Catalytic Oxidation of Soot on a Novel Active Ca-Co Dually-Doped Lanthanum Tin Pyrochlore Oxide
Abstract
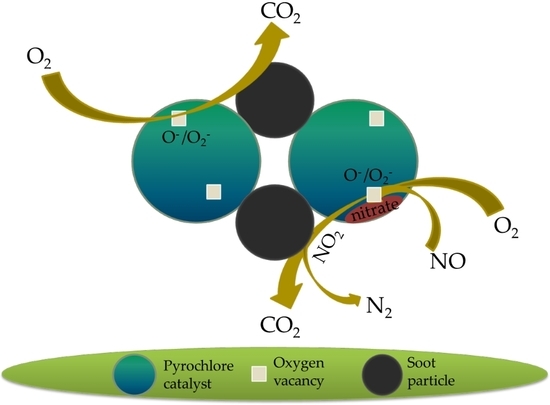
:1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalytic Activity Measurement
2.4. In Situ DRIFTS Experiments
3. Results and Discussion
3.1. XRD Analysis
3.2. Catalyst Morphologies
3.3. N2 Adsorption-Desorption Characterization
3.4. FT-IR
3.5. H2-TPR
3.6. Catalyst Chemical States and Surface Oxygen Vacancies
3.7. Catalytic Soot Combustion over La2−xCaxSn2−yCoyO7 Catalysts
3.8. Catalytic Activities for NO Oxidation
3.9. In Situ DRIFTS
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, G.; Zhao, Z.; Xu, J.; Zheng, J.; Liu, J.; Jiang, G.; Duan, A.; He, H. Comparative study on the preparation, characterization and catalytic performances of 3DOM Ce-based materials for the combustion of diesel soot. Appl. Catal. B Environ. 2011, 107, 302–315. [Google Scholar] [CrossRef]
- Jampaiah, D.; Venkataswamy, P.; Tur, K.M.; Ippolito, S.J.; Bhargava, S.K.; Reddy, B.M. Effect of mnoxloading on structural, surface, and catalytic properties of CeO2-mnoxmixed oxides prepared by sol-gel method. Z. Anorg. Allg. Chem. 2015, 641, 1141–1149. [Google Scholar] [CrossRef]
- Russo, N.; Fino, D.; Saracco, G.; Specchia, V. Promotion effect of au on perovskite catalysts for the regeneration of diesel particulate filters. Catal. Today 2008, 137, 306–311. [Google Scholar] [CrossRef]
- Lee, C.; Park, J.-I.; Shul, Y.-G.; Einaga, H.; Teraoka, Y. Ag supported on electrospun macro-structure CeO2 fibrous mats for diesel soot oxidation. Appl. Catal. B Environ. 2015, 174–175, 185–192. [Google Scholar] [CrossRef]
- Fino, D.; Russo, N.; Saracco, G.; Specchia, V. Catalytic removal of nox and diesel soot over nanostructured spinel-type oxides. J. Catal. 2006, 242, 38–47. [Google Scholar] [CrossRef]
- Guo, X.; Meng, M.; Dai, F.; Li, Q.; Zhang, Z.; Jiang, Z.; Zhang, S.; Huang, Y. Nox-assisted soot combustion over dually substituted perovskite catalysts La1−xKxCo1−yPdyO3−δ. Appl. Catal. B Environ. 2013, 142–143, 278–289. [Google Scholar] [CrossRef]
- Castoldi, L.; Matarrese, R.; Lietti, L.; Forzatti, P. Intrinsic reactivity of alkaline and alkaline-earth metal oxide catalysts for oxidation of soot. Appl. Catal. B Environ. 2009, 90, 278–285. [Google Scholar] [CrossRef]
- Venkataswamy, P.; Jampaiah, D.; Rao, K.N.; Reddy, B.M. Nanostructured Ce0.7Mn0.3O2−δ and Ce0.7Fe0.3O2−δ solid solutions for diesel soot oxidation. Appl. Catal. A Gen. 2014, 488, 1–10. [Google Scholar] [CrossRef]
- Lin, F.; Wu, X.; Weng, D. Effect of barium loading on CuOx–CeO2 catalysts: NOx storage capacity, NO oxidation ability and soot oxidation activity. Catal. Today 2011, 175, 124–132. [Google Scholar] [CrossRef]
- Oliveira, C.F.; Garcia, F.A.C.; Araújo, D.R.; Macedo, J.L.; Dias, S.C.L.; Dias, J.A. Effects of preparation and structure of cerium-zirconium mixed oxides on diesel soot catalytic combustion. Appl. Catal. A Gen. 2012, 413–414, 292–300. [Google Scholar] [CrossRef]
- Zahir, M.H.; Suzuki, T.; Fujishiro, Y.; Awano, M. Hydrothermal synthesis of Sr–Ce–Sn–Mn–O mixed oxidic/stannate pyrochlore and its catalytic performance for NO reduction. Mater. Chem. Phys. 2009, 116, 273–278. [Google Scholar] [CrossRef]
