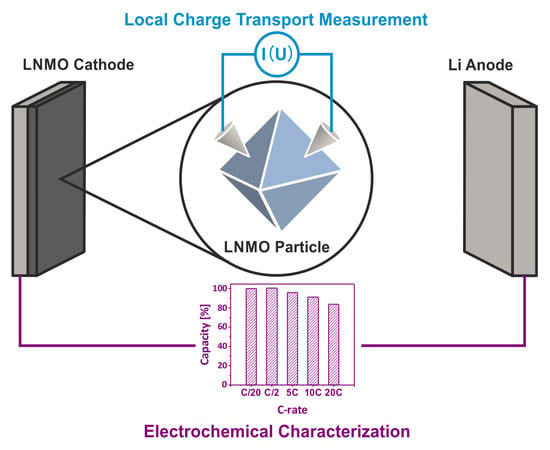
Electrochemical and Electronic Charge Transport Properties of Ni-Doped LiMn2O4 Spinel Obtained from Polyol-Mediated Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polyol-Mediated Synthesis of LNMO
2.2. Physicochemical Characterization
2.3. Cathode Preparation and Cell Assembly
2.4. Electrochemical Characterization
3. Results and Discussion
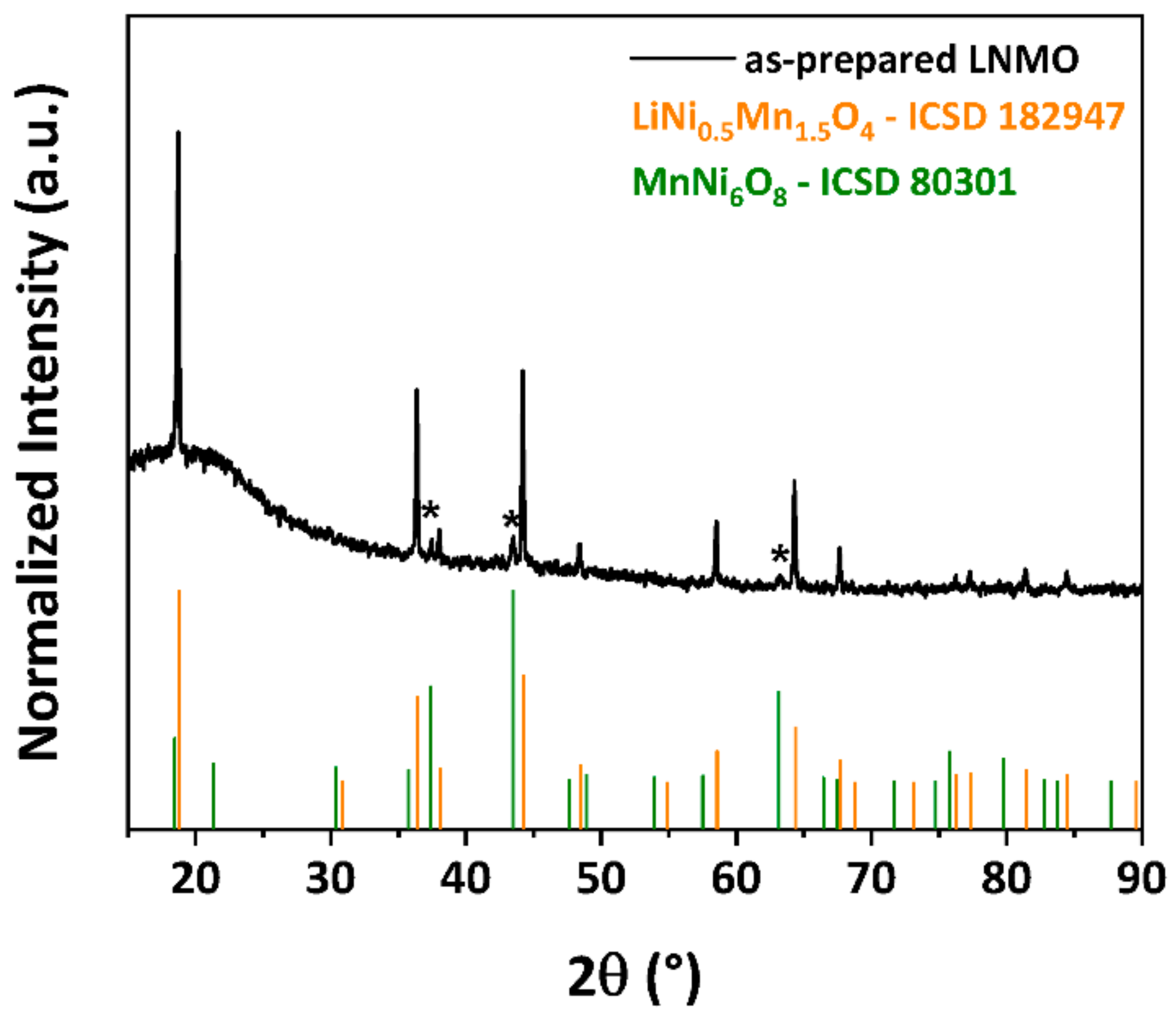
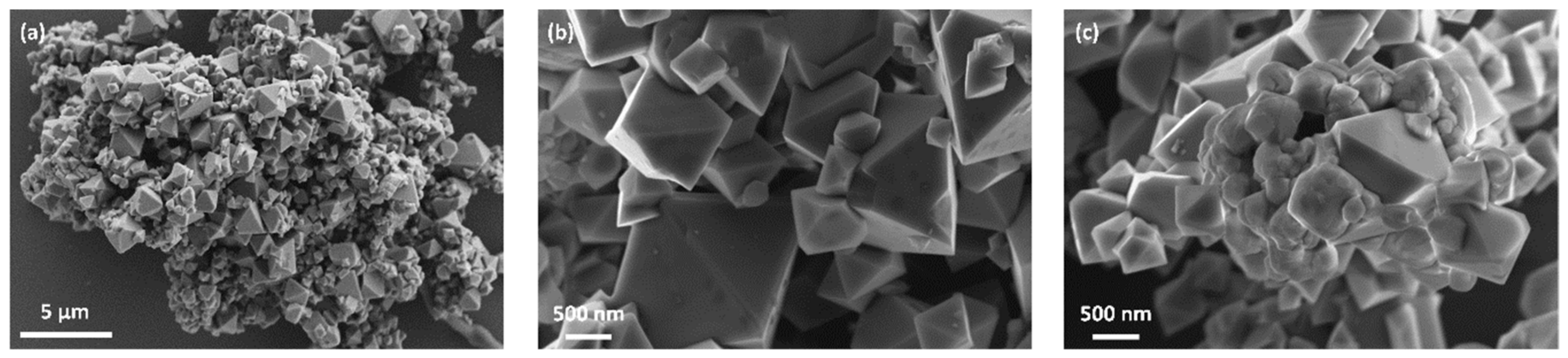
3.1. Crystal Structure and Particle Morphology of As-Prepared LNMO
3.2. Electronic Charge Transport Properties of As-Prepared LNMO
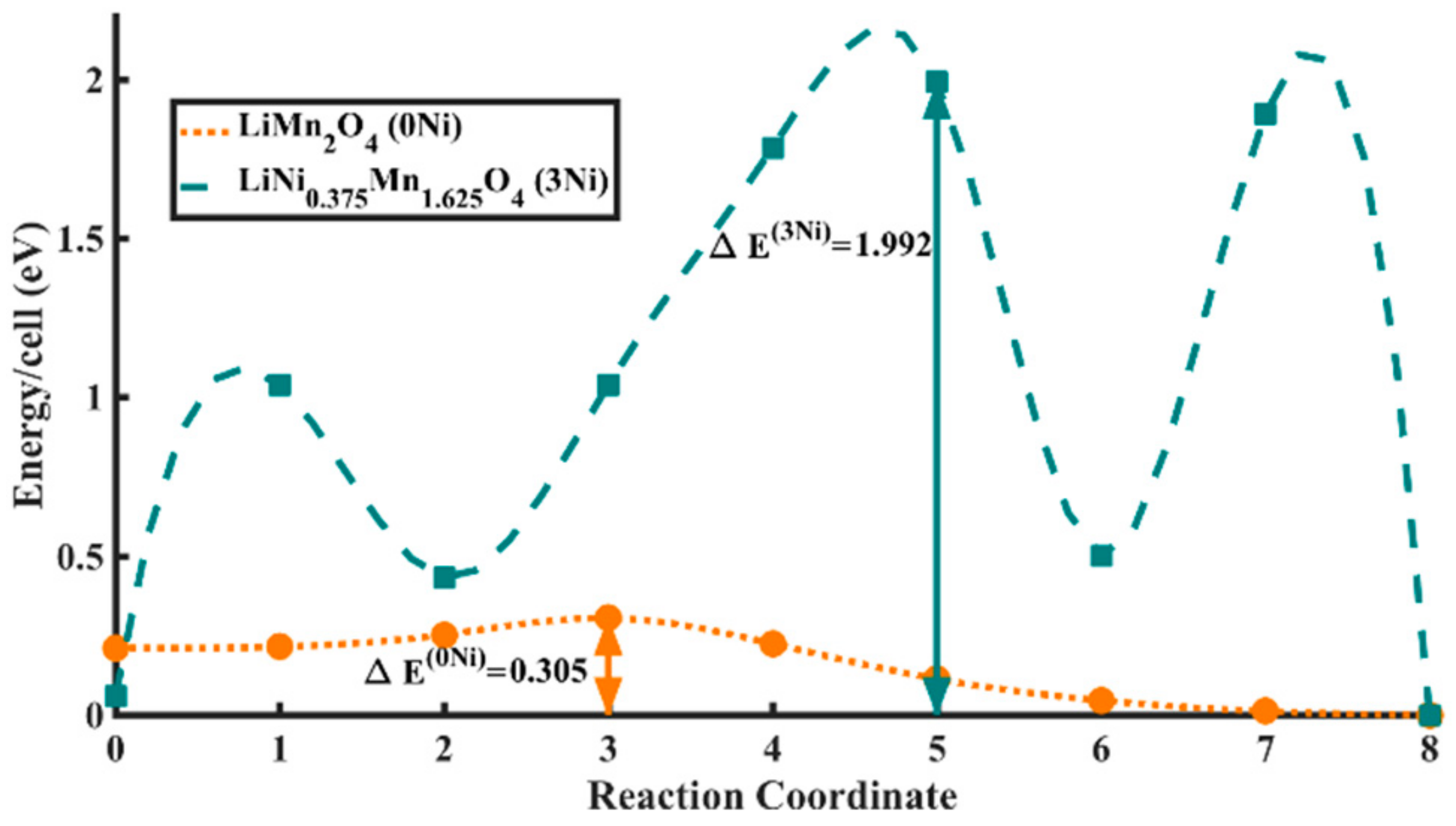
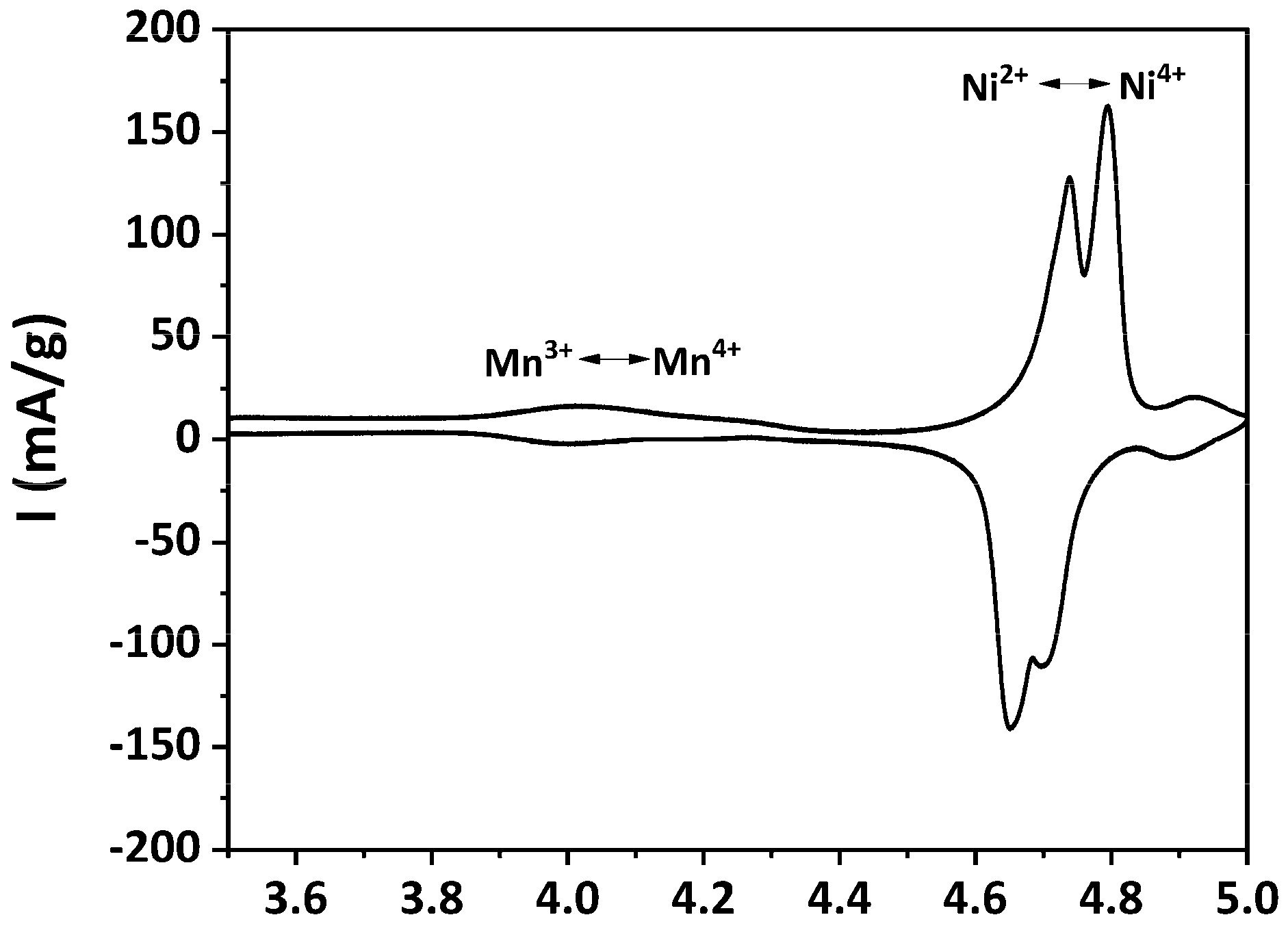
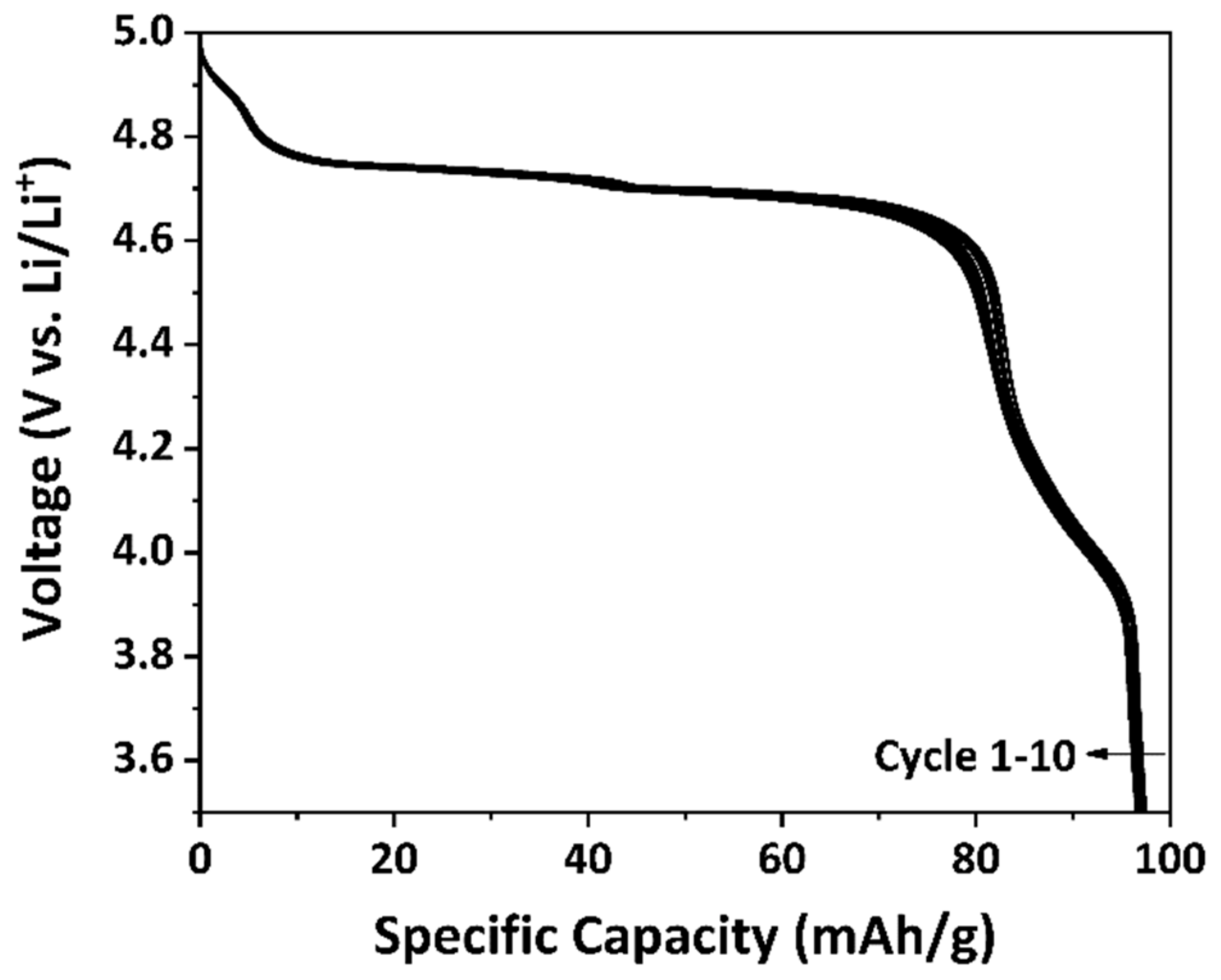
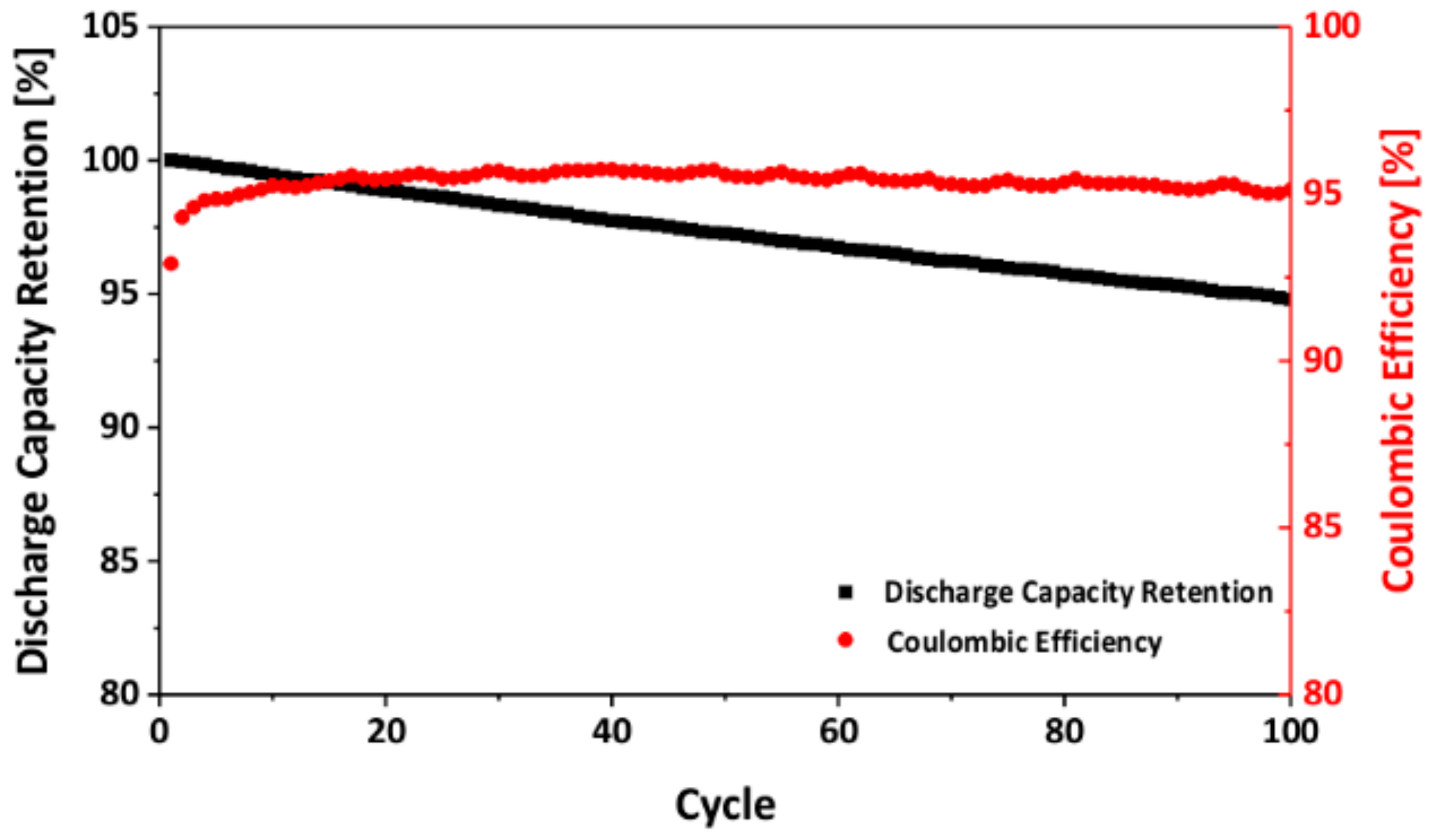
3.3. Electrochemical Performance of As-Prepared LNMO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.D.; Markevich, E.; Salitra, G.; Sharon, D.; Aurbach, D. On the challenge of developing advanced technologies for electrochemical energy storage and conversion. Mater. Today 2014, 17, 110–121. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Johnson, P.J.; de Picciotto, L.A.; Bruce, P.G.; Goodenough, J.B. Electrochemical extraction of lithium from LiMn2O4. Mater. Res. Bull. 1984, 19, 179–187. [Google Scholar] [CrossRef]
- Thackeray, M.M. Manganese oxides for lithium batteries. Prog. Solid State Chem. 1997, 25, 1–71. [Google Scholar] [CrossRef]
- Manthiram, A. Materials Challenges and Opportunities of Lithium Ion Batteries. J. Phys. Chem. Lett. 2011, 2, 176–184. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.-C.; Feldmann, C. Polyol synthesis of nanoparticles: Status and options regarding metals, oxides, chalcogenides, and non-metal elements. Green Chem. 2015, 17, 4107–4132. [Google Scholar] [CrossRef]
- Lu, J.; Lee, K.S. Spinel cathodes for advanced lithium ion batteries: A review of challenges and recent progress. Mater. Technol. 2016, 31, 628–641. [Google Scholar] [CrossRef]
