Effects of Pre-Treatments on Bioactivity of High-Purity Titanium
Abstract
:1. Introduction
2. Experimental
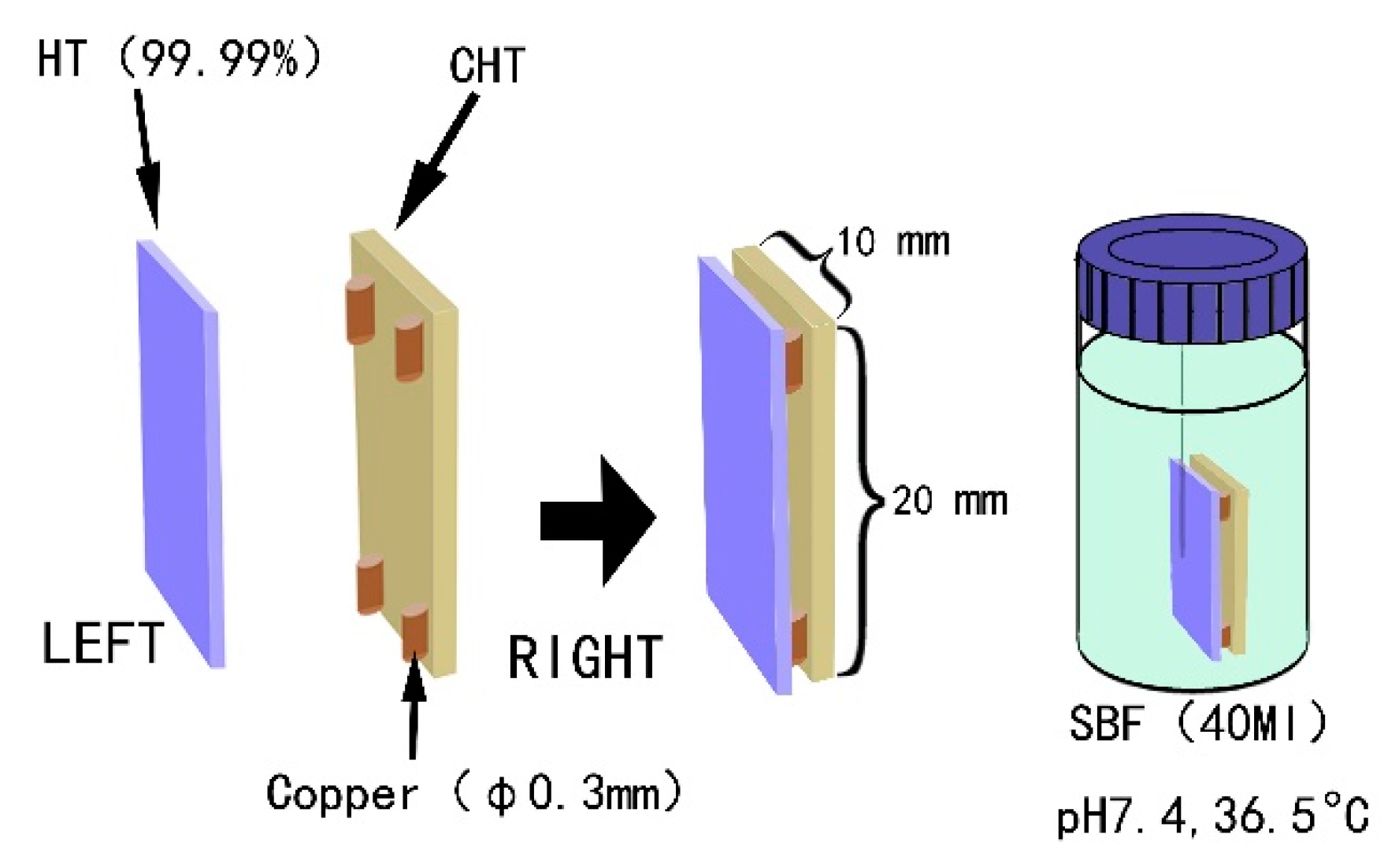
2.1. Material, Specimens, and Chemical/Heat Treatment
2.2. Assessing the In Vitro Bioactivity
3. Results and Discussion
4. Conclusions
- (1)
- Rutile-type titania formed on high-purity titanium possesses splendid bioactivity.
- (2)
- A porous microstructure of rutile-type titania plays an important role in promoting apatite forming ability.