- Peng, H.; Xu, J.; Tian, J.; Liu, Y.; He, Y.; Tan, J.; Xu, X.; Liu, W.; Zhang, N.; Wang, X. Mesoporous Y2Sn2O7pyrochlore with exposed (111) facets: An active and stable catalyst for CO oxidation. RSC Adv. 2016, 6, 71791–71799. [Google Scholar] [CrossRef]
- Modeshia, D.R.; Walton, R.I. Cheminform abstract: Solvothermal synthesis of perovskites and pyrochlores: Crystallization of functional oxides under mild conditions. Chem. Soc. Rev. 2010, 42, 4303–4325. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, Y.; Torigoshi, K.I.; Yamaguchi, H.; Ikeda, T.; Kagawa, S. Direct decomposition of nitric oxide over stannate pyrochlore oxides: Relationship between solid-state chemistry and catalytic activity. J. Mol. Catal. A Chem. 2000, 155, 73–80. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, H.; Hao, Z.; Wang, S. Catalytic combustion of methane over cobalt doped lanthanum stannate pyrochlore oxide. Catal. Commun. 2008, 9, 690–695. [Google Scholar] [CrossRef]
- Shangguan, W.F.; Teraoka, Y.; Kagawa, S. Promotion effect of potassium on the catalytic property of CuFe2O4 for the simultaneous removal of NOx and diesel soot particulate. Appl. Catal. B Environ. 1998, 16, 149–154. [Google Scholar] [CrossRef]
- Lv, M.; Guo, X.; Wang, Z.; Wang, L.; Li, Q.; Zhang, Z. Synthesis and characterization of Co–Al–Fe nonstoichiometric spinel-type catalysts for catalytic CO oxidation. RSC Adv. 2016, 6, 27052–27059. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, L.; Wang, X.; Zou, W.; Cao, Y.; Sun, J.; Tang, C.; Gao, F.; Deng, Y.; Dong, L. Synthesis, characterization and catalytic performance of FeMnTiOx mixed oxides catalyst prepared by a CTAB-assisted process for mid-low temperature NH3-SCR. Appl. Catal. A Gen. 2015, 505, 235–242. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, H.; Ai, L.; Liu, X.; Lv, M.; Wang, L.; Ma, Z.; Zhang, Z. Catalytic combustion of soot particulates over rare-earth substituted Ln2Sn2O7 pyrochlores (Ln = La, Nd and Sm). J. Colloid Interface Sci. 2016, 478, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhang, X.; Guo, Y.; Chen, M.; Liu, W.; Xu, X.; Peng, H.; Gao, Z.; Wang, X.; Li, C. Highly active and stable Ni/Y2Zr2O7 catalysts for methane steam reforming: On the nature and effective preparation method of the pyrochlore support. Int. J. Hydrog. Energy 2016, 41, 11141–11153. [Google Scholar] [CrossRef]
- Kim, C.H.; Qi, G.; Dahlberg, K.; Li, W. Strontium-doped perovskites rival platinum catalysts for treating nox in simulated diesel exhaust. Science 2010, 327, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- López-Suárez, F.E.; Bueno-López, A.; Illán-Gómez, M.J.; Trawczynski, J. Potassium-copper perovskite catalysts for mild temperature diesel soot combustion. Appl. Catal. A Gen. 2014, 485, 214–221. [Google Scholar] [CrossRef]
- Li, K.W.; Zhang, T.T.; Wang, H.; Yan, H. Low-temperature synthesis and structure characterization of the serials Y2−δBiδSn2O7 (δ = 0–2.0 δ = 0–2.0 mathcontainer loading mathjax) nanocrystals. J. Solid State Chem. 2006, 179, 1029–1034. [Google Scholar] [CrossRef]
