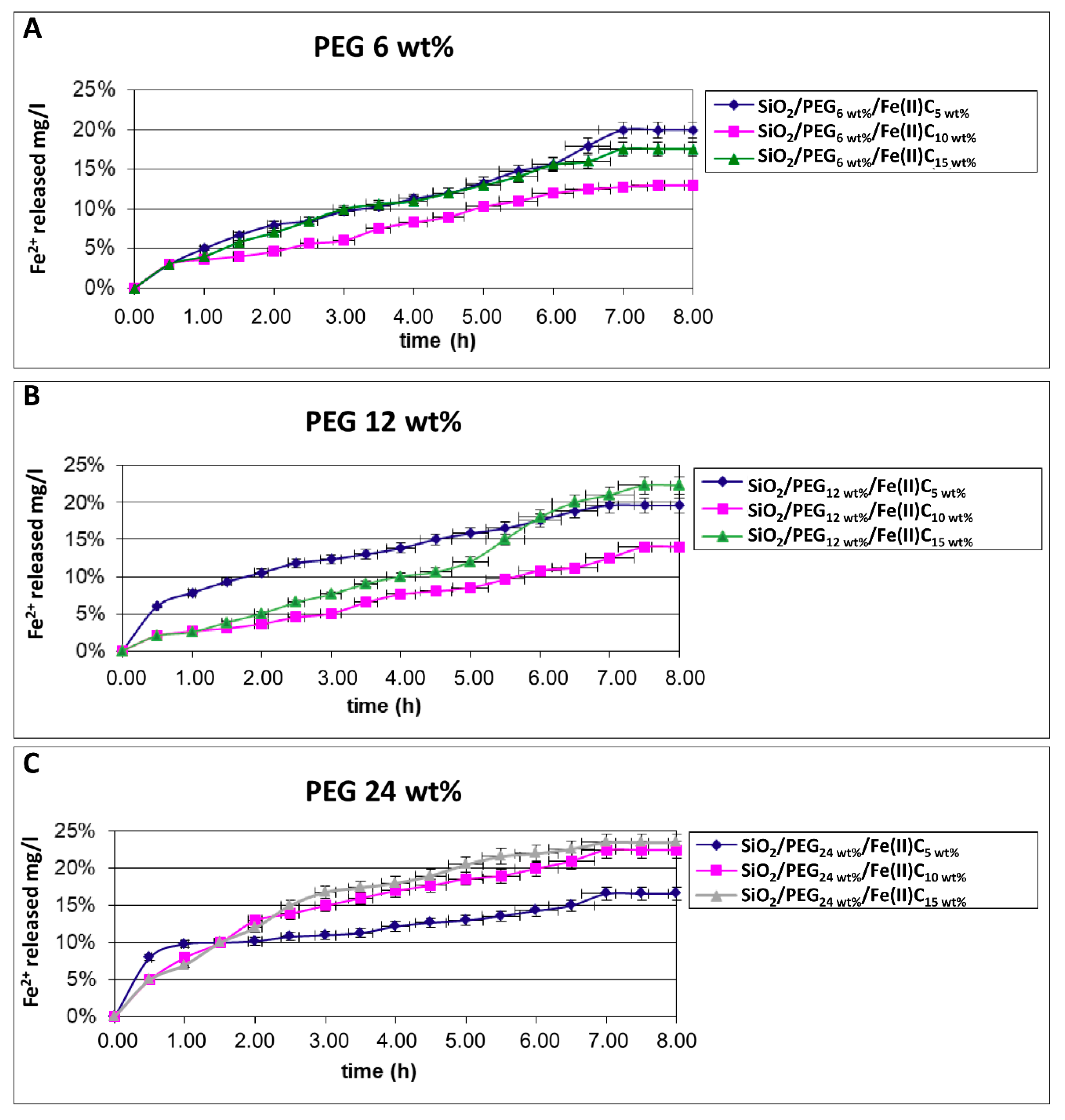
3.2. Study of In Vitro Release
3.3. Bioactivity Test
3.4. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; De Benoist, B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Pundir, S.; Badola, A.; Sharma, D. Sustained release matrix technology and recent advance in matrix drug delivery system: A review. Int. J. Drug Deliv. Res. Technol. 2017, 3, 8. [Google Scholar]
- Fragoulakis, V.; Kourlaba, G.; Goumenos, D.; Konstantoulakis, M.; Maniadakis, N. Economic evaluation of intravenous iron treatments in the management of anemia patients in Greece. ClinicoEconomics Outcomes Res. 2012, 4, 127–134. [Google Scholar] [CrossRef]
- Brinker, C.; Scherer, G. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: San Diego, CA, USA, 1989. [Google Scholar]
- Amiri, S.; Rahimi, A. Hybrid nanocomposite coating by sol–gel method: A review. Iran. Polym. J. 2016, 25, 559–577. [Google Scholar] [CrossRef]
- Judeinstein, P.; Sanchez, C. Hybrid organic–inorganic materials: A land of multidisciplinarity. J. Mater. Chem. 1996, 6, 511–525. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Renella, R.A.; Papale, F. Sol-gel synthesis of SiO2-CaO-P2O5 glasses: Influence of the heat treatment on their bioactivity and biocompatibility. Ceram. Int. 2015, 41, 12578–12588. [Google Scholar] [CrossRef]
- Blanco, I. Polysiloxanes in theranostics and drug delivery: A review. Polymers 2018, 10, 755. [Google Scholar] [CrossRef]
- Kim, G.; Hong, L.Y.; Jung, J.; Kim, D.-P.; Kim, H.; Kim, I.J.; Kim, J.R.; Ree, M. The biocompatability of mesoporous inorganic–organic hybrid resin films with ionic and hydrophilic characteristics. Biomaterials 2010, 31, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Garcia, A.; Sani, M.A.; Diaz, D.; Dubey, V.; Clayton, D.; Dal Poggetto, G.; Cornelius, F.; Payne, R.J.; Separovic, F.; et al. Interaction of N-terminal peptide analogues of the Na+,K+-ATPase with membranes. Biochim. Biophys. Acta 2018, 1860, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Tranquillo, E.; Salzillo, A.; Capasso, L.; Illiano, M.; Sapio, L.; Naviglio, S. Silica/Polyethylene Glycol Hybrid Materials Prepared by a Sol-Gel Method and Containing Chlorogenic Acid. Molecules 2018, 23, 2447. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Szebeni, J. Stealth liposomes and long circulating nanoparticles: Critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog. Lipid Res. 2003, 42, 463–478. [Google Scholar] [CrossRef]
