Microarchitecture of the Augmented Bone Following Sinus Elevation with an Albumin Impregnated Demineralized Freeze-Dried Bone Allograft (BoneAlbumin) versus Anorganic Bovine Bone Mineral: A Randomized Prospective Clinical, Histomorphometric, and Micro-Computed Tomography Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
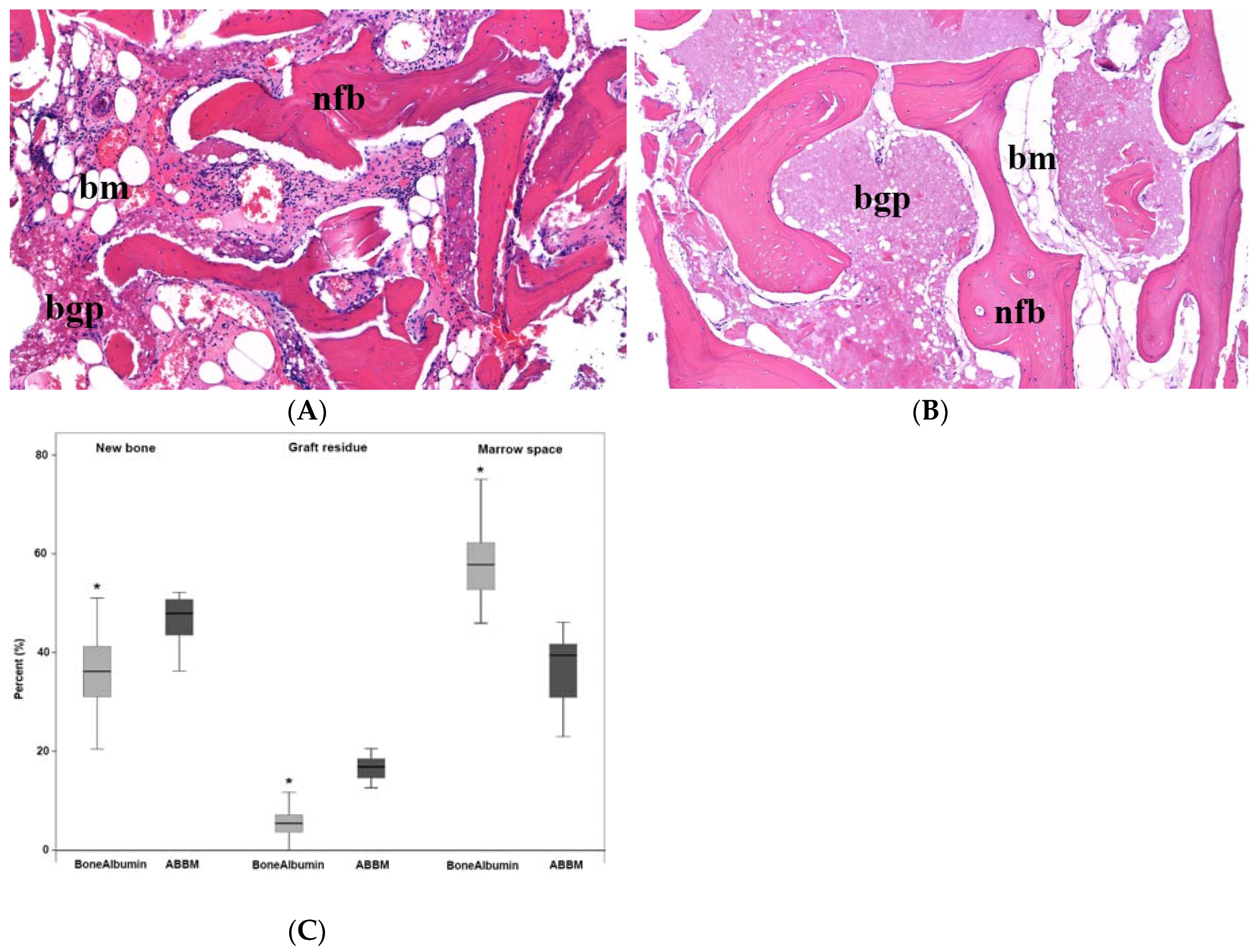
3.1. Histology and Histomorphometry
3.2. μCT Results
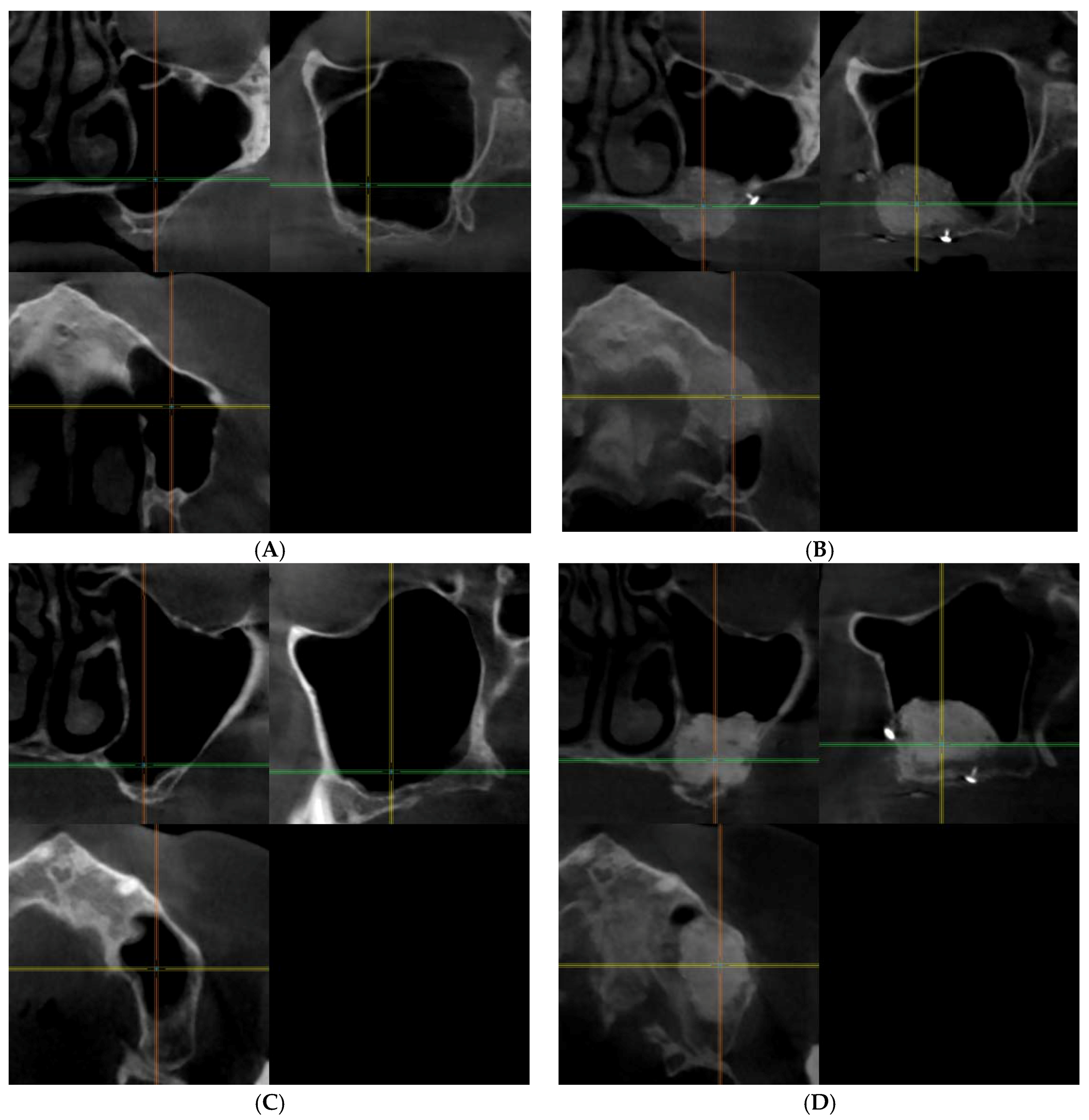
3.2.1. Qualitative Analysis of the μCT Images
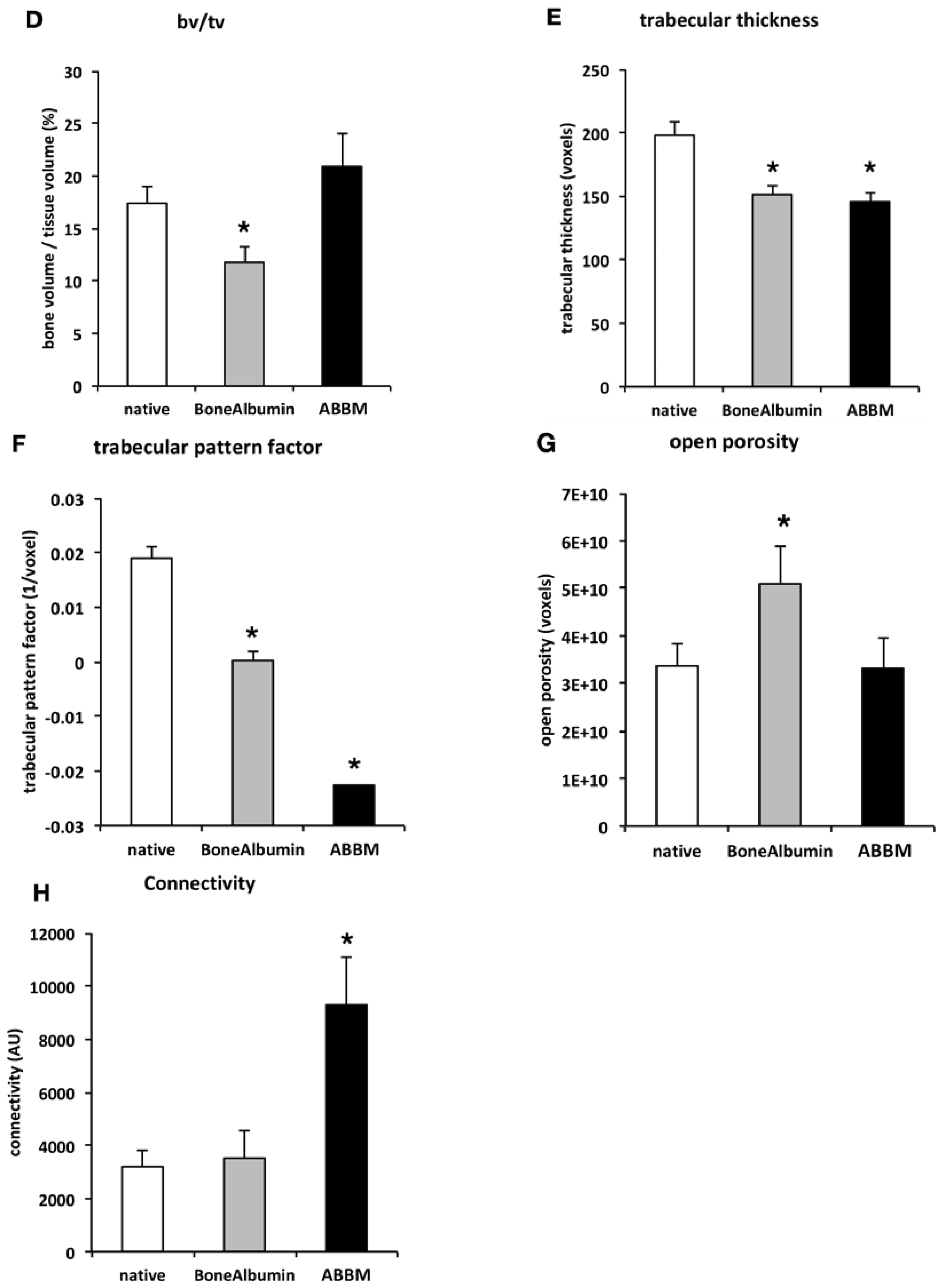
3.2.2. Quantitative Analysis of the μCT Images, Bone Microarchitecture Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baldini, N.; De Sanctis, M.; Ferrari, M. Deproteinized bovine bone in periodontal and implant surgery. Dent. Mater. 2011, 27, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Mardas, N.; Mezzomo, L.A.; Needleman, I.G.; Donos, N. Alveolar ridge preservation. A systematic review. Clin. Oral Investig. 2013, 17, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K. A study of the dimensional changes occurring in the maxilla following tooth extraction. Aust. Dent. J. 1969, 14, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Kivovics, M.; Szabo, B.T.; Nemeth, O.; Tari, N.; Dori, F.; Nagy, P.; Dobo-Nagy, C.; Szabo, G. Microarchitectural study of the augmented bone following ridge preservation with a porcine xenograft and a collagen membrane: Preliminary report of a prospective clinical, histological, and micro-computed tomography analysis. Int. J. Oral Maxillofac. Surg. 2017, 46, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.G.; Sukekava, F.; Wennstrom, J.L.; Lindhe, J. Ridge alterations following implant placement in fresh extraction sockets: An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar] [CrossRef]
- Lana, J.P.; Carneiro, P.M.; Machado Vde, C.; de Souza, P.E.; Manzi, F.R.; Horta, M.C. Anatomic variations and lesions of the maxillary sinus detected in cone beam computed tomography for dental implants. Clin. Oral Implant. Res. 2012, 23, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Schuh, E.; Schmiedl, R.; Vogel, G. Anatomic limits of endosseous dental implantation. Z. Stomatol. 1984, 81, 81–90. [Google Scholar] [PubMed]
- Tatum, O.H., Jr.; Lebowitz, M.S.; Tatum, C.A.; Borgner, R.A. Sinus augmentation. Rationale, development, long-term results. N. Y. State Dent. J. 1993, 59, 43–48. [Google Scholar] [PubMed]