- Ariyoshi, K.; Yamamoto, H.; Yamada, Y. High dimensional stability of LiCoMnO4 as positive electrodes operating at high voltage for lithium-ion batteries with a long cycle life. Electrochim. Acta 2018, 260, 498–503. [Google Scholar] [CrossRef]
- Sivakumar, P.; Nayak, P.K.; Markovsky, B.; Aurbach, D.; Gedanken, A. Sonochemical synthesis of LiNi0.5Mn1.5O4 and its electrochemical performance as a cathode material for 5 V Li-ion batteries. Ultrason. Sonochem. 2015, 26, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Chitrakar, R.; Makita, Y.; Ooi, K.; Sonoda, A. Synthesis of Iron-Doped Manganese Oxides with an Ion-Sieve Property: Lithium Adsorption from Bolivian Brine. Ind. Eng. Chem. Res. 2014, 53, 3682–3688. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Review of 5-V electrodes for Li-ion batteries: Status and trends. Ionics 2013, 19, 951–988. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Zaghib, K.; Liu, D. High Voltage Cathode Materials. In Rechargeable Batteries; Zhang, Z., Zhang, S.S., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2015; pp. 477–509. ISBN 978-3-319-15457-2. [Google Scholar]
- Patoux, S.; Daniel, L.; Bourbon, C.; Lignier, H.; Pagano, C.; Le Cras, F.; Jouanneau, S.; Martinet, S. High voltage spinel oxides for Li-ion batteries: From the material research to the application. J. Power Sources 2009, 189, 344–352. [Google Scholar] [CrossRef]
- Lee, E.-S.; Nam, K.-W.; Hu, E.; Manthiram, A. Influence of Cation Ordering and Lattice Distortion on the Charge–Discharge Behavior of LiMn1.5Ni0.5O4 Spinel between 5.0 and 2.0 V. Chem. Mater. 2012, 24, 3610–3620. [Google Scholar] [CrossRef]
