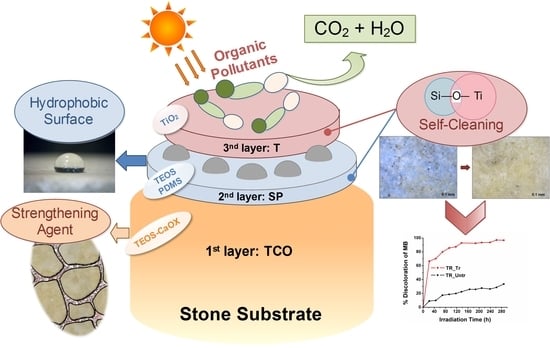
2.2. Treatments with Consolidant (TCO) and Hydrophobic (SP)/Self-Cleaning (T) Agents
A consolidation treatment with TCO was performed on the different lithoid substrates in 2013 [
16]. The treated samples were kept in laboratory conditions until now and the evaluation of their physicochemical and mechanical properties was carried out after three years of curing, in 2016, in order to assess the long-term efficiency of the consolidation in these stable conditions.
A protection treatment, with a different application protocol, followed by testing a simplified synthesis of the already studied STP [
22]. The new protocol, illustrated in detail in
Scheme 1a, consists of the separation of the hydrophobic phase (SP) of the nanocomposite from the self-cleaning phase (T). This was achieved by using tetraethyl orthosilicate (TEOS denoted as S, Sigma Aldrich, Darmstad, Germany) and hydroxyl-terminated polydimethylsiloxane (named as P, Sigma Aldrich, Darmstad, Germany) as main ingredients in the intermediate layer designated as SP, while the external layer consisted of TiO
2 (T) from titanium (IV) isopropoxide (TTIP, Sigma Aldrich, Darmstad, Germany). In both SP and T, oxalic acid dihydrate (Ox, Panreac AppliChem, Barcelona, Spain) was used as a catalyst, chelating agent, and hole-scavenger. The final molar ratio of the precursors and solvents TEOS/EtOH/H
2O/PDMS/TTIP was equal to 1/5.6/4/0.04/0.017, according to the synthesis of the SiO
2-TiO
2-PDMS STP-2 nanocomposite [
22]. The result of the consequent application of the wet T layer on the wet surface pre-treated with SP layer will be denoted from now on as SP&T treatment. All the treatments, included TCO applications in 2013, were applied successively with the aid of a brush, as indicated in
Scheme 1a.
2.3. Characterization of the Nanocomposites
The TCO nanocomposite synthesized in 2013 was fully characterized regarding its physicochemical, textural, and micro-structural properties and the obtained results are discussed in our previous scientific article in detail [
16]. The TCO consolidant, in xerogel form, synthesized in 2013 remained under laboratory conditions up to 2017. Then it was microscopically assessed with the aid of a digital microscope USB Dino-Lite AM4515T5 Edge (AnMo Electronics Corporation, New Taipei, Taiwan) with a resolution of 1.3 megapixels and a zoom of 500×–550× using a color CMOS sensor and assisted by the software Dino Capture 2.0 (2017Q2 Copyright © 2017IDCP BV, AnMo Electronics Corporation, New Taipei, Taiwan). In order to study the nanostructure of the SP&T nanocomposite, SP and T dispersions were applied on a glass slide and analyzed using a Scanning Electron Microscopy (SEM, FEI Quanta Inspect D8334 instrument, Hillsboro, Oregon, United States,, operating at 25 kV) equipped with energy X-ray energy dispersive spectroscopy (X-EDS, Oxford Instruments 2000 ISIS, Oxford Instruments, Abingdon, Oxfordshire, UK) and Transmission Electron Microscopy (TEM, Jeol JEM 2010F, Jeol, Tokyo, Japan), equipped with X-ray energy dispersive spectroscopy (X-EDS) detector. For the TEM observations, SP and T dispersions applied on the glass slide were allowed until the solvent evaporated under ambient conditions. Afterwards, the film derived was smoothly grinded in an agate pestle and mortar and dispersed in high purity ethanol. A drop of the suspension was deposited onto a lacey carbon film supported on a 3 mm Cu grid and was allowed to evaporate under ambient conditions.
