Electrochemical Behaviour and Galvanic Effects of Titanium Implants Coupled to Metallic Suprastructures in Artificial Saliva
Abstract
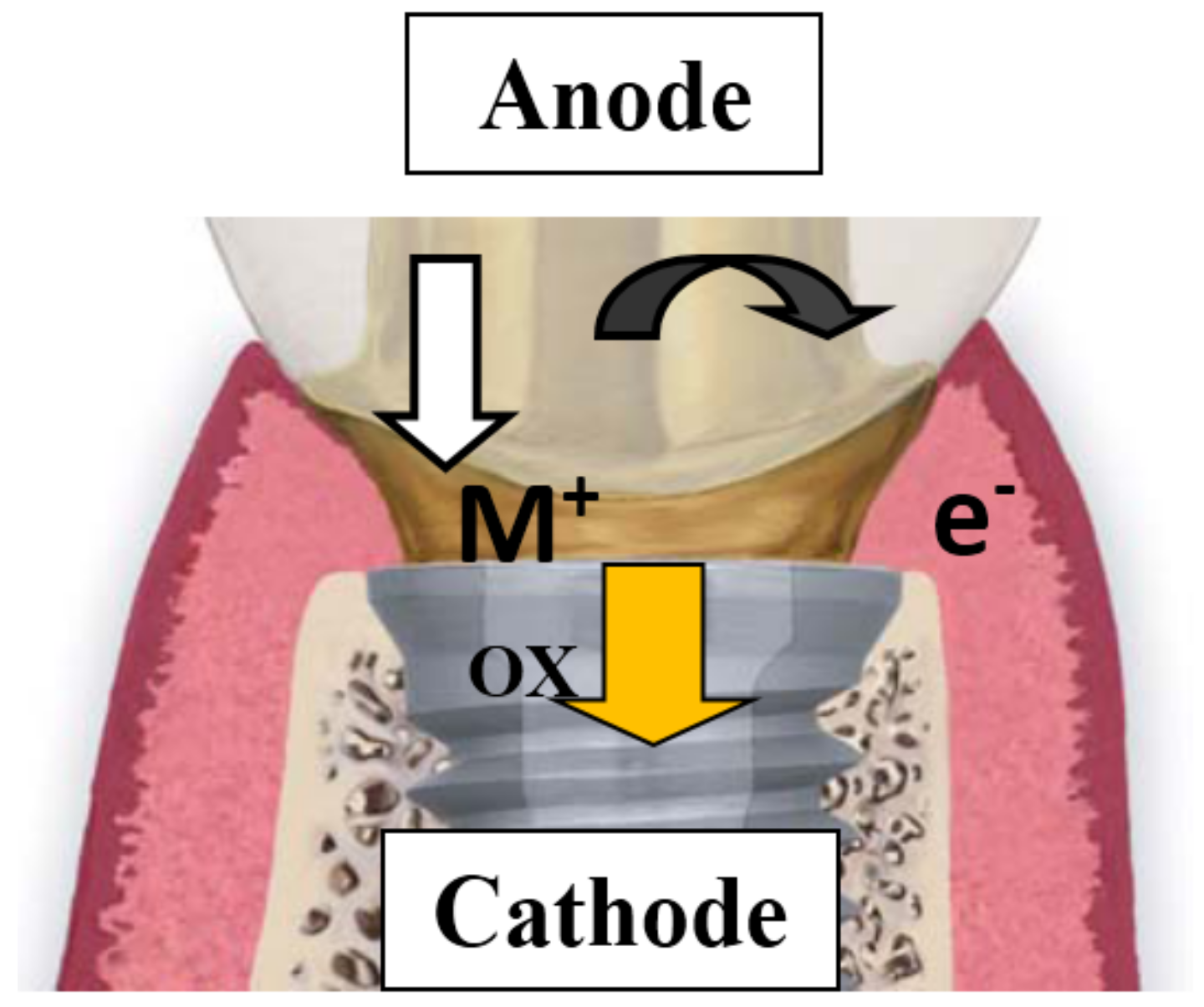
:1. Introduction
- ▪
- Passive dissolution is the main corrosion mechanism of titanium and CoCr alloys and the corresponding passive dissolution rate was not accelerated by the presence of fluorides. NiCrTi alloy is the less corrosion resistant alloy among the studied materials in artificial saliva and fluorides critically accelerate its corrosion rate due to the susceptibility of nickel towards fluorides.
- ▪
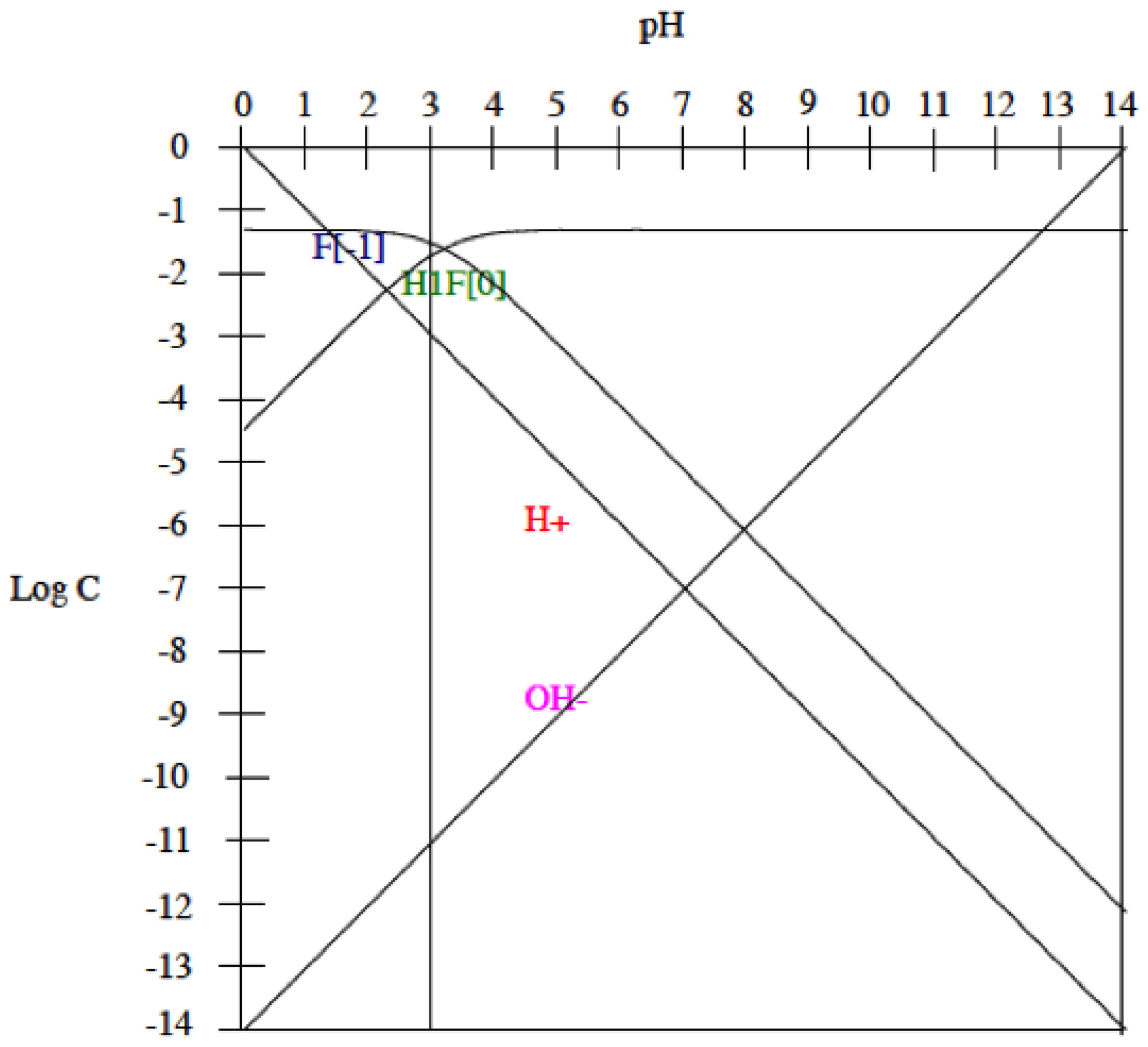
- Titanium alloys starts actively dissolving when the pH of the artificial saliva is below 3 and the fluoride content is 1000 ppm. Under these conditions, HF concentration is sufficiently high to form soluble titanium complexes in all studied titanium alloys.
- ▪
- Measurement of galvanic corrosion of TiG2 implant coupled to different materials can only be carried out by zero-resistance ammeter and the direct measurement of the galvanic current and potential due to the passive nature of the biomedical alloys (CoCr, NiTiCr and Ti6Al4V).
- ▪
- Acceleration of corrosion due to galvanic effects was only observed between titanium alloys and CoCr suprastructures in fluoride-containing acidic solutions. This galvanic effect is highly dependent on the solution chemistry and the coupled material, increasing when the suprastructure is Ti6Al4V.
2. Results
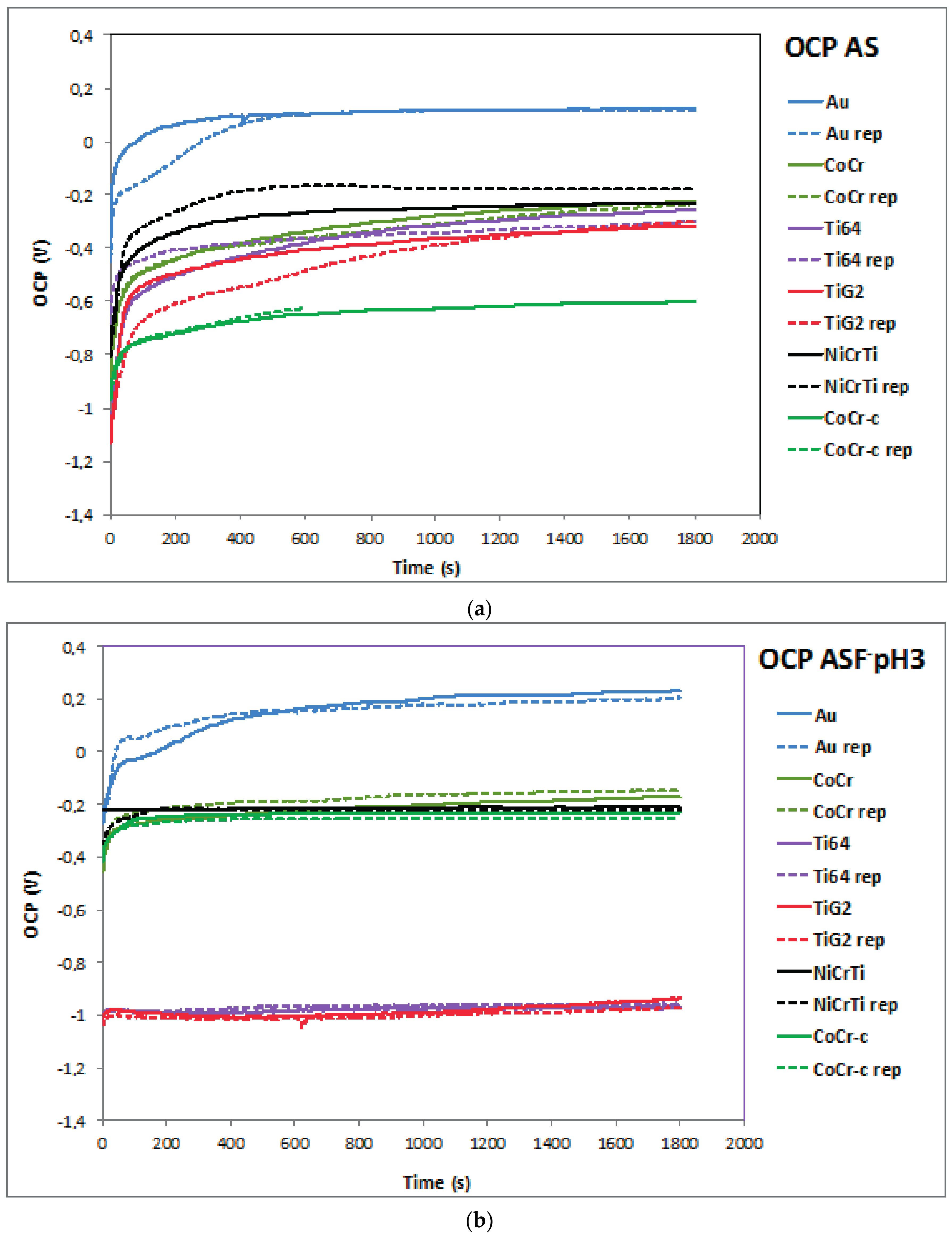
2.1. Open Circuit Potential (OCP)
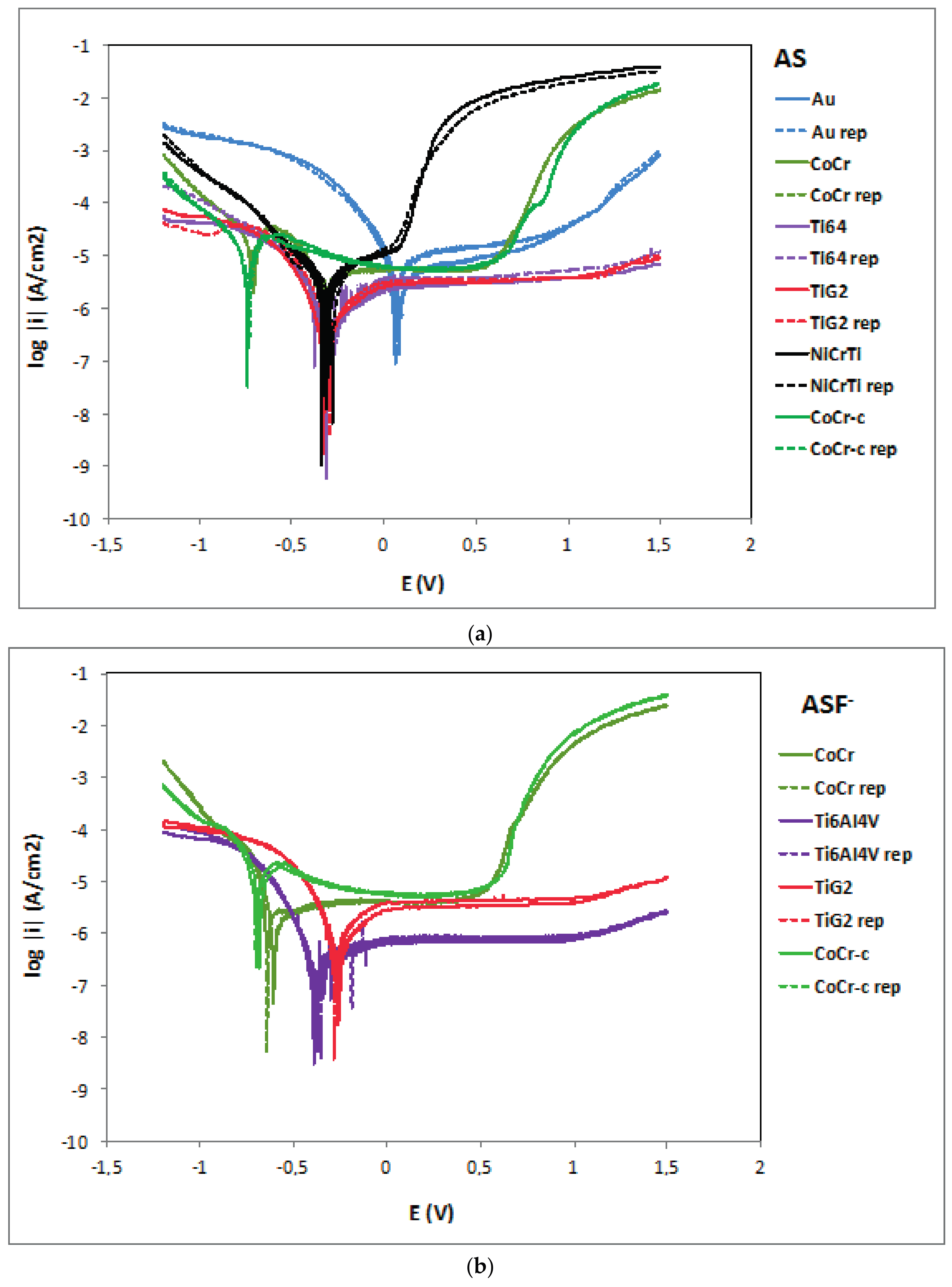
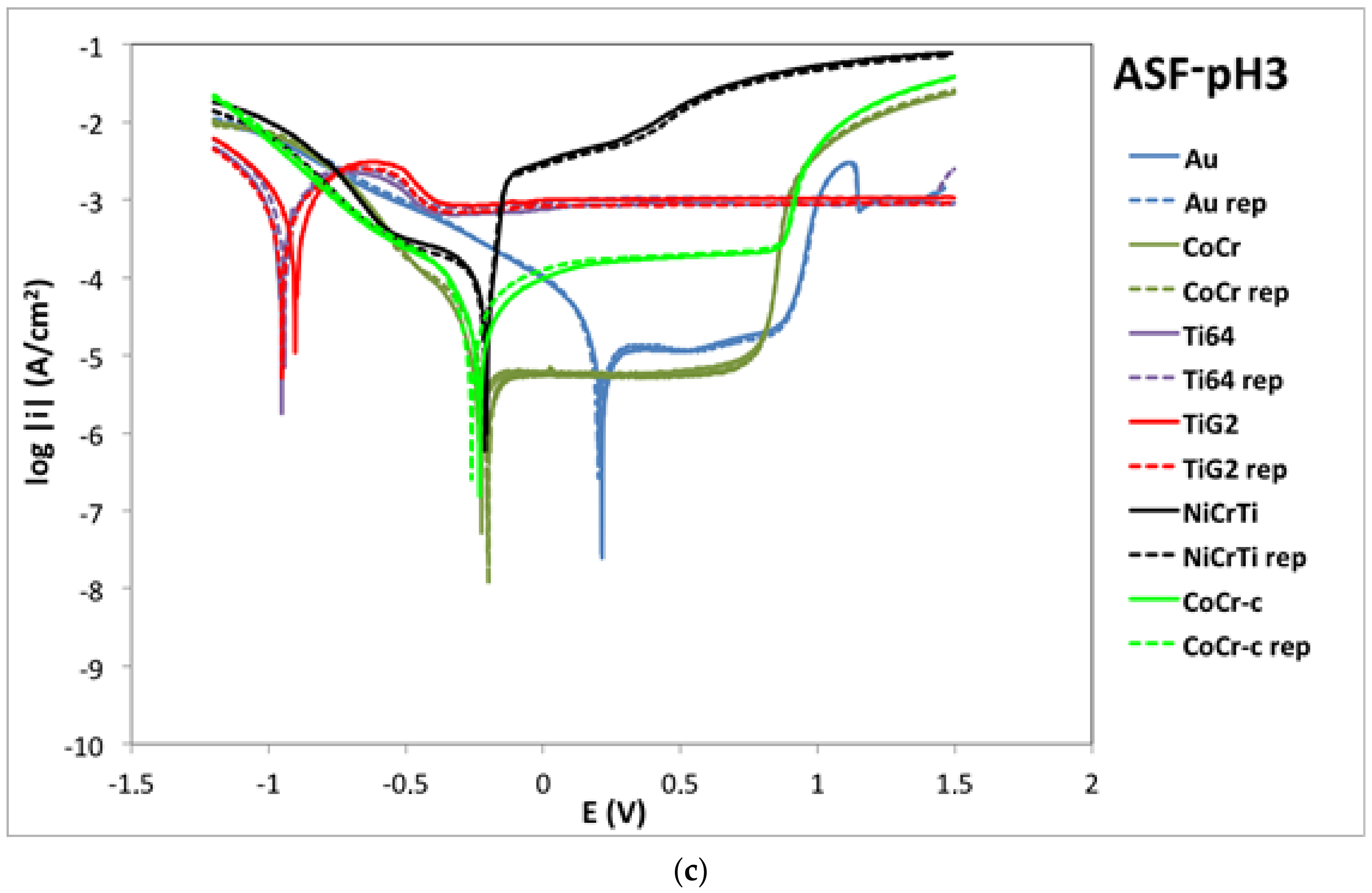
2.2. Potentiodynamic Curves
2.3. Zero Resistance Ammeter
3. Discussion
3.1. Corrosion Mechanisms
3.2. Influence of Fluoride Presence on the Corrosion Behaviour
3.3. Galvanic Corrosion
4. Materials and Methods
4.1. Electrolytes and Materials
4.2. Electrochemical Equipment and Experiments