- Maridati, P.; Stoffella, E.; Speroni, S.; Cicciu, M.; Maiorana, C. Alveolar antral artery isolation during sinus lift procedure with the double window technique. Open Dent. J. 2014, 8, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Beretta, M.; Cicciu, M.; Bramanti, E.; Maiorana, C. Schneider membrane elevation in presence of sinus septa: Anatomic features and surgical management. Int. J. Dent. 2012, 2012, 261905. [Google Scholar] [CrossRef] [PubMed]
- Rancitelli, D.; Borgonovo, A.E.; Cicciu, M.; Re, D.; Rizza, F.; Frigo, A.C.; Maiorana, C. Maxillary sinus septa and anatomic correlation with the schneiderian membrane. J. Craniofac. Surg. 2015, 26, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Belouka, S.M.; Strietzel, F.P. Sinus floor elevation and augmentation using synthetic nanocrystalline and nanoporous hydroxyapatite bone substitute materials: Preliminary histologic results. Int. J. Oral Maxillofac. Implant. 2016, 31, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Bornstein, M.M.; Carrel, J.P.; Buser, D.; Bernard, J.P. Maxillary sinus grafting with a synthetic, nanocrystalline hydroxyapatite-silica gel in humans: Histologic and histomorphometric results. Int. J. Periodontics Restor. Dent. 2014, 34, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Boyne, P.J.; James, R.A. Grafting of the maxillary sinus floor with autogenous marrow and bone. J. Oral Surg. 1980, 38, 613–616. [Google Scholar] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (prf): A second-generation platelet concentrate. Part V: Histologic evaluations of prf effects on bone allograft maturation in sinus lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Frenken, J.W.; Bouwman, W.F.; Bravenboer, N.; Zijderveld, S.A.; Schulten, E.A.; ten Bruggenkate, C.M. The use of straumann bone ceramic in a maxillary sinus floor elevation procedure: A clinical, radiological, histological and histomorphometric evaluation with a 6-month healing period. Clin. Oral Implant. Res. 2010, 21, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Jeong, T.M.; Lee, J.K. The efficacy of the graft materials after sinus elevation: Retrospective comparative study using panoramic radiography. Plast. Reconstr. Surg. 2014, 36, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Kubler, N.R.; Will, C.; Depprich, R.; Betz, T.; Reinhart, E.; Bill, J.S.; Reuther, J.F. Comparative studies of sinus floor elevation with autologous or allogeneic bone tissue. Mund Kiefer Gesichtschir. 