The chemical interaction and the effects of the UV ageing of the SP&T nanocomposite were evaluated through Fourier-Transform Infrared Spectroscopy (FTIR, Perkin-Elmer 1000 spectrometer, Perkin-Elmer, MA, USA) in the spectral range from 4000 to 400 cm−1, with spectral resolution of 4 cm−1 and 20 consecutive scans. The measured pellets derived from the powdering of xerogel samples and KBr (Sigma Aldrich, Sigma Aldrich, Darmstad, Germany, FTIR grade), in a weight ratio equal to 1:100.
Finally, the diffuse reflectance spectra (DRS) of the SP&T nanocomposite was measured using a Cary 5000 UV-Vis-NIR spectrophotometer (Varian, Palo Alto, CA, USA) in the range of 200–800 nm equipped with an integrating sphere DRA 2500. The Energy gap of the SP&T nanomaterials was calculated through the Tauc plots of (α·hv)
1/2 versus E, allowing indirect transition, where α is the absorption coefficient (cm
−1), h is the Planck’s constant (J.s), v is the light frequency (s
−1) and E expresses the energy (eV) [
27].
2.4. Assessment of the Consolidation and Protective Efficiency
An outline of the techniques applied for the assessing of the TCO and SP&T treatment is presented in
Scheme 1b. After each layer of the TCO&SP&T treatment, the specimens were kept under laboratory conditions (RH = 60 ± 5%, T = 20 ± 2 °C) until a constant weight was achieved. The dry matter of the products absorbed was calculated, subtracting the weight before treatment from the constant weight of treated specimens. The microscopic appearance of the treated specimens was observed using the digital microscope USB Dino-Lite.
The aesthetic compatibility of the three-layered treatment was studied using a Konica Minolta, CM-2600d (Konica Minolta Optics, INS, Osaka, Japan), Vis spectrophotometer, adapted with a D65 illuminant at 8-degree viewing, in a wavelength range from 360 to 740 nm. The average values of the color parameters
L*,
α* and
b*, derived from ten measurements of random areas of untreated and treated specimens used for the calculation of the total color difference expressed as Δ
Ε* [
21,
28].
As far as the morphological structure of the substrates is concerned, both treated and untreated specimens, coated with a conducting layer of graphite, were observed by Scanning Electron Microscopy using a SEM Jeol JSM-5400 (Jeol, Tokyo, Japan) and FEI Quanta Inspect D8334 (Hillsboro, OR, USA).
Mercury Intrusion Porosimetry (MIP) using an Autoscan Porosimeter (Quantachrome Corporation, FL, USA), in the range from 0.005 to 200 μm, was used for the determination of both porosity and pore size distribution before and after consolidation treatment with TCO. The analyzed samples were previously kept at 70 °C for 180 min, aiming to remove the entrapped air and humidity in the pores.
The hydrophobic protection provided by the laboratory SP&T synthesized products was evaluated through the assessment of the water absorption by capillarity (WAC) according to the EN 15801:2010) [
29] and the static contact angle (CA). Regarding the WAC, before and after treatment, the maximum water absorption per unit area (Qi, expressed in mg·cm
−1) and the capillary water absorption coefficients (CA) in mg·cm
−1·s
−1/2 were calculated for all the substrates. Moreover, according to the literature [
4,
30] both the capillary index (CI) and the relative capillary index (CI
Rel) were obtained from the WAC measurements. The CI was calculated through the formula: CI =
, where
expresses the area under the absorption curve and the
denotes the total absorbed water per unit area at final time (t
f). The CI
Rel further evaluates the effectiveness of the hydrophobic layer, as it compares the areas under the absorption curves observed from untreated and treated specimens. In particular, the CI
Rel was calculated by the equation: CI
Rel =
, where
indicates the area under curve of the treated samples, whereas the
expresses the corresponding area of the untreated specimens. As for the CA measurements, an optical tensionmeter (Thetalite TL 101, KSV, Biolin Scientific Attension, Gothenburg, Sweden) was used, determining the static contact angle (θs) of three treated and three untreated specimen surfaces according to EN Standard Procedure [
31]. On different points of each specimen, several water droplets (~5 μL volume) were deposited close to the surfaces using a needle. Afterwards, the images of the droplets were further processed, revealing the average CA values for 0 and 20 s.