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Venugopalan, R.; Lucas, L.C. Evaluation of restorative and implant alloys galvanically coupled to titanium. Dent. Mater. 1998, 14, 165–172. [Google Scholar] [CrossRef]
- Geis, G.J.; Weber, J.G.; Sauer, K.H. In vitro substance loss due to galvanic corrosión in titanium implant/Ni-Cr supraconstruction systems. Int. J. Oral Maxillofac. Implants 1989, 4, 119–123. [Google Scholar]
- Lucas, L.C.; Lemons, J.E. Biodegradation of restorative metallic systems. Adv. Dent. Res. 1992, 6, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, D.; Fernandez, M.M.; Guglielmotti, M.B.; Cabrini, R.L. Macrophages related to dental implant failure. Implant Dent. 2003, 12, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Guglielmotti, M.B.; Cabrini, R.L. Evaluación biológica de implantes dentales fracasados. Rev. Asoc. Odontol. Argent. 1997, 85, 313–317. [Google Scholar]
- Hallab, N.; Merrit, K.; Jacobs, J. Metal sensitivity in patients with orthopaedic implants. J. Bone Jt. Surg. 2001, 83, 428–436. [Google Scholar] [CrossRef]
- Schmalz, G.; Garhammer, P. Biological interactions of dental cast alloys with oral tissues. Dent. Mater. 2002, 18, 396–406. [Google Scholar] [CrossRef]
- Balkin, B.E. Implant dentistry: Historical overview with current perspective. Int. J. Oral Implantol. 1988, 5, 27–28. [Google Scholar] [PubMed]
- Al-Mayouf, A.M.; Al-Swayih, A.A.; Al-Mobarak, N.A.; Al-Jabab, A.S. Corrosion behavior of a new titanium alloy for dental implant applications in fluoride media. Mater. Chem. Phys. 2004, 86, 320–329. [Google Scholar] [CrossRef]
- Reclaru, L.; Meyer, J.M. Study of corrosion between a titanium implant and dental alloys. J. Dent. 1994, 22, 159–168. [Google Scholar] [CrossRef]
- Nakagawa, M.; Matsuya, S.; Shiraishi, T.; Ohta, M. Effect of fluoride concentration and pH on corrosion behavior of titanium for dental use. J. Dent. Res. 1999, 78, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- ToumelinChemla, F.; Rouelle, F.; Burdairon, G. Corrosive properties of fluoride-containing odontologic gels against titanium. J. Dent. 1996, 24, 109–115. [Google Scholar] [CrossRef]