1999, 3 (Suppl. S1), S53–S60. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Fernandez, M.P.; Calvo-Guirado, J.L.; Mate-Sanchez Del Val, J.E.; Delgado-Ruiz, R.A.; Negri, B.; Barona-Dorado, C. Ultrastructural study by backscattered electron imaging and elemental microanalysis of bone-to-biomaterial interface and mineral degradation of porcine xenografts used in maxillary sinus floor elevation. Clin. Oral Implant. Res. 2013, 24, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Rickert, D.; Slater, J.J.; Meijer, H.J.; Vissink, A.; Raghoebar, G.M. Maxillary sinus lift with solely autogenous bone compared to a combination of autogenous bone and growth factors or (solely) bone substitutes. A systematic review. Int. J. Oral Maxillofac. Surg. 2012, 41, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Degidi, M.; Iezzi, G.; Pecora, G.; Piattelli, M.; Orsini, G.; Caputi, S.; Perrotti, V.; Mangano, C.; Piattelli, A. Maxillary sinus augmentation with different biomaterials: A comparative histologic and histomorphometric study in man. Implant Dent. 2006, 15, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Tadjoedin, E.S.; de Lange, G.L.; Lyaruu, D.M.; Kuiper, L.; Burger, E.H. High concentrations of bioactive glass material (biogran) vs. Autogenous bone for sinus floor elevation. Clin. Oral Implant. Res. 2002, 13, 428–436. [Google Scholar] [CrossRef]
- Taschieri, S.; Corbella, S.; Weinstein, R.; Di Giancamillo, A.; Mortellaro, C.; Del Fabbro, M. Maxillary sinus floor elevation using platelet-rich plasma combined with either biphasic calcium phosphate or deproteinized bovine bone. J. Craniofac. Surg. 2016, 27, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Ricci, M.; Covani, U.; Nannmark, U.; Azarmehr, I.; Calvo-Guirado, J.L. Maxillary sinus augmentation using prehydrated corticocancellous porcine bone: Hystomorphometric evaluation after 6 months. Clin. Implant Dent. Relat. Res. 2012, 14, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Huys, L.; Coulthard, P.; Maiorana, C.; Garagiola, U.; Barabas, J.; Nemeth, Z.; Hrabak, K.; Suba, Z. A prospective multicenter randomized clinical trial of autogenous bone versus beta-tricalcium phosphate graft alone for bilateral sinus elevation: Histologic and histomorphometric evaluation. Int. J. Oral Maxillofac. Implant. 