- Salama, A.; El-Sakhawy, M. Preparation of polyelectrolyte/calcium phosphate hybrids for drug delivery application. Carbohydr. Polym. 2014, 113, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Son, K.D.; Kim, Y.-J. Anticancer activity of drug-loaded calcium phosphate nanocomposites against human osteosarcoma. Biomater. Res. 2017, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-F.; Sun, T.-W.; Chen, F.; Zuo, D.-Q.; Wang, H.-S.; Hua, Y.-Q.; Cai, Z.-D.; Tan, J. Calcium phosphate-phosphorylated adenosine hybrid microspheres for anti-osteosarcoma drug delivery and osteogenic differentiation. Biomaterials 2017, 121, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M. The Lambert-Beer Law; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Tranquillo, E.; Risoluti, R.; Ciprioti, S.V. Sol-Gel synthesis, spectroscopic and thermal behavior study of SiO2/PEG composites containing different amount of chlorogenic acid. Polymers 2018, 10, 682. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared spectroscopy of sol–gel derived silica-based films: A spectra-microstructure overview. J. Non-Cryst. Solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Catauro, M.; Pacifico, S. Synthesis of bioactive chlorogenic acid-silica hybrid materials via the sol-gel route and evaluation of their biocompatibility. Materials 2017, 10, 840. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of infrared spectra, a practical approach. Encycl. Anal. Chem. 2000, 12, 10815–10837. [Google Scholar]
- Catauro, M.; Renella, R.; Papale, F.; Ciprioti, S.V. Investigation of bioactivity, biocompatibility and thermal behavior of sol–gel silica glass containing a high PEG percentage. Mater. Sci. Eng. C 2016, 61, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Bichara, L.C.; Lanús, H.E.; Ferrer, E.G.; Gramajo, M.B.; Brandán, S.A. Vibrational study and force field of the citric acid dimer based on the SQM methodology. Adv. Phys. Chem. 2011, 2011, 347072. [Google Scholar] [CrossRef]
- Tsimbler, S.; Shevchenko, L.; Grigor’eva, V. The IR absorption spectra of the tartrate and citrate complexes of nickel, cobalt, and iron. J. Appl. Spectrosc. 1969, 11, 1096–1101. [Google Scholar] [CrossRef]
- Catauro, M.; Naviglio, D.; Risoluti, R.; Vecchio Ciprioti, S. Sol–gel synthesis and thermal behavior of bioactive ferrous citrate–silica hybrid materials. J. Therm. Anal. Calorim. 2018, 133, 1085–1092. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Gallicchio, M.; Pacifico, S. Synthesis and chemical characterization of new silica polyethylene glycol hybrid nanocomposite materials for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2014, 24, 320–325. [Google Scholar] [CrossRef]
- Catauro, M.; Melisi, D.; Curcio, A.; Rimoli, M.G. Sol-gel processing of anti-inflammatory entrapment in silica, release kinetics, and bioactivity. J. Biomed. Mater. Res. A 2008, 87, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Kruppke, B.; Hose, D.; Schnettler, R.; Seckinger, A.; Rößler, S.; Hanke, T.; Heinemann, S. Drug Release as a function of bioactivity, incubation regime, liquid, and initial load: Release of bortezomib from calcium phosphate-containing silica/collagen xerogels. J. Biomed. Mater. Res. B 2018, 106, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, C.; Kokubo, T.; Yamamuro, T. Mechanism of apatite formation on CaOSiO2P2O5 glasses in a simulated body fluid. J. Non-Cryst. Solids 1992, 143, 84–92. [Google Scholar] [CrossRef]