- Lemons, J.E.; Lucas, L.C.; Johansson, B. Intraoral corrosion resulting from coupling dental implants and restorative metallic systems. Implant Dent. 1992, 1, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, S.; Oshida, Y.; Andres, C.J.; Barco, M.T.; Brown, D.T.; Hovijitra, S.; Hito, M.; Nagasawa, S.; Yoshida, T. Effects of coupling methods on galvanic corrosion behavior of commercially pure titanium with dental precious alloys. Biomed. Mater. Eng. 2005, 15, 307–316. [Google Scholar] [PubMed]
- Ciszewski, A.; Baraniak, M.; Urbanek-Brychczyńska, M. Corrosion by galvanic coupling between amalgam and different chromium-based alloys. Dent. Mater. 2007, 23, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Grosgogeat, B.; Reclaru, L.; Lissac, M.; Dalard, F. Measurement and evaluation of galvanic corrosion between titanium/Ti6Al4V implants and dental alloys by electrochemical techniques and auger spectrometry. Biomaterials 1999, 20, 933–941. [Google Scholar] [CrossRef]
- Horasawa, N.; Takahashi, S.; Marek, M. Galvanic interaction between titanium and gallium alloy or dental amalgam. Dent. Mater. 1999, 15, 318–322. [Google Scholar] [CrossRef]
- Oh, K.-T.; Kim, K.-N. Electrochemical properties of supraestructures galvanically coupled to a titanium implant. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 70, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Landolt, D. Corrosion reaction rates. In Corrosion and Surface Chemistry of Metals, 1st ed.; Taylor and Francis Group, LLC CRC Press: Boca Raton, FL, USA, 2007; pp. 119–178. [Google Scholar]
- Taher, N.M.; Al Jabab, A.S. Galvanic corrosion behavior of implant suprastructure dental alloys. Dent. Mater. 2003, 19, 54–59. [Google Scholar] [CrossRef]
- Cortada, M.; Giner, L.L.; Costa, S.; Gil, F.J.; Rodríguez, D.; Planell, J.A. Galvanic corrosion behaviour of titanium implants coupled to dental alloys. J. Mater. Sci. Mater. Med. 2000, 11, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Kim, H.J.; Jeong, Y.T.; Son, M.K.; Jeong, Y.H.; Choe, H.C. Electrochemical behavior of dental implant system before and after clinical use. Trans. Nonferrous Met. Soc. China 2009, 19, 846–851. [Google Scholar] [CrossRef]