2005, 20, 371–381. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Tan, W.C.; Zwahlen, M.; Lang, N.P. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J. Clin. Periodontol. 2008, 35, 216–240. [Google Scholar] [CrossRef] [PubMed]
- Handschel, J.; Simonowska, M.; Naujoks, C.; Depprich, R.A.; Ommerborn, M.A.; Meyer, U.; Kubler, N.R. A histomorphometric meta-analysis of sinus elevation with various grafting materials. Head Face Med. 2009, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Horvathy, D.B.; Vacz, G.; Cselenyak, A.; Weszl, M.; Kiss, L.; Lacza, Z. Albumin-coated bioactive suture for cell transplantation. Surg. Innov. 2013, 20, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Horvathy, D.B.; Vacz, G.; Szabo, T.; Szigyarto, I.C.; Toro, I.; Vamos, B.; Hornyak, I.; Renner, K.; Klara, T.; Szabo, B.T.; et al. Serum albumin coating of demineralized bone matrix results in stronger new bone formation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Horvathy, D.B.; Vacz, G.; Toro, I.; Szabo, T.; May, Z.; Duarte, M.; Hornyak, I.; Szabo, B.T.; Dobo-Nagy, C.; Doros, A.; et al. Remineralization of demineralized bone matrix in critical size cranial defects in rats: A 6-month follow-up study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Klara, T.; Csonge, L.; Janositz, G.; Csernatony, Z.; Lacza, Z. Albumin-coated structural lyophilized bone allografts: A clinical report of 10 cases. Cell Tissue Bank. 2014, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Klara, T.; Csonge, L.; Janositz, G.; Pap, K.; Lacza, Z. The use of structural proximal tibial allografts coated with human albumin in treating extensive periprosthetic knee-joint bone deficiency and averting late complications. Case report. Orv. Hetil. 2015, 156, 67–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schandl, K.; Horvathy, D.B.; Doros, A.; Majzik, E.; Schwarz, C.M.; Csonge, L.; Abkarovits, G.; Bucsi, L.; Lacza, Z. Bone-albumin filling decreases donor site morbidity and enhances bone formation after anterior cruciate ligament reconstruction with bone-patellar tendon-bone autografts. Int. Orthop. 