- (3)
- The presence of copper ions in SBF and copper particles on top of the titania layer can hinder apatite formation.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, X.X.; Yan, W.; Hayakawa, S.; Tsuru, K.; Osaka, A. Apatite deposition on thermally and anodically oxidized titanium surfaces in a simulated body fluid. Biomaterials 2003, 24, 4631–4637. [Google Scholar] [CrossRef]
- Yang, B.; Uchida, M.; Kim, H.M.; Zhang, X.; Kokubo, T. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 2004, 25, 1003–1008. [Google Scholar] [CrossRef]
- Liu, X.; Chu Paul, K.; Ding, C. Surface nano-functionalization of biomaterials. Mater. Sci. Eng. R. Rep. 2010, 70, 275–302. [Google Scholar] [CrossRef]
- Duan, K.; Wang, R. Surface modifications of bone implants through wet chemistry. J. Mater. Chem. 2006, 16, 2309–2321. [Google Scholar] [CrossRef]
- Le Guehennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Sugino, A.; Uetsuki, K.; Tsuru, K.; Hayakawa, S.; Ohtsuki, C.; Osaka, A. Gap effect on the heterogeneous nucleation of apatite on thermally oxidized titanium substrate. Key Eng. Mater. 2008, 361–363, 621–624. [Google Scholar] [CrossRef]
- Sugino, A.; Uetsuki, K.; Tsuru, K.; Hayakawa, S.; Osaka, A.; Ohtsuki, C. Surface topography designed to provide osteoconductivity to titanium after thermal oxidation. Mater. Trans. 2008, 49, 428–434. [Google Scholar] [CrossRef]
- Sugino, A.; Ohtsuki, C.; Tsuru, K.; Hayakawa, S.; Nakano, T.; Okazaki, Y.; Osaka, A. Effect of spatial design and thermal oxidation on apatite formation on Ti-15Zr-4Ta-4Nb alloy. Acta Biomater. 2009, 5, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Masuda, Y.; Okamoto, K.; Shirosaki, Y.; Kato, K.; Osaka, A. Liquid phase deposited titania coating to enable in vitro apatite formation on Ti6Al4V alloy. J. Mater. Sci. Mater. Med. 2014, 25, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.B.; Nakanishi, K.; Kokubo, T.; Soga, N.; Ohtsuki, C.; Nakamura, T. Apatite formation on silica gel in simulated body fluid: Its dependence on structures of silica gels prepared in different media. J. Biomed. Mater. Res. 1996, 33, 145–151. [Google Scholar] [CrossRef]
- Peltola, T.; Jokinen, M.; Rahiala, H.; Pätsi, M.; Heikkilä, J.; Kangasniemi, I.; Yli-Urpo, A. Effect of aging time of sol on structure and in vitro calcium phosphate formation of sol-gel-derived titania films. J. Biomed. Mater. Res. 2000, 51, 200–208. [Google Scholar] [CrossRef]
- Uchida, M.; Kim, H.M.; Kokubo, T.; Fujibayashi, S.; Nakamura, T. Structural dependence of apatite formation on titania gels in a simulated body fluid. J. Biomed. Mater. Res. 2003, 64, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Hayakawa, S.; Tsuru, K.; Osaka, A. Bioactive titania-gel layers formed by chemical treatment of Ti substrate with a H2O2/HCl solution. Biomaterials 2002, 23, 1353–1357. [Google Scholar] [CrossRef]
- Kaijser, T. Titania derived from combined chemical and thermal treatments of titanium: in vitro apatite forming ability. Phosphorus Res. Bull. 2004, 17, 131–140. [Google Scholar]
- Uetsuki, K.; Nakai, S.; Shirosaki, Y.; Hayakawa, S.; Osaka, A. Nucleation and growth of apatite on an anatase layer irradiated with UV light under different environmental conditions. J. Biomed. Mater. Res. 2013, 101A, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kim, H.M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Lopez-Heredia, M.; Enkel, B.; Weiss, P.; Amouriq, Y.; Layrolle, P. Osteoblastic cell behaviour on different titanium implant surfaces. Acta Biomater. 2008, 4, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, V.B.; Bhatnagar, D.; Leszczak, V.; Popat, K.C. Titania nanostructures: A biomedical perspective. RSC Adv. 2015, 5, 37149–37171. [Google Scholar] [CrossRef]