- Yamazoe, M. Study of corrosion of combinations of titanium/Ti-6Al-4V implants and dental alloys. Dent. Mater. J. 2010, 29, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Foti, B.; Tavitian, P.; Tosello, A.; Bonfil, J.J.; Franquin, J.C. Polymetallism and osseointegration in oral implantology: A pilot study on primate. J. Oral Rehabil. 1999, 26, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Sutow, E.J.; Wayne, W.A.; Taylor, J.C.; Hall, G.C. In vivo galvanic currents of intermittently contacting dental amalgam and other metallic restorations. Dent. Mater. 2004, 20, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C. Alloys for prosthodontic restorations. J. Prosthet. Dent. 2002, 87, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Manaranche, C.; Hornberger, H. A proposal for the classification of dental alloys according to their resistance to corrosion. Dent. Mater. 2007, 23, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Tuna, S.H.; Pekmez, N.Ö.; Keyf, F.; Canli, F. The influence of the pure metal components of four different casting alloys on the electrochemical properties of the alloys. Dent. Mater. 2009, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Al-Hity, R.R.; Kappert, H.F.; Viennot, S.; Dalard, F.; Grosgogeat, B. Corrosion resistance measurements of dental alloys, are they correlated? Dent. Mater. 2007, 23, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Milosev, I.; Kosec, T.; Strehblow, H.H. XPS and EIS study of the passive film formed on orthopaedic Ti6Al7Nb alloy in Hank’s physiological solution. Electrochim. Acta 2008, 53, 3547–3558. [Google Scholar] [CrossRef]
- Milosev, I.; Strehblow, H.H. The composition of the surface passive film formed on CoCrMo alloy in simulated physiological solution. Electrochim. Acta 2003, 48, 2767–2774. [Google Scholar] [CrossRef]