2016, 40, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Skaliczki, G.; Schandl, K.; Weszl, M.; Major, T.; Kovacs, M.; Skaliczki, J.; Szendroi, M.; Dobo-Nagy, C.; Lacza, Z. Serum albumin enhances bone healing in a nonunion femoral defect model in rats: A computer tomography micromorphometry study. Int. Orthop. 2013, 37, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Weszl, M.; Skaliczki, G.; Cselenyak, A.; Kiss, L.; Major, T.; Schandl, K.; Bognar, E.; Stadler, G.; Peterbauer, A.; Csonge, L.; et al. Freeze-dried human serum albumin improves the adherence and proliferation of mesenchymal stem cells on mineralized human bone allografts. J. Orthop. Res. 2012, 30, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Brkovic, B.M.; Prasad, H.S.; Rohrer, M.D.; Konandreas, G.; Agrogiannis, G.; Antunovic, D.; Sandor, G.K. Beta-tricalcium phosphate/type i collagen cones with or without a barrier membrane in human extraction socket healing: Clinical, histologic, histomorphometric, and immunohistochemical evaluation. Clin. Oral Investig. 2012, 16, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Egan, K.P.; Brennan, T.A.; Pignolo, R.J. Bone histomorphometry using free and commonly available software. Histopathology 2012, 61, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.J.; Boyce, R.W.; Nyengaard, J.R.; Odgaard, A. The conneulor: Unbiased estimation of connectivity using physical disectors under projection. Bone 1993, 14, 217–222. [Google Scholar] [CrossRef]
- Caubet, J.; Ramis, J.M.; Ramos-Murguialday, M.; Morey, M.A.; Monjo, M. Gene expression and morphometric parameters of human bone biopsies after maxillary sinus floor elevation with autologous bone combined with bio-oss or boneceramic. Clin. Oral Implant. Res. 2015, 26, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Chackartchi, T.; Iezzi, G.; Goldstein, M.; Klinger, A.; Soskolne, A.; Piattelli, A.; Shapira, L. Sinus floor augmentation using large (1–2 mm) or small (0.25–1 mm) bovine bone mineral particles: A prospective, intra-individual controlled clinical, micro-computerized tomography and histomorphometric study. Clin. Oral Implant. Res. 2011, 22, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Chappard, D.; Guillaume, B.; Mallet, R.; Pascaretti-Grizon, F.; Basle, M.F.; Libouban, H. Sinus lift augmentation and beta-tcp: A microct and histologic analysis on human bone biopsies. Micron 2010, 41, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chopra, P.M.; Johnson, M.; Nagy, T.R.; Lemons, J.E. Micro-computed tomographic analysis of bone healing subsequent to graft placement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 611–618. [Google Scholar] [CrossRef] [PubMed]