- Kim, J.-H.; Myung, S.-T.; Yoon, C.S.; Kang, S.G.; Sun, Y.-K. Comparative Study of LiNi0.5Mn1.5O4-δ and LiNi0.5Mn1.5O4 Cathodes Having Two Crystallographic Structures: Fd-3m and P4332. Chem. Mater. 2004, 16, 906–914. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Huang, X.; Baudrin, E. A comparative study of Fd-3m and P4332 “LiNi0.5Mn1.5O4”. Solid State Ion. 2011, 193, 32–38. [Google Scholar] [CrossRef]
- Yang, J.; Han, X.; Zhang, X.; Cheng, F.; Chen, J. Spinel LiNi0.5Mn1.5O4 cathode for rechargeable lithiumion batteries: Nano vs micro, ordered phase (P4332) vs disordered phase (Fd-3m). Nano Res. 2013, 6, 679–687. [Google Scholar] [CrossRef]
- Moorhead-Rosenberg, Z.; Huq, A.; Goodenough, J.B.; Manthiram, A. Electronic and Electrochemical Properties of Li1-xiMn1.5Ni0.5O4 Spinel Cathodes As a Function of Lithium Content and Cation Ordering. Chem. Mater. 2015, 27, 6934–6945. [Google Scholar] [CrossRef]
- Kim, J.-H.; Huq, A.; Chi, M.; Pieczonka, N.P.W.; Lee, E.; Bridges, C.A.; Tessema, M.M.; Manthiram, A.; Persson, K.A.; Powell, B.R. Integrated Nano-Domains of Disordered and Ordered Spinel Phases in LiNi0.5Mn1.5O4 for Li-Ion Batteries. Chem. Mater. 2014, 26, 4377–4386. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, J.; Yu, X.; Kovarik, L.; Gu, M.; Omenya, F.; Chen, X.; Yang, X.-Q.; Liu, J.; Graff, G.L.; et al. Enhanced Li+ ion transport in LiNi0.5Mn1.5O4 through control of site disorder. Phys. Chem. Chem. Phys. PCCP 2012, 14, 13515–13521. [Google Scholar] [CrossRef] [PubMed]
- Jafta, C.J.; Mathe, M.K.; Manyala, N.; Roos, W.D.; Ozoemena, K.I. Microwave-assisted synthesis of high-voltage nanostructured LiMn1.5Ni0.5O4 spinel: Tuning the Mn3+ content and electrochemical performance. ACS Appl. Mater. Interfaces 2013, 5, 7592–7598. [Google Scholar] [CrossRef] [PubMed]
- Kunduraci, M.; Amatucci, G.G. Synthesis and Characterization of Nanostructured 4.7 V LixMn1.5Ni0.5O4 Spinels for High-Power Lithium-Ion Batteries. J. Electrochem. Soc. 2006, 153, A1345–A1352. [Google Scholar] [CrossRef]