- Hodgson, A.W.E.; Kurz, S.; Virtanen, S.; Fervel, V.; Olsson, C.O.A.; Mischler, S. Passive and transpassive behaviour of CoCrMo in simulated biological solutions. Electrochim. Acta 2004, 49, 2167–2178. [Google Scholar] [CrossRef]
- Huang, H.H.; Lee, T.H. Electrochemical impedance spectroscopy study of Ti6Al4V alloy in artificial saliva with fluoride and/or bovine albumin. Dent. Mater. 2005, 21, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Wylie, C.M.; Shelton, R.M.; Fleming, G.J.; Davenport, A.J. Corrosion of nickel-based dental casting alloys. Dent. Mater. 2007, 23, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Bizar, J. Efecto de las Variaciones Térmicas Durante la Fusión de los Colados en Prótesis Fija. Ph.D. Thesis, Facultad de Odontología, Universidad de Barcelona, Barcelona, Spain, 1999. [Google Scholar]
- Wataha, J.C. Biocompatibility of dental casting alloys: A review. J. Prosthet. Dent. 2000, 83, 223–234. [Google Scholar] [CrossRef]
- Schiff, N.; Grosgogeat, B.; Lissac, M.L.; Dalard, F. Influence of fluoridated mouthwashes on corrosion resistance of orthodontics wires. Biomaterials 2004, 25, 4535–4542. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Han, E.; Ke, W. Influence of fluoride and chloride on corrosion behavior of NiTi orthodontic wires. Acta Biomater. 2007, 3, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Wang, C.C.; Huang, T.K.; Chen, L.K.; Chou, M.Y.; Huang, H.H. Corrosion resistance of titanium-containing dental orthodontic wires in fluoride-containing artificial saliva. J. Alloys Compd. 2009, 488, 482–489. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Seol, H.J.; Kim, H.I.; Hwang, K.J.; Lee, S.G.; Kim, K.H. Effect of acidic fluoride solution on beta titanium alloy wire. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 73, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, S.; Hattori, M.; Yoshinari, M.; Kawada, E.; Asami, K.; Oda, Y. Corrosion mechanism of TiCr alloys in solution containing fluoride. Dent. Mater. 2009, 25, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, D.G.; Tasat, D.R.; Duffo, G.; Guglielmotti, M.B.; Cabrini, R.L. The issue of corrosion in dental implants: A review. Acta Odontol. Latinoam. 2009, 22, 3–9. [Google Scholar] [PubMed]