- Petit, C.; Kaddouri, A.; Libs, S.; Kiennemann, A.; Rehspringer, J.L.; Poix, P. Bond energy effects in methane oxidative coupling on pyrochlore structures. J. Catal. 1993, 140, 328–334. [Google Scholar] [CrossRef]
- Bensaid, S.; Russo, N.; Fino, D. CeO2 catalysts with fibrous morphology for soot oxidation: The importance of the soot–catalyst contact conditions. Catal. Today 2013, 216, 57–63. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Xu, J.; Xu, C.; Duan, A.; Jiang, G.; He, H. The highly active catalysts of nanocomposite K-Co-CeO2 for soot combustion. Chem. Commun. 2011, 47, 11119–11121. [Google Scholar] [CrossRef] [PubMed]
- Pecchi, G.; Dinamarca, R.; Campos, C.M.; Garcia, X.; Jimenez, R.; Fierro, J.L.G. Soot oxidation on silver-substituted LaMn0.9Co0.1O3 perovskites. Ind. Eng. Chem. Res. 2014, 53, 10090–10096. [Google Scholar] [CrossRef]
- Yao, W.; Wang, R.; Yang, X. LaCo1−xPdxO3 perovskite-type oxides: Synthesis, characterization and simultaneous removal of NOx and diesel soot. Catal. Lett. 2009, 130, 613–621. [Google Scholar] [CrossRef]
- Tian, J.; Peng, H.; Xu, X.; Liu, W.; Ma, Y.; Wang, X.; Yang, X. High surface area La2Sn2O7 pyrochlore as a novel, active and stable support for Pd for CO oxidation. Catal. Sci. Technol. 2015, 5, 2270–2281. [Google Scholar] [CrossRef]
- Asuvathraman, R.; Gnanasekar, K.I.; Clinsha, P.C.; Ravindran, T.R.; Govindan Kutty, K.V. Investigations on the charge compensation on Ca and U substitution in CePO4 by using XPS, XRD and raman spectroscopy. Ceram. Int. 2015, 41, 3731–3739. [Google Scholar] [CrossRef]
- Natile, M.M.; Ugel, E.; Maccato, C.; Glisenti, A. LaCoO3: Effect of synthesis conditions on properties and reactivity. Appl. Catal. B Environ. 2007, 72, 351–362. [Google Scholar] [CrossRef]
- Tang, X.; Li, Y.; Huang, X.; Xu, Y.; Zhu, H.; Wang, J.; Shen, W. MnOx–CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: Effect of preparation method and calcination temperature. Appl. Catal. B Environ. 2006, 62, 265–273. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Investigations into nanostructured ceria–zirconia catalysts for soot combustion. Appl. Catal. B Environ. 2016, 180, 271–282. [Google Scholar] [CrossRef]
- Ura, B.; Trawczyński, J.; Kotarba, A.; Bieniasz, W.; Illán-Gómez, M.J.; Bueno-López, A.; López-Suárez, F.E. Effect of potassium addition on catalytic activity of SrTiO3 catalyst for diesel soot combustion. Appl. Catal. B Environ. 2011, 101, 169–175. [Google Scholar] [CrossRef]
- Jia, C.J.; Schwickardi, M.; Weidenthaler, C.; Schmidt, W.; Korhonen, S.; Weckhuysen, B.M.; Schüth, F. Co3O4-SiO2 nanocomposite: A very active catalyst for Co oxidation with unusual catalytic behavior. J. Am. Chem. Soc. 2011, 133, 11279–11288. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Meng, M.; Zha, Y.; Dai, F.; Hu, T.; Xie, Y.; Zhang, J. Highly efficient multifunctional dually-substituted perovskite catalysts La1−xKxCo1−yCuyO3−δ used for soot combustion, NOx storage and simultaneous NOx-soot removal. Appl. Catal. B Environ. 2012, 121–122, 65–74. [Google Scholar] [CrossRef]
- Li, Q.; Meng, M.; Tsubaki, N.; Li, X.; Li, Z.; Xie, Y.; Hu, T.; Zhang, J. Performance of k-promoted hydrotalcite-derived comgalo catalysts used for soot combustion, nox storage and simultaneous soot–nox removal. Appl. Catal. B Environ. 2009, 91, 406–415. [Google Scholar] [CrossRef]