- Kunduraci, M.; Al-Sharab, J.F.; Amatucci, G.G. High-Power Nanostructured LiMn2-xNixO4 High-Voltage Lithium-Ion Battery Electrode Materials: Electrochemical Impact of Electronic Conductivity and Morphology. Chem. Mater. 2006, 18, 3585–3592. [Google Scholar] [CrossRef]
- Molenda, J.; Marzec, J.; Ojczyk, W.; Palubiak, D.; Swierczek, K.; Ziemnicki, M. The effect of 3d substitutions in the manganese sublattice on the electrical and electrochemical properties of manganese spinel. Solid State Ion. 2004, 175, 297–304. [Google Scholar] [CrossRef]
- Marzec, J.; Świerczek, K.; Przewoźnik, J.; Molenda, J.; Simon, D.R.; Kelder, E.M.; Schoonman, J. Conduction mechanism in operating a LiMn2O4 cathode. Solid State Ion. 2002, 146, 225–237. [Google Scholar] [CrossRef]
- Amdouni, N.; Zaghib, K.; Gendron, F.; Mauger, A.; Julien, C.M. Structure and insertion properties of disordered and ordered LiNi0.5Mn1.5O4 spinels prepared by wet chemistry. Ionics 2006, 12, 117–126. [Google Scholar] [CrossRef]
- Sun, Q.; Li, X.-H.; Wang, Z.-X.; Ji, Y. Synthesis and electrochemical performance of 5V spinel LiNi0.5Mn1.5O4 prepared by solid-state reaction. Trans. Nonferrous Met. Soc. China 2009, 19, 176–181. [Google Scholar] [CrossRef]
- Noyong, M.; Blech, K.; Rosenberger, A.; Klocke, V.; Simon, U. In situ nanomanipulation system for electrical measurements in SEM. Meas. Sci. Technol. 2007, 18, N84–N89. [Google Scholar] [CrossRef]
- Timper, J.; Gutsmiedl, K.; Wirges, C.; Broda, J.; Noyong, M.; Mayer, J.; Carell, T.; Simon, U. Surface “click” reaction of DNA followed by directed metalization for the construction of contactable conducting nanostructures. Angew. Chem. Int. Ed. 2012, 51, 7586–7588. [Google Scholar] [CrossRef] [PubMed]