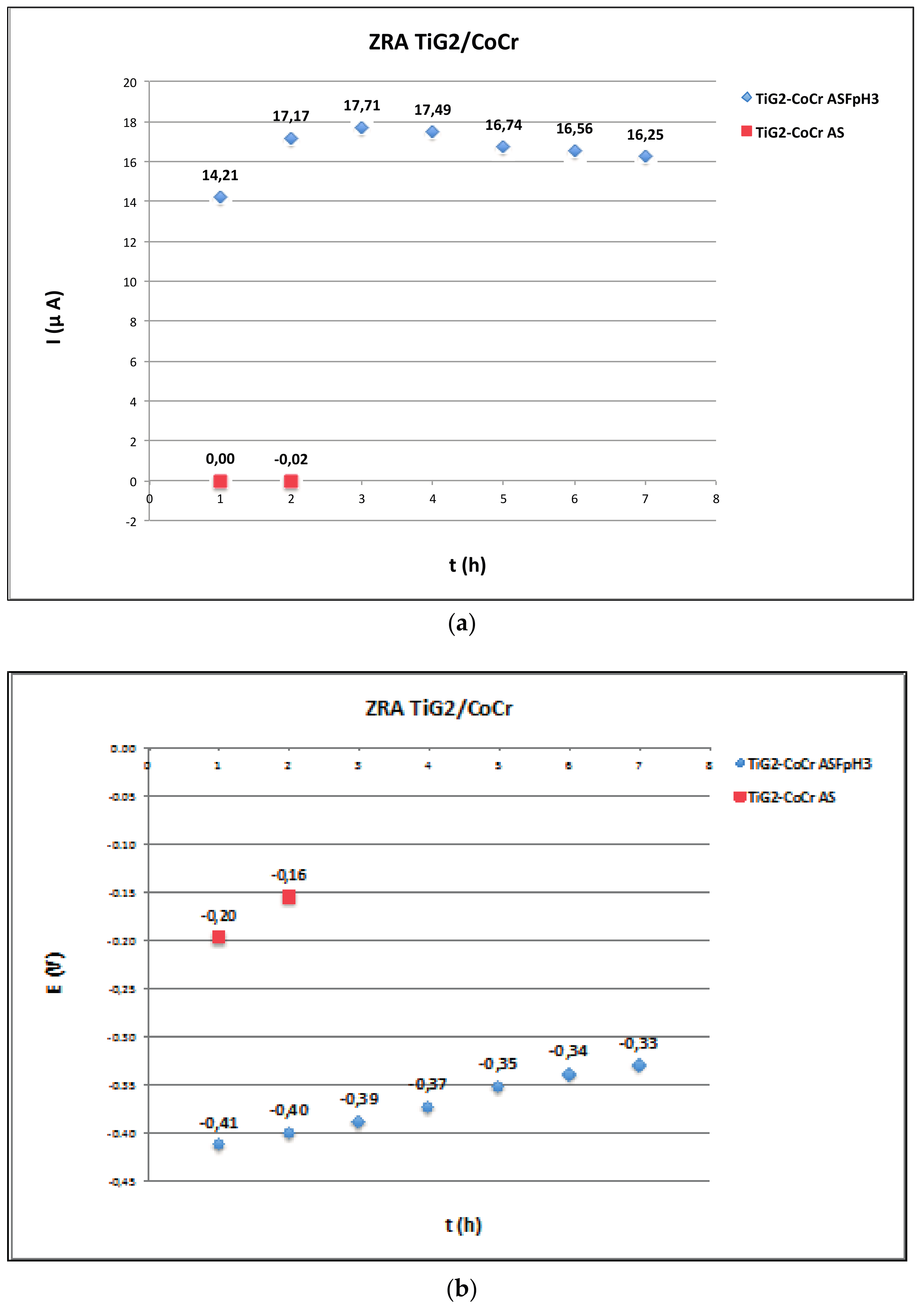
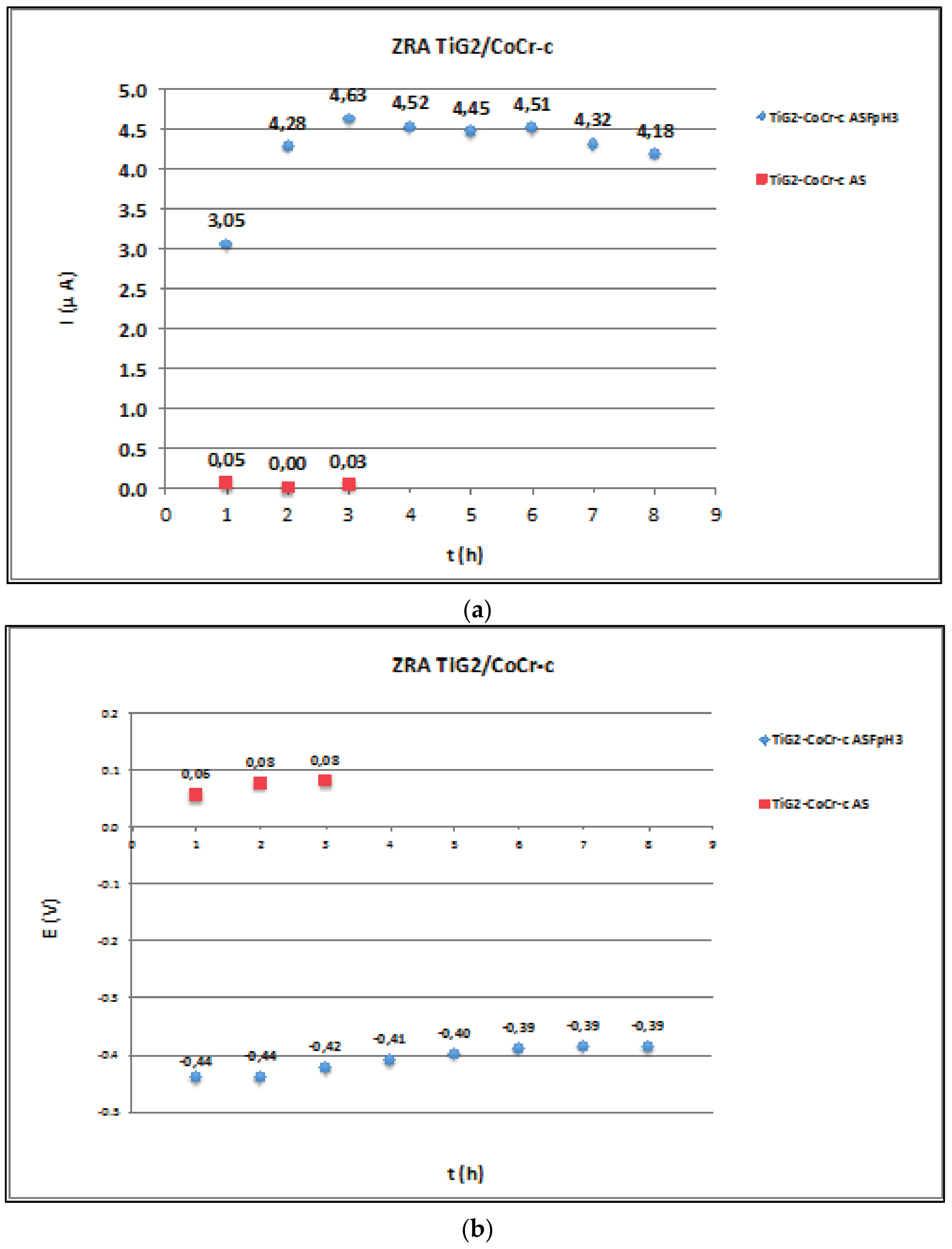
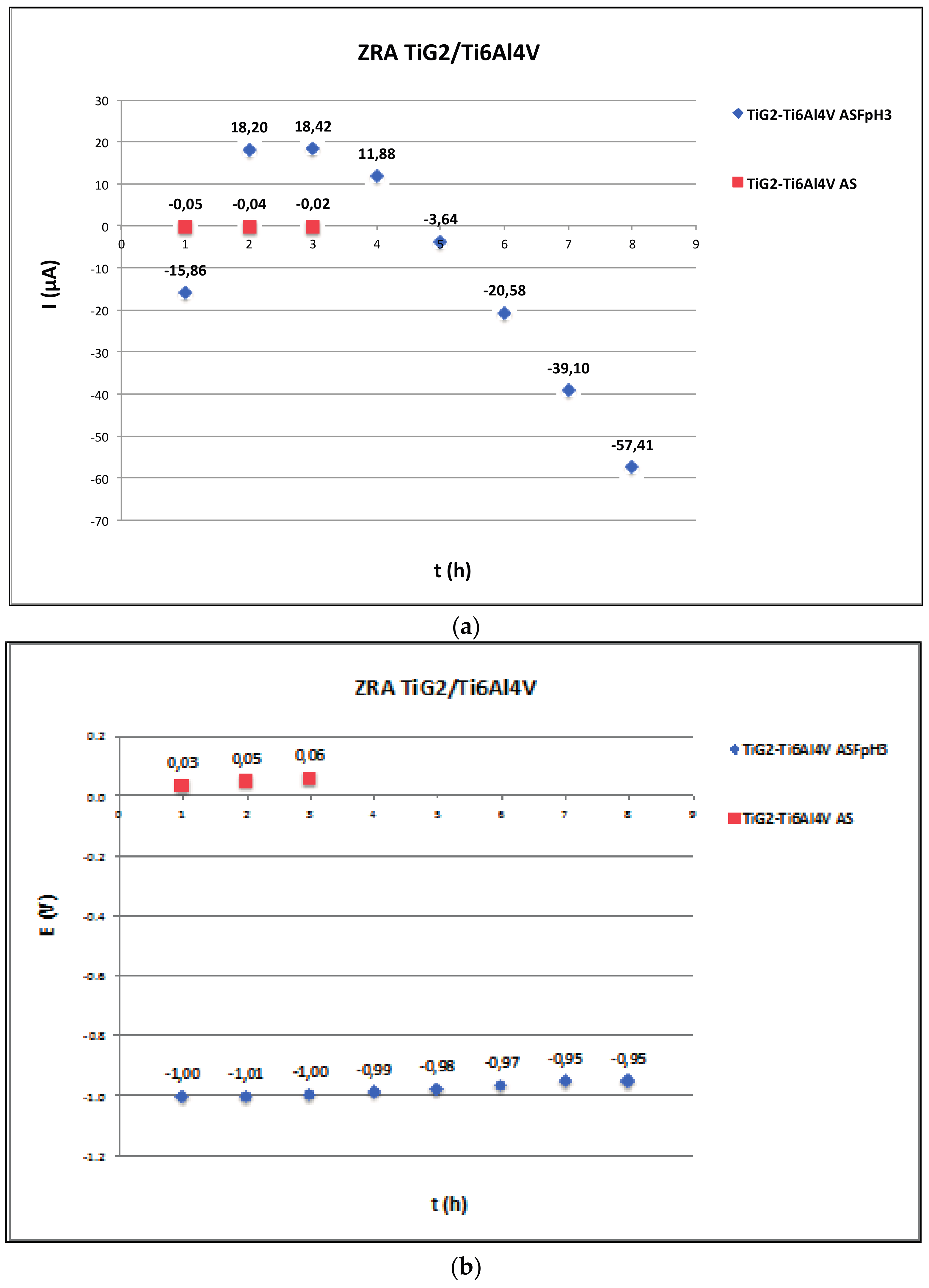
| Ref. | Materials Implant // Suprastructure | Solution pH, T (°C) | Electrochem. Technique | Anal. Tech | Studied Parameters |
|---|---|---|---|---|---|
| Geis, 1989 [2] | c.p. Ti // NiCrMo y NiCrMoBe | Asa1 1 2.3 | PD 2 | AAS 3 | Vcorr (µg/cm2/día) |
| Lemons, 1992 [13] | Ti, Ti6Al4V, CoCrMo, 316L SS // Au alloys, Pd, Ni, Cu Amalgam | 0.9% NaCl 7 ± 0.5, 37 ± 1 | PD 2 | Ecorr (mV), icorr (μA/cm2) | |
| Venugopalan, 1998 [1] | Ti cp grade 2 // Au alloys, AgPd, 316L SS, CoCr, Ni (67%), Ni (70.4%) y Ni (77.5%) Amalgam | AS1 4 without O2 | OCP PD 1 (Emax = 1.2 V) ZRA | E (mV) vs. t (6 h), Eb (mV), EM (mV) vs. t (6 h) Ig (μA/cm2) vs. t (6 h), EM (mV) | |
| Reclaru, 1998 [10] | Ti c.p. grade 4 // Au alloys, Ag, Pd, CoCr y 316L SS | (1) ASFm 5 y ASFm3 + 0.1% F− (without O2) (2) NaCl 1% y NaCl 1% + 0.1% F− 6.15/5/4/3.5/3 | OCP (24 h) PD 1 ZRA MPT6 Crevice corrosion (ASTM F746-81) | SEM 7 | E (mV) vs. t (24 h), i (μA/cm2) vs. E (V) EM (mV) vs. t (24 h), Ig (μA/cm2) vs. t (24 h) |
| Grosgogeat, 1999 [16] | Ti cp, Ti6Al4V // Au alloys, Ag, Pd, CoCr | (1) ASF-M 8: 5, 37 (2) AFNOR 9 with O2: 6.7, 37 | -OCP (24 h) -PD 1 -ZRA (15 h) -TPM 4 | SEM 5 AES 10 | E (mV) vs. t (s), EM (mV), ig (nA/cm2) |
| Foti, 1999 [24] | Ti c.p // Ti, Au alloys | In Vivo | Histology IO 11 | ||
| Horasawa, 1999 [17] | Ti grade 2 // copper alloys and Gallium alloy | AS2 12 6.8, 37 | OCP PD 1 | E (mV) vs. t (s), EM (V) ig (A/cm2) | |
| Cortada, 2000 [21] | Ti cp grade 1 // Ti cp grade 2, Ti cast cp grade 2, Au alloys, Pd, NiCr. | AS3 13 without O2 6.7, 37 | OCP ZRA (250 min) | ICP-MS 14 | Ez, Ecorr, icorr |