- De Lange, G.L.; Overman, J.R.; Farre-Guasch, E.; Korstjens, C.M.; Hartman, B.; Langenbach, G.E.; Van Duin, M.A.; Klein-Nulend, J. A histomorphometric and micro-computed tomography study of bone regeneration in the maxillary sinus comparing biphasic calcium phosphate and deproteinized cancellous bovine bone in a human split-mouth model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2014, 117, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Hsu, J.T.; Chen, M.Y.; Liu, C.; Chang, C.H.; Li, Y.F.; Chen, K.T. Microcomputed tomography analysis of particular autogenous bone graft in sinus augmentation at 5 months: Differences on bone mineral density and 3d trabecular structure. Clin. Oral Investig. 2013, 17, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, S.; Brochhausen, C.; Gotz, H.; Filippi, A.; Payer, M.; d’Hoedt, B.; Kreisler, M. The influence of bone substitute materials on the bone volume after maxillary sinus augmentation: A microcomputerized tomography study. Clin. Oral Investig. 2013, 17, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Rebaudi, A.; Maltono, A.A.; Pretto, M.; Benedicenti, S. Sinus grafting with magnesium-enriched bioceramic granules and autogenous bone: A microcomputed tomographic evaluation of 11 patients. Int. J. Periodontics Restor. Dent. 2010, 30, 53–61. [Google Scholar] [CrossRef]
- Vandeweghe, S.; Leconte, C.; Ono, D.; Coelho, P.G.; Jimbo, R. Comparison of histological and three-dimensional characteristics of porous titanium granules and deproteinized bovine particulate grafts used for sinus floor augmentation in humans: A pilot study. Implant Dent. 2013, 22, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Verket, A.; Lyngstadaas, S.P.; Rasmusson, L.; Haanaes, H.R.; Wallstrom, M.; Wall, G.; Wohlfahrt, J.C. Maxillary sinus augmentation with porous titanium granules: A microcomputed tomography and histologic evaluation of human biopsy specimens. Int. J. Oral Maxillofac. Implant. 