- Saltzmann, T.; Bornhöfft, M.; Mayer, J.; Simon, U. Shape without Structure: An Intriguing Formation Mechanism in the Solvothermal Synthesis of the Phase-Change Material Sb2Te3. Angew. Chem. Int. Ed. 2015, 54, 6632–6636. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.O.; Raab, N.; Noyong, M.; Santhanam, V.; Dittmann, R.; Simon, U. Resistive Switching of Sub-10 nm TiO2 Nanoparticle Self-Assembled Monolayers. Nanomaterials 2017, 7, 370. [Google Scholar] [CrossRef] [PubMed]
- Höweling, A.; Stoll, A.; Schmidt, D.O.; Geßwein, H.; Simon, U.; Binder, J.R. Influence of Synthesis, Dopants and Cycling Conditions on the Cycling Stability of Doped LiNi0.5Mn1.5O4 Spinels. J. Electrochem. Soc. 2017, 164, A6349–A6358. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J.; Rousse, G.; Masquelier, C.; Hervieu, M. Electronic Crystallization in a Lithium Battery Material: Columnar Ordering of Electrons and Holes in the Spinel LiMn2O4. Phys. Rev. Lett. 1998, 81, 4660–4663. [Google Scholar] [CrossRef]
- Ouyang, C.Y.; Shi, S.Q.; Lei, M.S. Jahn–Teller distortion and electronic structure of LiMn2O4. J. Alloys Compd. 2009, 474, 370–374. [Google Scholar] [CrossRef]
- Ouyang, C.; Du, Y.; Shi, S.; Lei, M. Small polaron migration in LixMn2O4: From first principles calculations. Phys. Lett. A 2009, 373, 2796–2799. [Google Scholar] [CrossRef]
- Xin, X.-G.; Shen, J.-Q.; Shi, S.-Q. Structural and magnetic properties of LiNi0.5Mn1.5O4 and LiNi0.5Mn1.5O4−δ spinels: A first-principles study. Chin. Phys. B 2012, 21, 128202. [Google Scholar] [CrossRef]
- Shiiba, H.; Zettsu, N.; Nakayama, M.; Oishi, S.; Teshima, K. Defect Formation Energy in Spinel LiNi0.5Mn1.5O4-δ Using Ab Initio DFT Calculations. J. Phys. Chem. C 2015, 119, 9117–9124. [Google Scholar] [CrossRef]
- Fang, J.-C.; Xu, Y.-F.; Xu, G.-L.; Shen, S.-Y.; Li, J.-T.; Huang, L.; Sun, S.-G. Fabrication of densely packed LiNi0.5Mn1.5O4 cathode material with excellent long-term cycleability for high-voltage lithium ion batteries. J. Power Sources 2016, 304, 15–23. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, F.; Zhang, K.; Liang, Y.; Yang, S.; Liang, J.; Chen, J. Facile polymer-assisted synthesis of LiNi0.5Mn1.5O4 with a hierarchical micro–nano structure and high rate capability. RSC Adv. 2012, 2, 5669–5675. [Google Scholar] [CrossRef]