| Taher, 2003 [20] | Ti cp grade 1 // Ter Ti 15, SSTi 16, Au, AgPd, NiCr, CoCr 1 (Co 61%-Cr 25%), CoCr 2 (Co 63.5%-Cr 30%) and amalgam | Asm 17 (ASTM, 1978) 7.2 | EM (mV) ig (µA/cm2) | ||
| Oh, 2004 [18] | Ti cp grade 3 // Ti cp grade 3, Au alloy, AgPd, CoCr, NiCr | AS1 2 37 | OCP (5000 s) PD 1, PS 18 | ip (µA/cm2) Eb (mV) i (μA/cm2) vs. E (mV) | |
| Sutow, 2004 [25] | amalgam - amalgam amalgam - noble metal noble metal - noble metal amalgam - non-noble metal amalgam - noble metal - non-noble metal Groups: - News: ≤12 months - Old: >12 months | In vivo 35.1 | -ZRA (15 s) | i-peak (µA) i-15 s (µA) | |
| Al-Ali, 2005 [14] | Ti cp grade 2 // Au alloy, Au-Ag-Pt, Ag-Au-Pd -LW 19 Ti/noble alloy -MA 20 Ti/noble alloy | Ringer Solution | OCP (24 h), Rp (polarization resistance) | Ecorr icorr Ecorr Mixto icorr Mixto | |
| Ciszewski, 2007 [15] | - NiCr and CoCr alloys - Amalgam Combinations: - amalgam/NiCr - amalgam/CoCr -NiCr/CoCr | ASF 21 con O2 5.6, 37 | OCP (6 h) PD 1 EIS 22 | VDA 23 (1, 2, 4, 6, 7 y 30 días) | Ecorr (mV) icorr (µA/cm2) i-peak (µA/cm2) i-15 s (µA/cm2) i-5000 s (µA/cm2) |
| Yamazoe, 2010 [23] | Ti cp and Ti6Al4V // Ti cp, Ti6Al4V, 5 aleaciones nobles MP 24, 5 aleaciones base Au, aleación Ag-Pd-Cu-Au, aleación base Ag -D I/S 25 -C I/S 26 | Lactic acid 1% | ICPE 27 SCLM 28 |
| Alloy | OCP (mV) | Ecorr (mV) | icorr (µA/cm2) | ip (µA/cm2) | Eb (mV) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| AS | ASF−pH3 | AS | ASF−pH3 | AS | ASF−pH3 | AS | ASF−pH3 | AS | ASF−pH3 | |
| Au | 121 ± 7 | 219 ± 18 | 63 ± 15 | 201 ± 1 | 1.7 ± 0.2 | 4.1 ± 0.7 | 12.2 ± 5 | 12.0 ± 0.1 | 1195 ± 7 | 940 ± 1 |
| CoCr | −229 ± 9 | −157 ± 16 | −342 ± 1 | −215 ± 17 | 1.3 ± 0.1 | 2.6 ± 0.4 | 5.3 ± 0.1 | 5.5 ± 0.1 | 790 ± 1 | 849 ± 1 |
| CoCr-c | −611 ± 17 | −237 ± 13 | −738 ± 7 | −246 ± 21 | 5.1 ± 0.5 | 10.3 ± 1.3 | 6.4 ± 0.1 | 189 ± 9 | 853 ± 6 | 890 ± 7 |
| Ti6Al4V | −281 ± 31 | −963 ± 9 | −305 ± 4 | −947 ± 7 | 0.1 ± 0.1 | 293.5 ± 67 | 3.3 ± 0.6 | 975 ± 114 | - | - |
| TiG2 | −309 ± 11 | −954 ± 27 | −311 ± 23 | −925 ± 35 | 0.2 ± 0.1 | 238.0 ± 39 | 3.3 ± 0.3 | 940 ± 112 | - | - |