- Anselme, K.; Bigerelle, M.; Noel, B.; Alain, I.; Hardouin, P. Effect of grooved titanium substratum on human osteoblastic cell growth. J. Biomed. Mater. Res. 2002, 60, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Bigerelle, M.; Anselme, K.; Noel, B.; Ruderman, I.; Hardouin, P.; Alain, I. Improvement in the morphology of Ti-based surfaces: A new process to increase in vitro human osteoblast response. Biomaterials 2002, 23, 1563–1577. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, J.; Scheideler, L.; Altebaeumer, T.; Geis-Gerstorfer, J.; Kern, D. Cellular reactions of osteoblasts to micron- and submicron-scale porous structures of titanium surfaces. Cells Tissues Organs. 2004, 178, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, X.; Elkhooly, T.A.; Zhang, R.; Yang, X.; Shen, Z.; Feng, Q. Preparation and characterization of TiO2/silicate hierarchical coating on titanium surface for biomedical applications. Mater. Sci. Eng. C 2016, 60, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Simon, C.G.; Kim, G. A mini-review: Cell response to microscale, nanoscale, and hierarchical patterning of surface structure. J. Biomed. Mater. Res. 2014, 102, 1580–1594. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, M.; Edalat, F.; Manoucheri, S.; Khademhosseini, A. Engineering microscale topographies to control the cell-substrate interface. Biomaterials 2012, 33, 5230–5246. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. The impact of oral implants—Past and future, 1966–2042. J. Can. Dent. Assoc. 2005, 71, 327. [Google Scholar] [PubMed]
- Becker, W.; Becker, B.E.; Ricci, A.; Bahat, O.; Rosenberg, E.; Rose, L.F.; Handelsman, M.; Israelson, H. A prospective multicenter clinical trial comparing one- and two-stage titanium screw-shaped fixtures with one-stage plasma-sprayed solid-screw fixtures. Clin. Implant. Dent. Relat. Res. 2000, 2, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sheng, X.X.; Dan, X.H.; Xiang, Q.J. Preparation of mica/apatite glass-ceramics biomaterials. Mater. Sci. Eng. C 2006, 26, 1390–1394. [Google Scholar] [CrossRef]
- Klein, P.A.T.; Driessen, A.A.; de Groot, K.; Van den Hooff, A. Biodegradation behavior of various calcium phosphate materials in bone tissue. J. Biomed. Mater. Res. 1983, 17, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Lou, J.; Zeng, L.L.; Xiang, J.H. Osteogenic potential of a novel microarc oxidized coating formed on Ti6Al4V alloys. Appl. Surf. Sci. 2017, 412, 29–36. [Google Scholar] [CrossRef]









| Element | Ti | Al | P | V | Cr | Fe | Ni | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|
| content (ppm) | substrate | 4.577 | 4.540 | 1.122 | 3.992 | 200.113 | 14.541 | 2.329 | 1.166 |
| Sample | Size (mm3) | Treatment Method | |
|---|---|---|---|
| Type A | CHT | 10 × 20 × 1 | Chemically treated with 30% wt H2O2 at 80 °C for 3 h, then heated at 400 °C for 1 h |
| HT | Heated at 400 °C for 5 h covered with 50 µm Na2O∙2B2O3 powder | ||
| Type B | GT | 10 × 10 × 3 | Machined micro-grooves of 50 µm both in depth and in width, then heated at 600 °C for 5 h |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, G.; Lu, Z.; Li, W.; Yan, Y.; Song, Y.; Akiyoshi, O. Effects of Pre-Treatments on Bioactivity of High-Purity Titanium. Materials 2018, 11, 675. https://doi.org/10.3390/ma11050675
Wang Y, Wang G, Lu Z, Li W, Yan Y, Song Y, Akiyoshi O. Effects of Pre-Treatments on Bioactivity of High-Purity Titanium. Materials. 2018; 11(5):675. https://doi.org/10.3390/ma11050675
Chicago/Turabian StyleWang, Yaming, Guangxin Wang, Zhi Lu, Wuhui Li, Yanfu Yan, Yongfa Song, and Osaka Akiyoshi. 2018. "Effects of Pre-Treatments on Bioactivity of High-Purity Titanium" Materials 11, no. 5: 675. https://doi.org/10.3390/ma11050675
APA StyleWang, Y., Wang, G., Lu, Z., Li, W., Yan, Y., Song, Y., & Akiyoshi, O. (2018). Effects of Pre-Treatments on Bioactivity of High-Purity Titanium. Materials, 11(5), 675. https://doi.org/10.3390/ma11050675