2013, 28, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhou, W.; Monje, A.; Huang, W.; Wang, Y.; Wu, Y. Influence of healing period upon bone turn over on maxillary sinus floor augmentation grafted solely with deproteinized bovine bone mineral: A prospective human histological and clinical trial. Clin. Implant Dent. Relat. Res. 2017, 19, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Kamitakahara, M.; Ohtsuki, C.; Miyazaki, T. Review paper: Behavior of ceramic biomaterials derived from tricalcium phosphate in physiological condition. J. Biomater. Appl. 2008, 23, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Mordenfeld, A.; Hallman, M.; Johansson, C.B.; Albrektsson, T. Histological and histomorphometrical analyses of biopsies harvested 11 years after maxillary sinus floor augmentation with deproteinized bovine and autogenous bone. Clin. Oral Implant. Res. 2010, 21, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.; Silvestri, M.; Forni, F.; Icaro Cornaglia, A.; Tesei, P.; Cattaneo, V. Ten-year follow-up in a maxillary sinus augmentation using anorganic bovine bone (bio-oss). A case report with histomorphometric evaluation. Clin. Oral Implant. Res. 2003, 14, 369–372. [Google Scholar] [CrossRef]
- Schlegel, A.K.; Donath, K. Bio-oss—A resorbable bone substitute? J. Long Term Eff. Med. Implant. 1998, 8, 201–209. [Google Scholar]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.D.; Smeets, R. Current trends and future perspectives of bone substitute materials—From space holders to innovative biomaterials. J. Craniomaxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Egusa, H. Current bone substitutes for implant dentistry. J. Prosthodont. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Perrotti, V.; Piattelli, A.; Iezzi, G. Eight-year results of site retention of anorganic bovine bone and anorganic bovine matrix. J. Oral Implantol. 2013, 39, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Hentunen, T.; Salonen, J.; Nagai, A.; Yamashita, K. Characterization of bone mineral-resembling biomaterials for optimizing human osteoclast differentiation and resorption. J. Biomed. Mater. Res. A 2013, 101, 3141–3151. [Google Scholar] [CrossRef] [PubMed]

| Abbreviation | Variable | Description | Standard Unit |
|---|---|---|---|
| BV/TV | Bone volume fraction | Relative volume of calcified tissue in the selected volume of interest (VOI). | % |
| Tb.Th | Trabecular thickness | Mean thickness of trabeculae, assessed using direct 3D methods. | mm |