- Arrebola, J.C.; Caballero, A.; Hernán, L.; Morales, J. Polymer-Mediated Growth of Highly Crystalline Nano- and Micro-Sized LiNi0.5Mn1.5O4 Spinels. Eur. J. Inorg. Chem. 2008, 2008, 3295–3302. [Google Scholar] [CrossRef]
- Arrebola, J.C.; Caballero, A.; Hernán, L.; Morales, J. PMMA-assisted synthesis of Li1−xNi0.5Mn1.5O4−δ for high-voltage lithium batteries with expanded rate capability at high cycling temperatures. J. Power Sources 2008, 180, 852–858. [Google Scholar] [CrossRef]
- Arrebola, J.C.; Caballero, A.; Cruz, M.; Hernán, L.; Morales, J.; Castellón, E.R. Crystallinity Control of a Nanostructured LiNi0.5Mn1.5O4 Spinel via Polymer-Assisted Synthesis: A Method for Improving Its Rate Capability and Performance in 5 V Lithium Batteries. Adv. Funct. Mater. 2006, 16, 1904–1912. [Google Scholar] [CrossRef]
- Yang, S.; Homberger, M.; Noyong, M.; Simon, U. Polyol mediated synthesis and electrochemical performance of nanostructured LiMn2O4 cathodes. Int. J. Electrochem. Sci. 2016, 10847–10862. [Google Scholar] [CrossRef]
- Feldmann, C. Polyol-Mediated Synthesis of Nanoscale Functional Materials. Adv. Funct. Mater. 2003, 13, 101–107. [Google Scholar] [CrossRef]
- Fiévet, F.; Brayner, R. The Polyol Process. In Nanomaterials: A Danger or a Promise? A Chemical and Biological Perspective; Brayner, R., Fiévet, F., Coradin, T., Eds.; Springer: London, UK, 2013; pp. 1–25. ISBN 978-1-4471-4212-6. [Google Scholar]
- Kaiser, R.E.; Mühlbauer, J.A. Elementare Tests zur Beurteilung von Meßdaten: Soforthilfe für Statististische Tests mit Wenigen Meßdaten; Bibliographisches Institut: Leipzig, Germany, 1983; ISBN 9783411057740. [Google Scholar]
- JEMS-SAAS; version 4.4831U2016Dr. P. Stadelmann: Saas-Fee, Switzerland, 2016.