- Kokubo, T.; Ito, S.; Huang, Z.; Hayashi, T.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Ca, P-rich layer formed on high-strength bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Y.; Huang, Y.; Cheng, H.; Seo, H.J. Surface reactivity and hydroxyapatite formation on Ca5MgSi3O12 ceramics in simulated body fluid. Appl. Surf. Sci. 2017, 423, 900–908. [Google Scholar] [CrossRef]
- Ben-David, Y.; Zlotnik, E.; Zander, I.; Yerushalmi, G.; Shoshani, S.; Banin, E. SawR a new regulator controlling pyomelanin synthesis in Pseudomonas aeruginosa. Microbiol. Res. 2018, 206, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.C.; Newman, D.K. A putative ABC transporter, hatABCDE, is among molecular determinants of pyomelanin production in Pseudomonas aeruginosa. J. Bacteriol. 2010, 192, 5962–5971. [Google Scholar] [CrossRef] [PubMed]
- Ketelboeter, L.M.; Potharla, V.Y.; Bardy, S.L. NTBC treatment of the pyomelanogenic Pseudomonas aeruginosa clinical isolate PA1111 inhibits pigment production and increases sensitivity to oxidative stress. Curr. Microbiol. 2014, 69, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Chatfield, C.H.; Cianciotto, N.P. The secreted pyomelanin pigment of Legionella pneumophila confers ferric reductase activity. Infect. Immun. 2007, 75, 4062–4070. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Lee, S.Y.; Kong, H.G.; Jo, E.J.; Choi, H.K.; Khan, R.; Lee, S.-W. Genetic determinants for pyomelanin production and its protective effect against oxidative stress in ralstonia solanacearum. PLoS ONE 2016, 11, e0160845. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catauro, M.; Tranquillo, E.; Barrino, F.; Blanco, I.; Dal Poggetto, F.; Naviglio, D. Drug Release of Hybrid Materials Containing Fe(II)Citrate Synthesized by Sol-Gel Technique. Materials 2018, 11, 2270. https://doi.org/10.3390/ma11112270
Catauro M, Tranquillo E, Barrino F, Blanco I, Dal Poggetto F, Naviglio D. Drug Release of Hybrid Materials Containing Fe(II)Citrate Synthesized by Sol-Gel Technique. Materials. 2018; 11(11):2270. https://doi.org/10.3390/ma11112270
Chicago/Turabian StyleCatauro, Michelina, Elisabetta Tranquillo, Federico Barrino, Ignazio Blanco, Francesco Dal Poggetto, and Daniele Naviglio. 2018. "Drug Release of Hybrid Materials Containing Fe(II)Citrate Synthesized by Sol-Gel Technique" Materials 11, no. 11: 2270. https://doi.org/10.3390/ma11112270