| Tb.Pf | Trabecular bone pattern factor | This is an index of connectivity of trabecular bone; it calculates an index of relative convexity or concavity of the total bone surface, on the principle that concavity indicates connectivity (and the presence of “nodes”), and convexity indicates isolated disconnected structures (struts). | 1/mm |
| Po(op) | Open porosity (percent) | Percent open porosity is the volume of open pores as a percent of the total VOI volume. | % |
| Conn. | Connectivity | One useful and fast algorithm for calculating the Euler connectivity in 3D is the “Conneulor”. It measures what might be called “redundant connectivity”, the degree to which parts of the object are multiply connected. It is a measure of how many connections in a structure can be severed before the structure falls into two separate pieces. | none |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márton, K.; Tamás, S.B.; Orsolya, N.; Béla, C.; Ferenc, D.; Péter, N.; Csaba, D.-N.; Lajos, C.; Zsombor, L.; Eitan, M.; et al. Microarchitecture of the Augmented Bone Following Sinus Elevation with an Albumin Impregnated Demineralized Freeze-Dried Bone Allograft (BoneAlbumin) versus Anorganic Bovine Bone Mineral: A Randomized Prospective Clinical, Histomorphometric, and Micro-Computed Tomography Study. Materials 2018, 11, 202. https://doi.org/10.3390/ma11020202
Márton K, Tamás SB, Orsolya N, Béla C, Ferenc D, Péter N, Csaba D-N, Lajos C, Zsombor L, Eitan M, et al. Microarchitecture of the Augmented Bone Following Sinus Elevation with an Albumin Impregnated Demineralized Freeze-Dried Bone Allograft (BoneAlbumin) versus Anorganic Bovine Bone Mineral: A Randomized Prospective Clinical, Histomorphometric, and Micro-Computed Tomography Study. Materials. 2018; 11(2):202. https://doi.org/10.3390/ma11020202
Chicago/Turabian StyleMárton, Kivovics, Szabó Bence Tamás, Németh Orsolya, Czinkóczky Béla, Dőri Ferenc, Nagy Péter, Dobó-Nagy Csaba, Csönge Lajos, Lacza Zsombor, Mijiritsky Eitan, and et al. 2018. "Microarchitecture of the Augmented Bone Following Sinus Elevation with an Albumin Impregnated Demineralized Freeze-Dried Bone Allograft (BoneAlbumin) versus Anorganic Bovine Bone Mineral: A Randomized Prospective Clinical, Histomorphometric, and Micro-Computed Tomography Study" Materials 11, no. 2: 202. https://doi.org/10.3390/ma11020202