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Duncan, H.; Abu-Lebdeh, Y.; Davidson, I.J. Study of the Cathode–Electrolyte Interface of LiMn1.5Ni0.5O4 Synthesized by a Sol–Gel Method for Li-Ion Batteries. J. Electrochem. Soc. 2010, 157, A528–A535. [Google Scholar] [CrossRef]
- Duncan, H.; Duguay, D.; Abu-Lebdeh, Y.; Davidson, I.J. Study of the LiMn1.5Ni0.5O4/Electrolyte Interface at Room Temperature and 60 °C. J. Electrochem. Soc. 2011, 158, A537–A545. [Google Scholar] [CrossRef]
- Kiziltaş-Yavuz, N. Influence of M-Doping (M = Ru, Fe, La, Nd, Sm) on the Performance of Lithium Nickel-Manganese Spinels as High-Voltage Cathode Materials for Lithium Ion Batteries. Ph.D. Thesis, Karlsruher Institut für Technologie (KIT), Karlsruhe, Germany, 2015. [Google Scholar]
- Chemelewski, K.R.; Lee, E.-S.; Li, W.; Manthiram, A. Factors Influencing the Electrochemical Properties of High-Voltage Spinel Cathodes: Relative Impact of Morphology and Cation Ordering. Chem. Mater. 2013, 25, 2890–2897. [Google Scholar] [CrossRef]
- Song, J.; Shin, D.W.; Lu, Y.; Amos, C.D.; Manthiram, A.; Goodenough, J.B. Role of Oxygen Vacancies on the Performance of Li[Ni0.5–xMn1.5+x]O4 (x = 0, 0.05, and 0.08) Spinel Cathodes for Lithium-Ion Batteries. Chem. Mater. 2012, 24, 3101–3109. [Google Scholar] [CrossRef]
- Chemelewski, K.R.; Shin, D.W.; Li, W.; Manthiram, A. Octahedral and truncated high-voltage spinel cathodes: The role of morphology and surface planes in electrochemical properties. J. Mater. Chem. A 2013, 1, 3347–3354. [Google Scholar] [CrossRef]
- Schmidt, D.O.; Hoffmann-Eifert, S.; Zhang, H.; La Torre, C.; Besmehn, A.; Noyong, M.; Waser, R.; Simon, U. Resistive Switching of Individual, Chemically Synthesized TiO2 Nanoparticles. Small 2015, 11, 6444–6456. [Google Scholar] [CrossRef] [PubMed]
- Waser, R. Nanoelectronics and Information Technology: Advanced Electronic Materials and Novel Devices, 3rd ed.; Waser, R., Ed.; Wiley-VCH: Weinheim, Germany, 2012; ISBN 978-3-527-40927-3. [Google Scholar]
- Goodenough, J.B.; Manthiram, A.; Wnetrzewski, B. Electrodes for lithium batteries. J. Power Sources 1993, 43, 269–275. [Google Scholar] [CrossRef]
- Nakayama, M.; Nogami, M. A first-principles study on phase transition induced by charge ordering of in spinel. Solid State Commun. 2010, 150, 1329–1333. [Google Scholar] [CrossRef]
- Lee, E.; Persson, K.A. Revealing the coupled cation interactions behind the electrochemical profile of LixNi0.5Mn1.5O4. Energy Environ. Sci. 2012, 5, 6047–6051. [Google Scholar] [CrossRef]
- Lee, E.; Persson, K.A. Solid-Solution Li Intercalation as a Function of Cation Order/Disorder in the High-Voltage LixNi0.5Mn1.5O4 Spinel. Chem. Mater. 2013, 25, 2885–2889. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- Yu, M.; Trinkle, D.R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys. 2011, 134, 64111. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Yasaka, K.; Nishikawa, F.; Konishi, T.; Yoshio, M.; Nakai, I. In Situ XAFS Analysis of Li(Mn, M)2O4 (M = Cr, Co, Ni) 5V Cathode Materials for Lithium-Ion Secondary Batteries. J. Solid State Chem. 2001, 156, 286–291. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, Y.; Kim, J.-H.; Wu, Y.; Li, G.-L.; Huang, Y.-H. The LiZnxNi0.5-xMn1.5O4 spinel with improved high voltage stability for Li-ion batteries. Electrochim. Acta 2014, 117, 76–83. [Google Scholar] [CrossRef]