| NiCrTi | −203 ± 39 | −215 ± 6 | −295 ± 22 | −207 ± 3 | 1.2 ± 0.01 | 43.7 ± 6 | 12.1 ± 1 | 2921 ± 213 | 158 ± 10 | 290 ± 38 |
| Elements | Alloys | |||||
|---|---|---|---|---|---|---|
| %wt | Ti Grade2 (TiG2) | Ti-6Al-4V (Ti6Al4V) | Co-Cr-Mo (CoCr) | Co-Cr-Mo Cast (CoCr-c) | Ni-Cr-Ti (NiCrTi) | Au-Pd (Au) |
| Ti | Bal. | Bal. | 0.006 | 4 | ||
| Al | 5.5–6.5 | 0.005 | ||||
| V | 3.5–4.5 | |||||
| N | 0.03 | 0.05 | 0.16 | |||
| C | 0.1 | 0.08 | 0.036 | |||
| H | 0.015 | 0.012 | ||||
| Fe | 0.3 | 0.25 | 0.07 | <1 | ||
| O | 0.25 | 0.13 | 0.01 | |||
| Co | 65.32 | 59 | ||||
| Cr | 27.42 | 25.5 | 14.5 | |||
| Mo | 5.51 | 5.5 | 9 | |||
| Mn | 0.68 | |||||
| Ni | 0.07 | 72 | ||||
| W | 0.02 | 5 | ||||
| Ga | 3.2 | 1 | ||||
| Nb | LT 0.02 | <1 | ||||
| B | LT 0.01 | <1 | ||||
| Si | 0.66 | <1 | ||||
| Au | 60 | |||||
| Pd | 30.6 | |||||
| In | 8.4 | |||||
| Cu | 0.01 | |||||
| P | 0.004 | |||||
| S | 0.002 | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mellado-Valero, A.; Muñoz, A.I.; Pina, V.G.; Sola-Ruiz, M.F. Electrochemical Behaviour and Galvanic Effects of Titanium Implants Coupled to Metallic Suprastructures in Artificial Saliva. Materials 2018, 11, 171. https://doi.org/10.3390/ma11010171
Mellado-Valero A, Muñoz AI, Pina VG, Sola-Ruiz MF. Electrochemical Behaviour and Galvanic Effects of Titanium Implants Coupled to Metallic Suprastructures in Artificial Saliva. Materials. 2018; 11(1):171. https://doi.org/10.3390/ma11010171
Chicago/Turabian StyleMellado-Valero, Ana, Anna Igual Muñoz, Virginia Guiñón Pina, and Ma Fernanda Sola-Ruiz. 2018. "Electrochemical Behaviour and Galvanic Effects of Titanium Implants Coupled to Metallic Suprastructures in Artificial Saliva" Materials 11, no. 1: 171. https://doi.org/10.3390/ma11010171
APA StyleMellado-Valero, A., Muñoz, A. I., Pina, V. G., & Sola-Ruiz, M. F. (2018). Electrochemical Behaviour and Galvanic Effects of Titanium Implants Coupled to Metallic Suprastructures in Artificial Saliva. Materials, 11(1), 171. https://doi.org/10.3390/ma11010171