The water vapor permeability experiments were performed on cylindrical specimens of 40 mm × 20 mm (diameter × height) according to EN 15803:2010 [
32]. The test was carried out before and after treatment, using a special home-made PVC apparatus. From the obtained results, the water vapor permeability δp expressed in kg·m
−2·s
−1·Pa
−1, was calculated before the treatment (δp
untr) and after the application of the materials (δp
tr). The ratio of δp
tr/δp
untr was calculated in order to assess the permeability of the substrates after the three layers were applied [
4].
The estimation of the penetration depth and the stability of the TCO consolidant was based on the FTIR spectra from the analysis of powders originating from different depths of the treated surfaces. The consolidated specimens were cut in half and powders from different depths of the perpendicular surfaces were extracted with the aid of a dentist wheel.
The mechanical properties used for the evaluation of the TCO effectiveness as a strengthening agent were: (i) the indirect tensile strength (Brazilian test) according to ASTM D3967-86 and (ii) the dynamic modulus of elasticity (E
dyn) calculated from ultrasonic measurements according to EN 14579 (2004). The
was calculated through the equation:
E, where
is the density of the sample and
the measured pulse velocity [
16,
28,
33].
Furthermore, Vickers hardness of the ceramic substrate was calculated as the average value of the 6 measurements under loading of 300 g using a microhardness tester Future-Tech FM-700 (FUTURE-TECH CORP, Kawasaki-City, Japan).
The cohesion and adherence of the three-layered treatment were assessed by a peeling test according to the described methodology in the bibliography [
34,
35]. As a peeling means, a double-sided Tesa Powerbond Indoor (55740) adhesive tape (Global Headquarters—tesa SE, Norderstedt, Germany) was used.
2.5. Assessment of the Self-Cleaning Performance
The efficiency of the SP&T as self-cleaning layer for lithoid substrates was evaluated by monitoring the photo-degradation of stains deposited on the surfaces of specimens. In order to ensure both the homogeneous deposition and the rapid evaporation of the solvent, ethanolic solution 1 mM of Methylene Blue (MB, Panreac AppliChem, Barcelona, Spain) was used as the staining agent of the substrates [
4]. 1 mL of the prepared MB solution was applied on each kind of specimen and the samples were kept in the dark for 42 h. Thereafter, all of the stained specimens, untreated and treated with SP&T, were subjected to a UV chamber equipped with four 8 W black light blue lamps, emitting in the near-UV range (315–400 nm, 3 mW·cm
−2). The % Discoloration (%
D) of the MB compound was calculated by monitoring the changes of the
b* color parameter, using the Konica Minolta Vis-spectrophotometer, as previously described. The %D was obtained through the formula:
%
D where
b is the value of the parameter after t hours of UV,
b*(int) represents the value of the coordinate
b* before the MB deposition and, finally,
b*(MB) expresses the
b* value at time equal to 0 h [
25]. Supporting evidence on the self-cleaning features of the stained specimens derived by capturing images under the optical Dino-Lite microscope (AnMo Electronics Corporation, New Taipei City, Taiwan).
Each assessment test for the product performance e.g., MIP, capillary water absorption, static contact angle, product penetration depth, spectrophotometric measurements, ultrasound velocity, indirect tensile strength, photocatalytic activity measurement, peeling test, referred to the average value of three samples per treatment.