- Caballero, A.; Hernán, L.; Melero, M.; Morales, J.; Angulo, M. Oxygen Lattice Instability as a Capacity Fading Mechanism for 5 V Cathode Materials. Electrochem. Solid-State Lett. 2005, 152, A6–A12. [Google Scholar] [CrossRef]
- Xia, H.; Meng, Y.S.; Lu, L.; Ceder, G. Electrochemical Properties of Nonstoichiometric LiNi0.5Mn1.5O4−δ Thin-Film Electrodes Prepared by Pulsed Laser Deposition. J. Electrochem. Soc. 2007, 154, A737–A743. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Jarry, A.; Gottis, S.; Yu, Y.-S.; Roque-Rosell, J.; Kim, C.; Cabana, J.; Kerr, J.; Kostecki, R. The formation mechanism of fluorescent metal complexes at the LixNi0.5Mn1.5O4-δ/carbonate ester electrolyte interface. J. Am. Chem. Soc. 2015, 137, 3533–3539. [Google Scholar] [CrossRef] [PubMed]
- Pieczonka, N.P.W.; Liu, Z.; Lu, P.; Olson, K.L.; Moote, J.; Powell, B.R.; Kim, J.-H. Understanding Transition-Metal Dissolution Behavior in LiNi0.5Mn1.5O4 High-Voltage Spinel for Lithium Ion Batteries. J. Phys. Chem. C 2013, 117, 15947–15957. [Google Scholar] [CrossRef]
- Kim, J.-H.; Pieczonka, N.P.W.; Li, Z.; Wu, Y.; Harris, S.; Powell, B.R. Understanding the capacity fading mechanism in LiNi0.5Mn1.5O4/graphite Li-ion batteries. Electrochim. Acta 2013, 90, 556–562. [Google Scholar] [CrossRef]
- Hirayama, M.; Ido, H.; Kim, K.; Cho, W.; Tamura, K.; Mizuki, J.; Kanno, R. Dynamic structural changes at LiMn2O4/electrolyte interface during lithium battery reaction. J. Am. Chem. Soc. 2010, 132, 15268–15276. [Google Scholar] [CrossRef] [PubMed]
- Leung, K. First-Principles Modeling of the Initial Stages of Organic Solvent Decomposition on LixMn2O4 (100) Surfaces. J. Phys. Chem. C 2012, 116, 9852–9861. [Google Scholar] [CrossRef]
- Hai, B.; Shukla, A.K.; Duncan, H.; Chen, G. The effect of particle surface facets on the kinetic properties of LiMn1.5Ni0.5O4 cathode materials. J. Mater. Chem. A 2013, 1, 759–769. [Google Scholar] [CrossRef]
- Ariyoshi, K.; Maeda, Y.; Kawai, T.; Ohzuku, T. Effect of Primary Particle Size upon Polarization and Cycling Stability of 5-V Lithium Insertion Material of Li[Ni1/2Mn3/2]O4. J. Electrochem. Soc. 2011, 158, A281–A284. [Google Scholar] [CrossRef]

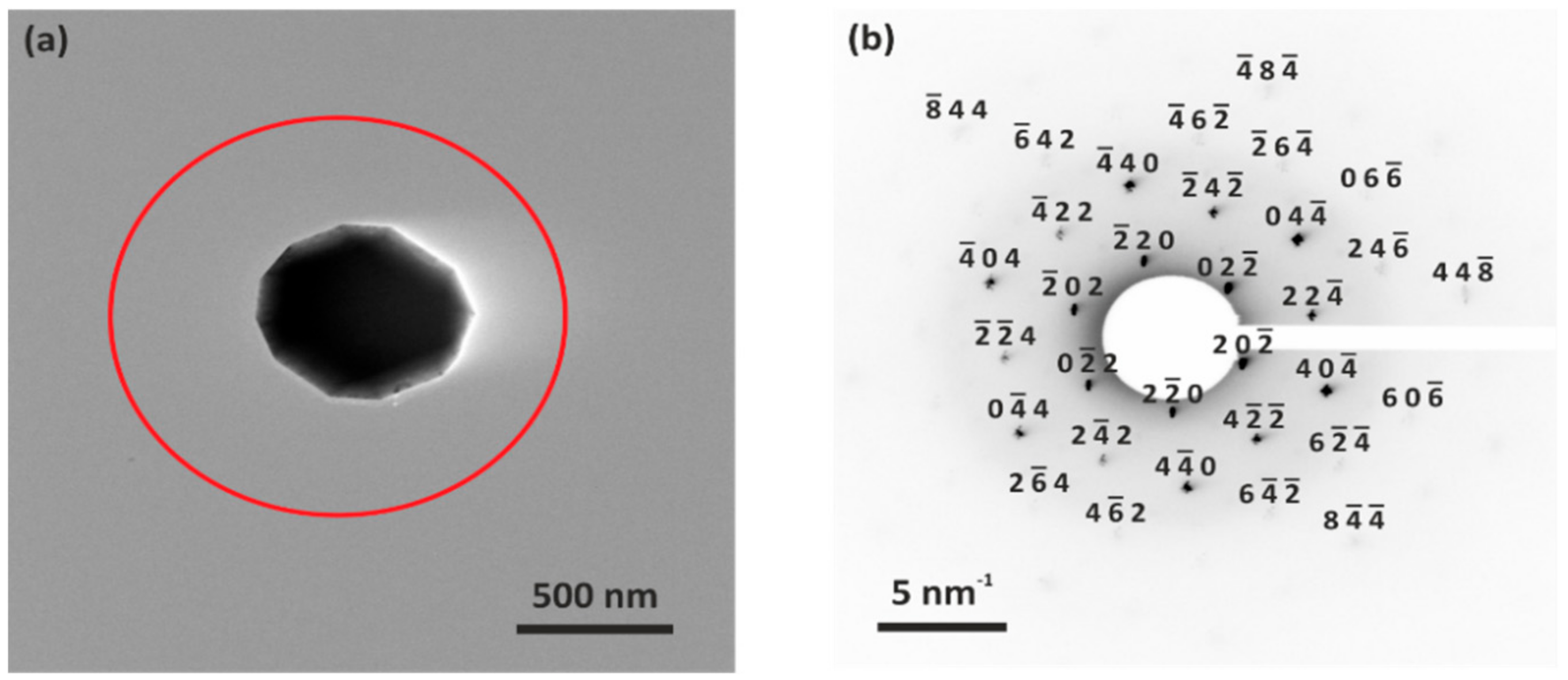



| Particle | Mn Content (At %) | Ni Content (At %) | Stoichiometry |
|---|---|---|---|
| 1 | 81 | 19 | LiNi0.38Mn1.62O4 |
| 2 | 80 | 20 | LiNi0.40Mn1.60O4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Schmidt, D.O.; Khetan, A.; Schrader, F.; Jakobi, S.; Homberger, M.; Noyong, M.; Paulus, A.; Kungl, H.; Eichel, R.-A.; et al. Electrochemical and Electronic Charge Transport Properties of Ni-Doped LiMn2O4 Spinel Obtained from Polyol-Mediated Synthesis. Materials 2018, 11, 806. https://doi.org/10.3390/ma11050806
Yang S, Schmidt DO, Khetan A, Schrader F, Jakobi S, Homberger M, Noyong M, Paulus A, Kungl H, Eichel R-A, et al. Electrochemical and Electronic Charge Transport Properties of Ni-Doped LiMn2O4 Spinel Obtained from Polyol-Mediated Synthesis. Materials. 2018; 11(5):806. https://doi.org/10.3390/ma11050806
Chicago/Turabian StyleYang, Shuo, Dirk Oliver Schmidt, Abhishek Khetan, Felix Schrader, Simon Jakobi, Melanie Homberger, Michael Noyong, Anja Paulus, Hans Kungl, Rüdiger-Albert Eichel, and et al. 2018. "Electrochemical and Electronic Charge Transport Properties of Ni-Doped LiMn2O4 Spinel Obtained from Polyol-Mediated Synthesis" Materials 11, no. 5: 806. https://doi.org/10.3390/ma11050806