- Li, Q.; Meng, M.; Zou, Z.Q.; Li, X.G.; Zha, Y.Q. Simultaneous soot combustion and nitrogen oxides storage on potassium-promoted hydrotalcite-based comgalo catalysts. J. Hazard. Mater. 2009, 161, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Giménez, A.M.; Xavier, L.P.d.S.; Bueno-López, A. Improving ceria-zirconia soot combustion catalysts by neodymium doping. Appl. Catal. A Gen. 2013, 462–463, 100–106. [Google Scholar]
- Wu, X.; Lin, F.; Xu, H.; Weng, D. Effects of adsorbed and gaseous NOx species on catalytic oxidation of diesel soot with MnOx–CeO2 mixed oxides. Appl. Catal. B Environ. 2010, 96, 101–109. [Google Scholar] [CrossRef]
- Yang, G.; Li, Y.; Men, Y. Synergistic catalysis effect of mn-promoted BaAl2O4 catalysts on catalytic performance for soot combustion. Catal. Commun. 2015, 69, 202–206. [Google Scholar] [CrossRef]
- Sedlmair, C. Elementary steps of nox adsorption and surface reaction on a commercial storage–reduction catalyst. J. Catal. 2003, 214, 308–316. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Weng, D.; Li, M.; Ran, R. Roles of acid sites on Pt/H-ZSM5 catalyst in catalytic oxidation of diesel soot. ACS Catal. 2015, 5, 909–919. [Google Scholar] [CrossRef]









| Samples | 2θ (°) a | a (Å) b | Xs (nm) c | SBET (m2/g) d | Vp (cm3/g) e | Dp (nm) f |
|---|---|---|---|---|---|---|
| LS | 28.88 | 10.6965 ± 0.0011 | 22.2 | 19.59 | 0.08 | 17.91 |
| LCS | 28.92 | 10.6773 ± 0.0021 | 12.1 | 27.22 | 0.12 | 18.21 |
| LSC | 28.96 | 10.6863 ± 0.0020 | 21.4 | 17.92 | 0.06 | 13.36 |
| LCSC | 28.96 | 10.6711 ± 0.0038 | 8.9 | 21.18 | 0.10 | 18.17 |
| Samples | La (at %) | Sn (at %) | Theoretical La/Sn | Practical La/Sn | Ca (at %) | Co (at %) | Oads/Olatt |
|---|---|---|---|---|---|---|---|
| LS | 14.05 | 21.22 | 1.00 | 0.66 | - | - | 1.02 |
| LCS | 14.17 | 17.24 | 0.8 | 0.82 | 0.66 | - | 2.44 |
| LSC | 11.56 | 20.7 | 1.11 | 0.56 | - | 1.17 | 1.66 |
| LCSC | 11.31 | 12.12 | 0.89 | 0.93 | 0.98 | 0.88 | 4.25 |
| Samples | Soot + O2 | Soot + NO + O2 | ||||||
|---|---|---|---|---|---|---|---|---|
| T5 (°C) | T50 (°C) | T90 (°C) | SCO2 (%) | T5 (°C) | T50 (°C) | T90 (°C) | SCO2 (%) | |
| Blank | 470 | 590 | 633 | 37.7 | 460 | 600 | 647 | 49.1 |
| LS | 372 | 492 | 531 | 86.6 | 360 | 489 | 530 | 85.8 |
| LCS | 346 | 474 | 516 | 85.1 | 343 | 473 | 514 | 86.8 |
| LSC | 341 | 449 | 487 | 96.9 | 313 | 424 | 463 | 96.2 |
| LCSC | 317 | 451 | 491 | 96.4 | 305 | 432 | 473 | 95.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, L.; Wang, Z.; Cui, C.; Liu, W.; Wang, L. Catalytic Oxidation of Soot on a Novel Active Ca-Co Dually-Doped Lanthanum Tin Pyrochlore Oxide. Materials 2018, 11, 653. https://doi.org/10.3390/ma11050653
Ai L, Wang Z, Cui C, Liu W, Wang L. Catalytic Oxidation of Soot on a Novel Active Ca-Co Dually-Doped Lanthanum Tin Pyrochlore Oxide. Materials. 2018; 11(5):653. https://doi.org/10.3390/ma11050653
Chicago/Turabian StyleAi, Lijie, Zhongpeng Wang, Chenchen Cui, Wei Liu, and Liguo Wang. 2018. "Catalytic Oxidation of Soot on a Novel Active Ca-Co Dually-Doped Lanthanum Tin Pyrochlore Oxide" Materials 11, no. 5: 653. https://doi.org/10.3390/ma11050653